Background: Clozapine is the only medication approved for those patients with schizophrenia who do not achieve a clinical response to standard antipsychotic treatment, yet it is still underused. Furthermore, in the case of a partial or minimal response to clozapine treatment, there is no clarity on the next pharmacologic intervention.
Methods: The National Psychosis Service is a tertiary referral inpatient unit for individuals with refractory psychosis. Data from 2 pooled data sets (for a total of 325 medical records) were analyzed for treatment trajectories between admission and discharge (2001-2016). Effectiveness of pharmacologic treatment was determined using change in symptoms, assessed using the Operational Criteria (OPCRIT) system applied retrospectively to the medical records. Analysis was focused on identifying the optimal medication regimens impacting clinical status during the admission.
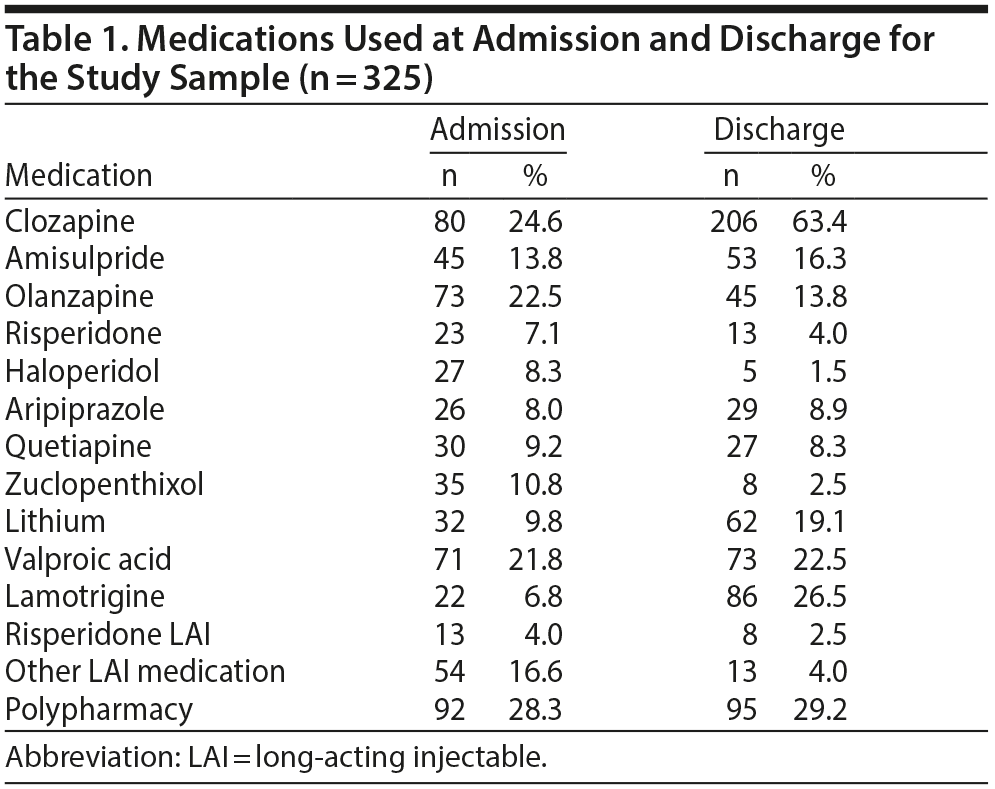
Results: Less than a quarter of the patients were on clozapine treatment at the time of admission; this rate increased to 63.4% at the time of discharge. Initiating clozapine during admission (n = 136) was associated with a 47.9% reduction of symptoms as reflected by their OPCRIT score. In cases in which clozapine monotherapy did not achieve sufficient improvement in symptoms, the most effective clozapine augmentation strategy was adding amisulpride (n = 22, 60.8% reduction of symptoms), followed by adding a mood stabilizer (n = 36, 53.7% reduction). A less favorable option was addition of quetiapine (n = 15, 26.7% reduction).
Conclusions: Many people with longer-term and complex refractory illness do respond to clozapine treatment with suitable augmentation strategies when necessary. Furthermore, it is possible to advance clozapine prescribing in these complex patients when they are supported by a skilled and dedicated multidisciplinary team. The optimal therapeutic approach relies on confirmation of diagnosis and compliance and optimization of clozapine dose using therapeutic drug monitoring, followed by augmentation of clozapine with amisulpride or mood stabilizers. There is some preliminary evidence suggesting that augmentation strategies may impact differentially depending on the symptom profile.

CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation. A $10 processing fee will apply.
CME Objective
After studying this article, you should be able to:
‘ ¢Initiate, optimize, and monitor treatment for refractory psychosis
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Creditâ„¢. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Academy of Physician Assistants (AAPA) accepts certificates of participation for educational activities certified for AMA PRA Category 1 Creditâ„¢ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 1 hour of Category I credit for completing this program.
Release, Expiration, and Review Dates
This educational activity was published in September 2019 and is eligible for AMA PRA Category 1 Creditâ„¢ through October 31, 2021. The latest review of this material was August 2019.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Marlene P. Freeman, MD, Editor in Chief, has received research funding from JayMac and Sage; has been a member of the advisory boards for Otsuka, Alkermes, and Sunovion; has been a member of the Independent Data Safety and Monitoring Committee for Janssen; and, as a Massachusetts General Hospital (MGH) employee, works with the MGH National Pregnancy Registry, which is sponsored by Teva, Alkermes, Otsuka, Actavis, and Sunovion, and works with the MGH Clinical Trials Network and Institute, which receives research funding from multiple pharmaceutical companies and the National Institute of Mental Health. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
ABSTRACT
Background: Clozapine is the only medication approved for those patients with schizophrenia who do not achieve a clinical response to standard antipsychotic treatment, yet it is still underused. Furthermore, in the case of a partial or minimal response to clozapine treatment, there is no clarity on the next pharmacologic intervention.
Methods: The National Psychosis Service is a tertiary referral inpatient unit for individuals with refractory psychosis. Data from 2 pooled data sets (for a total of 325 medical records) were analyzed for treatment trajectories between admission and discharge (2001-2016). Effectiveness of pharmacologic treatment was determined using change in symptoms, assessed using the Operational Criteria (OPCRIT) system applied retrospectively to the medical records. Analysis was focused on identifying the optimal medication regimens impacting clinical status during the admission.
Results: Less than a quarter of the patients were on clozapine treatment at the time of admission; this rate increased to 63.4% at the time of discharge. Initiating clozapine during admission (n = 136) was associated with a 47.9% reduction of symptoms as reflected by their OPCRIT score. In cases in which clozapine monotherapy did not achieve sufficient improvement in symptoms, the most effective clozapine augmentation strategy was adding amisulpride (n = 22, 60.8% reduction of symptoms), followed by adding a mood stabilizer (n = 36, 53.7% reduction). A less favorable option was addition of quetiapine (n = 15, 26.7% reduction).
Conclusions: Many people with longer-term and complex refractory illness do respond to clozapine treatment with suitable augmentation strategies when necessary. Furthermore, it is possible to advance clozapine prescribing in these complex patients when they are supported by a skilled and dedicated multidisciplinary team. The optimal therapeutic approach relies on confirmation of diagnosis and compliance and optimization of clozapine dose using therapeutic drug monitoring, followed by augmentation of clozapine with amisulpride or mood stabilizers. There is some preliminary evidence suggesting that augmentation strategies may impact differentially depending on the symptom profile.
J Clin Psychiatry 2019;80(5):18m12716
To cite: Krivoy A, Joyce D, Tracy D, et al. Real-world outcomes in the management of refractory psychosis. J Clin Psychiatry. 2019;80(5):18m12716.
To share: https://doi.org/10.4088/JCP.18m12716
© Copyright 2019 Physicians Postgraduate Press, Inc.
aNational Psychosis Service, South London, and Maudsley NHS Foundation Trust, London, United Kingdom
bDepartment of Psychosis Studies, the Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, United Kingdom
cSackler Faculty of Medicine, Tel-Aviv University, Ramat-Aviv, Israel
dOxleas NHS Foundation Trust, Green Parks House, Orpington, Kent, London, United Kingdom
eDepartment of Psychiatry, Royal College of Surgeons in Ireland, Beaumont Hospital, Dublin, Ireland
fDepartment of Psychiatry, School of Medicine and Medical Sciences, University College Dublin, St Vincent’s University Hospital, Dublin, Ireland
gCentral and North West London NHS Foundation Trust, London, United Kingdom
*Corresponding author: Amir Krivoy, MD, Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, PO63, De Crespigny Park, London SE5 8AF, UK ([email protected]).
Individuals with schizophrenia have a varied clinical trajectory.1,2 About a third of this cohort do not achieve sufficient clinical benefit from non-clozapine antipsychotic agents,2,3 which all act through blocking the dopamine receptors. Clozapine is the only evidence-based treatment for treatment-resistant schizophrenia (TRS).4,5 However, not only is it underutilized,6 but it is also used very late in the illness, and only 40%-50% of those receiving treatment will experience a sufficiently robust clinical response to be able to achieve recovery.7,8 Patients with treatment-refractory illness suffer from an enormous personal burden as well as a marked socioeconomic impact on them, on their carers, and on society.9
Current guidelines for pharmacologic management of clozapine-resistant schizophrenia illustrate conflicting evidence with little consensus.10,11 The current Maudsley prescribing guidelines12 suggest that there are no optimal evidence-based options following failure of clozapine treatment. Data from previous research13 suggested that the augmentation of clozapine with sodium valproate, lithium, amisulpride, or quetiapine has been useful in clozapine-refractory patients. A meta-analysis14 of 14 clozapine augmentation studies showed that adding a second antipsychotic conferred a small benefit over placebo (effect size = −0.239, P = .028); another meta-analysis15 of 46 studies concluded that the most effective augmentation agents for total psychosis symptoms were aripiprazole (standardized mean difference = 0.48), fluoxetine (standardized mean difference = 0.73), and sodium valproate (standardized mean difference = 2.36). However, many of these attempts to synthesize the data included poor-quality studies. A more robust recent Cochrane review16 of different antipsychotic drugs used to augment clozapine concluded that a formal meta-analysis could not be undertaken since the reliability of results is limited, with evidence being of low or very low quality. Despite the enormous personal and clinical burden, there is a lack of adequate pharmacologic research in this cohort. Thus, the clinician is faced with a lack of evidence, rather than evidence of absence of effect, in what may constitute one-sixth of psychosis patients.
The National Psychosis Service (NPS) is a tertiary-level specialist service located in London, United Kingdom, designed to offer expert care for individuals with psychosis,13,17 largely TRS, clozapine-resistant schizophrenia, and schizoaffective disorder. The NPS offers a holistic approach to the wide range of difficulties observed in these complex patients; this approach includes an active psychology treatment package delivered by experienced cognitive-behavioral therapists and family work, a comprehensive occupational therapy program, and pharmacologic management by academic consultant psychiatrists affiliated to the Psychosis Clinical Academic Group at the Institute of Psychiatry, Psychology and Neuroscience, King’s College London. The team also includes specialist mental health pharmacists, doctors in specialist training, mental health nurses, and a social worker. There is a close collaboration with hematology, cardiology, and other medical specialists within the local academic health center, King’s Health Partners.
This unique constellation allows a clinical effectiveness exploration of outcomes in a large cohort of treatment-refractory patients being managed in the same unit with a common philosophy. Furthermore, treatment approaches are bespoke to each patient and are refined according to response, so patients go through several personalized medication trials during their admission; thus, this cohort allows assessment of effectiveness of different treatment options in a real-world setting. Herein we set out to (a) evaluate the feasibility of systematically rationalizing pharmacologic treatment (indexed by increased rates of clozapine prescribing either as monotherapy or with augmentation during admission) in this unique population; (b) identify the most common clozapine augmentation strategies (medications prescribed at discharge, as a posited index of their optimal tolerated treatment); and (c) conduct an exploratory analysis of the impact of these different medications on overall outcome and on specific symptom domains.
METHODS
Population
We retrospectively examined clinical effectiveness outcomes from all consecutive admissions between 2001 and 2017 to the National Psychosis Service, South London and Maudsley NHS Foundation Trust. We pooled together data from 2 cohorts; the first was described earlier13 and included 153 medical records of patients admitted between the years 2001 and 2007 (mean [SD] age = 33.5 [10.9] years, 54.2% males), whereas the second included 172 medical records of patients admitted between the years 2008 and 2016 (mean [SD] age = 33.6 [12.1] years, 51.7% males). The study was performed as part of an audit approved by the Clinical Academic Group of South London and Maudsley NHS Foundation Trust, United Kingdom.
Medical records were reviewed by 4 independent medical raters affiliated to the team, and demographic and clinical data were extracted using the Operational Criteria (OPCRIT) system. Interrater reliability was optimized by common rating of a set of 10 different medical records and discussion around any differences; any subsequent uncertainty in any ratings was discussed with one of the authors (S.S.S). All participants had a chronic psychotic disorder, and most of them met ICD-10 criteria for a primary diagnosis of schizophrenia. The only exclusion criterion for patient admission to the National Psychosis Service is if an individual poses a threat to others through significant violence or has severe drug or alcohol addiction problems.
Measures
The OPCRIT is a widely used, reliable, and validated tool18 to extract symptom-level data from medical records, utilizing an inventory of psychopathological symptoms, demographics, and disease course variables that are scored with algorithms for clinical diagnosis.19 The medical records on admission to and discharge from the NPS were assessed to yield OPCRIT mental state examination (MSE) severity scores for each time point, across 5 domains. Each symptom domain score is ordinal (with zero indicating an absence of those symptoms and higher values indicating increasing symptom severity): affective symptoms (16 items: maximum score = 58), abnormal perceptions (6 items: maximum score = 12), abnormal beliefs (18 items: maximum score = 41), speech and thought disorders (6 items: maximum score = 13), and appearance and behavior (9 items: maximum score = 24). Demographic information was collated for all participants, and medications, including dose, on admission and discharge were recorded. Complete data at both time points were available for analysis of OPCRIT in 314 of 325 patients. Scores were missing due to lack of sufficient clinical data to deduce severity.
Statistical Analysis
Patients were divided into groups according to their medications at admission and discharge. OPCRIT scores were calculated for each of the 5 symptom domains. To overcome missing data for specific items in the domains, we calculated each domain score as a mean of available items (ie, not missing). Total score was calculated as the mean of all 5 domains’ scores. For each group, the change in total OPCRIT score was calculated as a percentage (baseline score – discharge score)/(baseline score). Each medication change (“journey”) group included patients treated with medication when there was a minimum of 15 patients treated with the drug at admission or discharge. A difference in mean clozapine dose between groups, when available, was performed using the Student t test.
RESULTS
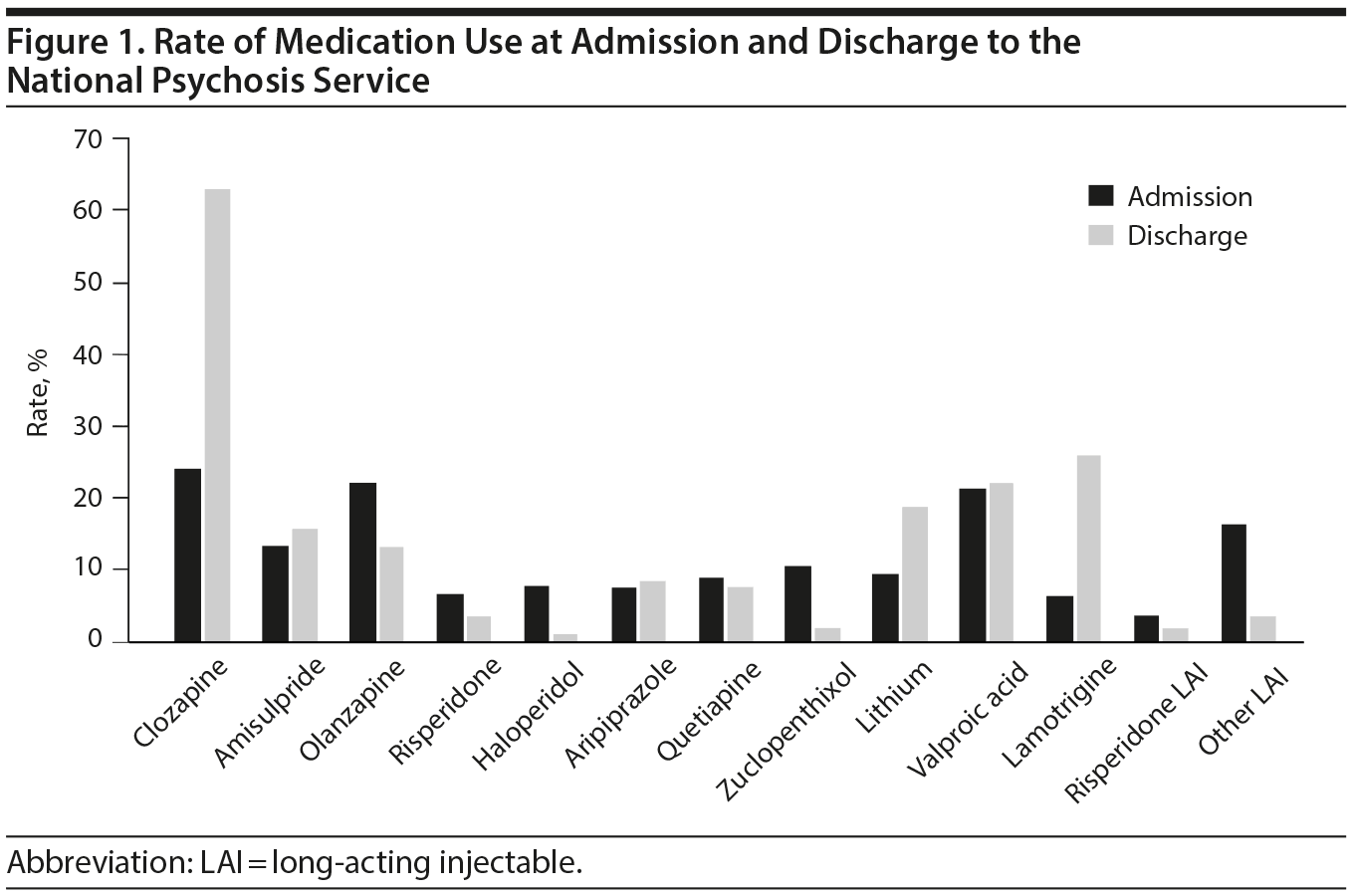
The 2 pooled samples comprised 325 medical records, composed of 153 females (47.1%) and 172 males (52.9%) with a mean (SD) age of 35.4 (11.7) years at admission (range, 16-78 years). Mean (SD) length of admission was 299 (206) days, the median was 251 days, and admissions ranged between 10 and 1,233 days. Whereas approximately 25% of patients were treated with clozapine on admission, this rate increased to 63.4% on discharge. Table 1 and Figure 1 depict the frequencies of commonly prescribed antipsychotic drugs and mood stabilizer treatments at admission and discharge. Patients admitted with clozapine had a mean (SD) dose of 448 (192) mg daily, whereas those discharged with clozapine had a mean (SD) dose of 445 (196) mg daily. Other notable changes in medication use were a decreased use of LAIs and first-generation antipsychotic medications at discharge and an increase in the frequency of use of mood stabilizers at discharge.
Change in Total OPCRIT Score
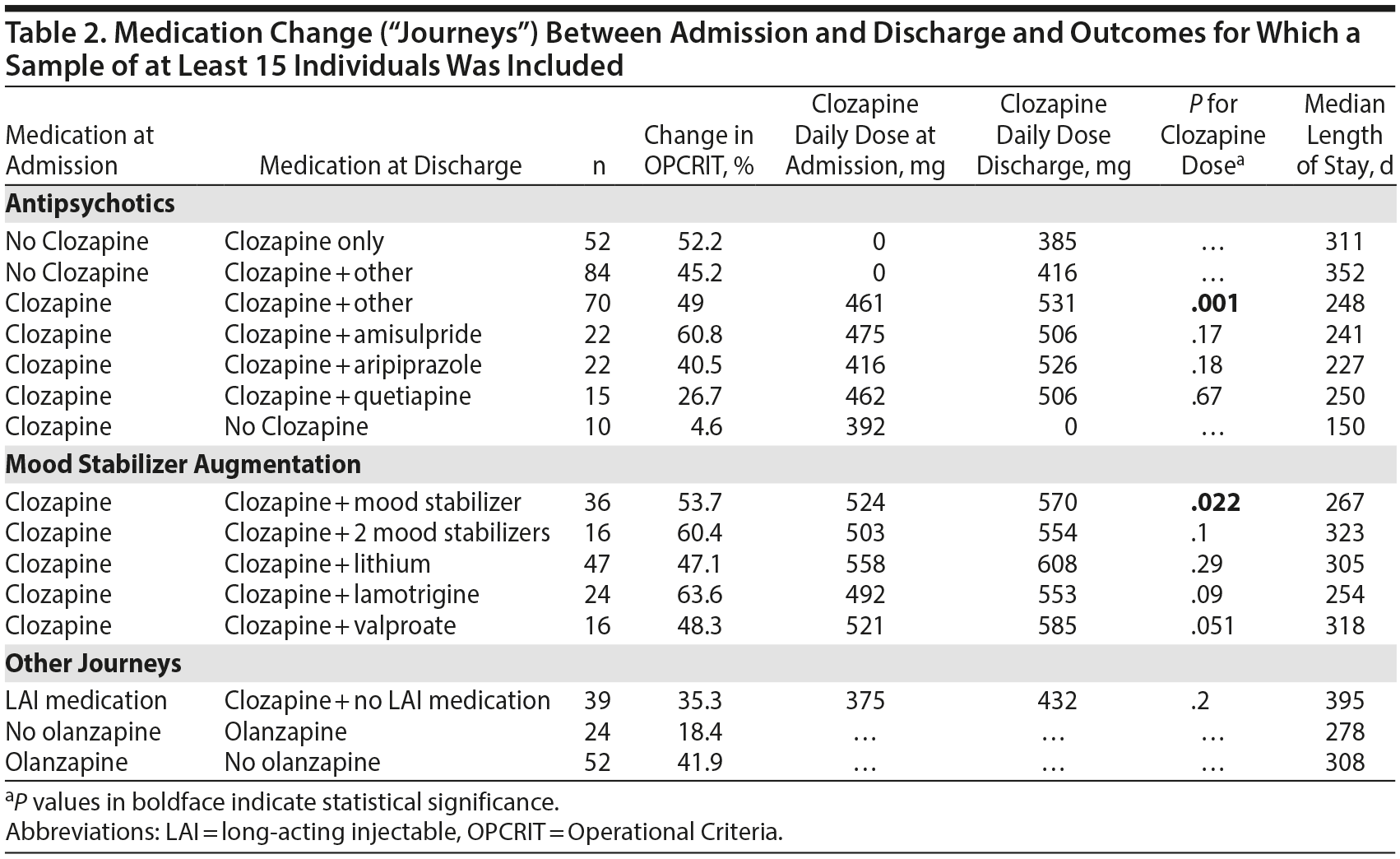
The change in the mean (SD) total OPCRIT score of the entire sample was significant from admission to discharge (0.52 [0.32] to 0.29 [0.27]; t = 15.95; P < .0001, respectively, a reduction of 44.2%). The largest medication journey group was composed of patients who were admitted without clozapine and were discharged on clozapine treatment (n = 136, 41.8% of the sample, 47.9% change in total OPCRIT score); of these, 84 (61.8%) had augmentation of clozapine with another antipsychotic at discharge. Table 2 shows the mean change in OPCRIT total scores from admission to discharge in the most prevalent medication journeys (ie, at least 15 patients). Less symptomatic change was seen with augmentation of clozapine with quetiapine (27%) and from groups with smaller size, eg, with risperidone augmentation of clozapine (n = 6) demonstrating more modest changes of around 35%.
OPCRIT Domains’ Change
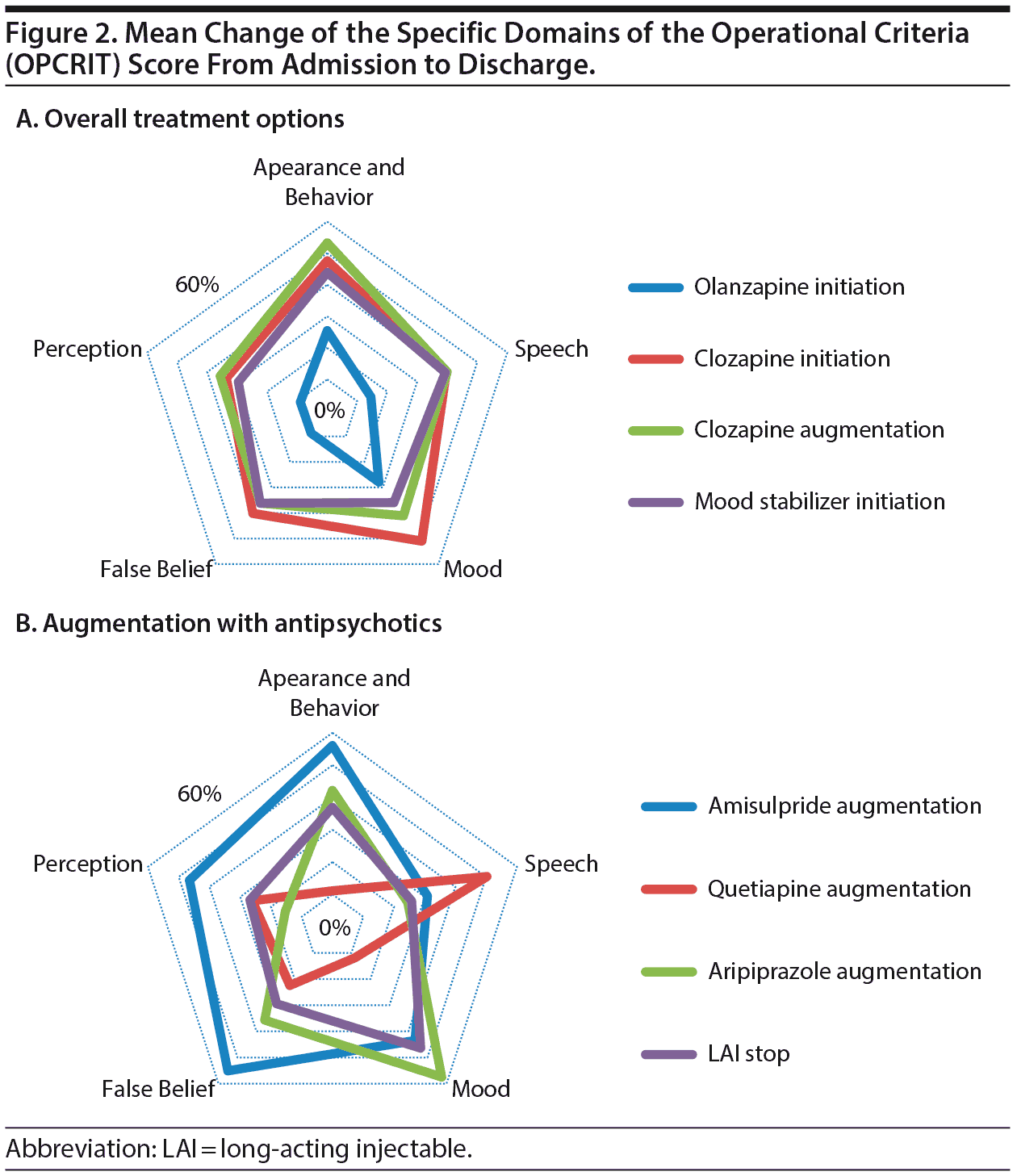
We examined the impact of the different treatment strategies on the 5 specific domains’ scores on the MSE as reflected by the OPCRIT: appearance and behavior, speech, mood, belief, and perceptual disturbance. Initiating clozapine or augmenting clozapine with other compounds had similar spread of effect, as shown in Figure 2, with the most striking elements being that clozapine treatment is effective across all of these symptom domains and clearly more effective across all domains compared with olanzapine. Clozapine augmentation with 3 of the main antipsychotic compounds (amisulpride, aripiprazole, and quetiapine) demonstrated some differential effects; amisulpride augmentation appeared to be associated with managing positive symptoms such as delusions and hallucinations; aripiprazole augmentation was more associated with improved mood symptoms, whereas quetiapine was associated with improvement in thought-disordered speech (see Figure 2). Augmenting clozapine with mood stabilizers was also linked to broad symptomatic benefit, but perhaps surprisingly, there was no differential impact on mood symptoms.
DISCUSSION
In this study, we described a real-world approach to the pharmacologic management of refractory psychosis based on personalized clinical decision making within a specialized inpatient unit. The main finding in our study is that it is possible to safely initiate or re-challenge with clozapine in 43% of refractory psychosis patients who were not treated with clozapine at admission and that this intervention was highly effective in reducing their symptom load (reduction in MSE symptoms of 47.9%). Almost all patients admitted to the NPS either had been treated with clozapine in the past, having been noncompliant or suffered significant adverse effects, or had been considered for treatment and refused it. This finding speaks to the need for a positive therapeutic environment encouraging clozapine use, supported by skilled psychological and occupational therapy. Clozapine augmentation might be effective in those for whom monotherapy is not an option.

- Clozapine is underused in continuous complex psychosis, and in the case of a partial or minimal response, there is no clarity on the next pharmacologic intervention.
- Real-world data suggest that precision in diagnosis should be sought and addressed, followed by optimization of clozapine treatment before declaring clinical resistance to clozapine.
- In the case of insufficient clinical response to clozapine monotherapy, augmentation with amisulpride, aripiprazole, or a mood stabilizer might help in achieving clinical stabilization.
The primary intervention in the NPS cohort was initiating (or re-challenging with) clozapine, as only a quarter of patients were treated with clozapine on admission and almost two-thirds were being treated with clozapine on discharge. This evidence-based approach was associated with overall improvement in the OPCRIT score of 47.9%, with improvement across the entire spectrum of symptoms. While there is a consensus on the clinical efficacy of clozapine in TRS4,5 and the importance of early intervention,20,21 in practice, clozapine prescribing rates vary greatly across different countries and even between different regions within a country.6,22 Patients with TRS coming to the NPS have usually been treated with several antipsychotic medications, including clozapine, with limited effective clinical response or limiting adverse effects. The data strongly support the case for re-initiating clozapine, even in cases in which clozapine was deemed ineffective or had to be stopped due to adverse effects. Interestingly, when patients were admitted while receiving clozapine and were discharged with clozapine augmentation, there was an increase in the dose of clozapine at discharge. This finding may strengthen the notion that optimization of clozapine dose is a crucial aspect in the management of TRS23; the standard practice of the NPS is to monitor plasma clozapine levels on a regular basis and to titrate the dose according to clinical response. When clozapine is only partially effective, a plasma level of above 0.5 mg/L is sought. Data from the toxicology laboratory that monitors plasma clozapine level requests across the United Kingdom24 indicate that up to 40% of samples have a level below 0.35 mg/L.
However, in the case of a partial or minimal response to clozapine treatment, there is no clarity in the wider literature on the next pharmacologic intervention.16,25 Following clozapine dose optimization and confirmation of diagnosis and compliance, the usual clinical approach is to augment clozapine with another psychotropic compound, usually another antipsychotic; however, there is no consensus on which compound is the most effective in this setting.14,15 The standard approach in the NPS is to use clozapine as a baseline intervention for subsequent augmentation. In the absence of comparative studies between different augmentation strategies, these real-world data suggest that augmenting clozapine with amisulpride or with mood stabilizers such as lithium, valproic acid, or lamotrigine if mood disturbance is indicated was associated with the greatest clinical improvement (as reflected by OPCRIT score at discharge). Clozapine augmentation with aripiprazole was associated with an intermediate success and may confer some benefit in managing metabolic side effects,26 whereas augmenting with quetiapine or risperidone was associated with less improvement in clinical status. Augmentation strategy was associated with longer length of stay, as it most probably reflects the time needed to achieve clinical stabilization, and therefore the numbers and complexity of medication changes during admission. Perhaps unsurprisingly, the data show that patients who had their clozapine stopped or could not be treated with clozapine and were treated with other antipsychotics had the poorest outcomes, with little change from baseline. This finding provides some evidence supporting the real-life methodology used in the study. Indeed, there was a large decrease in the use of LAI and first-generation antipsychotic medication over the admission; this decrease could also be anticipated because if there is no response to antipsychotic medications—such as LAIs, with which compliance is assured—then continuing treatment is unlikely to confer any further benefit.
In the NPS cohort, the most prevalent clozapine augmentation strategy at discharge was with amisulpride. Twenty-two patients were given clozapine at admission and were discharged with the combination of clozapine and amisulpride, with no significant change in clozapine daily dose. This combination was associated with about 60% improvement in the OPCRIT score. This intervention is usually motivated by the pharmacologic hypothesis that adding a high-affinity dopamine receptor type 2 antagonist to clozapine (a weak D2 blocker) may enhance the combined clinical efficacy.27 Several studies have investigated amisulpride for augmentation of clozapine. A recent study28 randomized 68 TRS patients to amisulpride or placebo as an augmentation for clozapine; however, in that study there was no significant difference in clinical response between groups. An earlier clinical trial29 comparing amisulpride and quetiapine augmentation of clozapine in 50 TRS patients found both interventions to be effective, with amisulpride having a greater effect. Although a more robust Cochrane review16 found no high-quality evidence for a benefit of any psychotropic addition to clozapine, our data suggest that some patients will benefit from the addition of a potent D2-blocker compound, such as amisulpride, as the next step in managing clozapine-refractory patients. Furthermore, the more exploratory analyses examining differential effects on specific symptom domains suggest that treatment with clozapine impacts across all symptom domains; clozapine augmentation with amisulpride impacts perceptual abnormalities and delusional beliefs, while augmentation with aripiprazole impacts mood symptoms. The difference between real-world outcomes and randomized clinical trial data can occur for several reasons. The real-world sample tends to be much more reflective of standard clinical practice (including patients with greater severity of symptoms, increasing degrees of physical comorbidity, and poorer insight and a lack of capacity to consent to treatment) and often consists of patients considered at risk of significant self-harm or who are already receiving treatment with a combination of other medications—all of whom are usually excluded from randomized clinical studies. However, the clinical trials will select a medication regimen or sequential regimens to apply to everyone, while the real-life studies will personalize the regimen to suit each individual patient. A formal statistical approach to maximize the value from real-world studies is to treat these studies as multiple n = 1 designs to ensure a more robust prospective treatment protocol with high-quality assessment.30
Augmentation of clozapine with aripiprazole (n = 22) was associated with a modest improvement of 40.5% in the OPCRIT total score, mostly on the mood symptoms domain. Some evidence supports aripiprazole augmentation in clozapine-refractory cases. Siskind et al15 reviewed 7 clinical trials of aripiprazole augmentation of clozapine and meta-analyzed a combined significant effect of 0.57 favoring aripiprazole versus placebo. However, the results were no longer significant for any of the psychosis outcomes when analyses were restricted to the higher-quality studies and those that used rating scales to define clozapine resistance. Another meta-analysis of aripiprazole augmentation31 showed only trends of benefit with a greater effect on weight gain and other metabolic parameters. Therefore, our results may point to a limited efficacy of aripiprazole addition, especially in cases in which it has been added for its metabolic advantage.
The addition of a mood stabilizer was associated with improvement (n = 36) during the NPS admission. One key aspect of clinical care is confirming the diagnosis and clarifying any masked comorbid diagnoses that may include comorbid mood disorders. Previous clinical trials of mood-stabilizer augmentation of clozapine have shown mixed results. Several meta-analyses15,32,33 demonstrated positive effects of sodium valproate augmentation of clozapine on total psychosis symptoms, although all of those studies were of low quality, while the high-quality studies of lamotrigine were nonsignificant. Lithium augmentation was again investigated only in small low-quality studies. It is worth noting that a significant proportion of patients admitted with low white blood cell counts are commenced on adjunct treatment with lithium to elevate these counts and prevent spurious blood monitoring warnings. Similarly, valproate or lamotrigine is used as seizure prophylaxis when clozapine levels are suprathreshold.
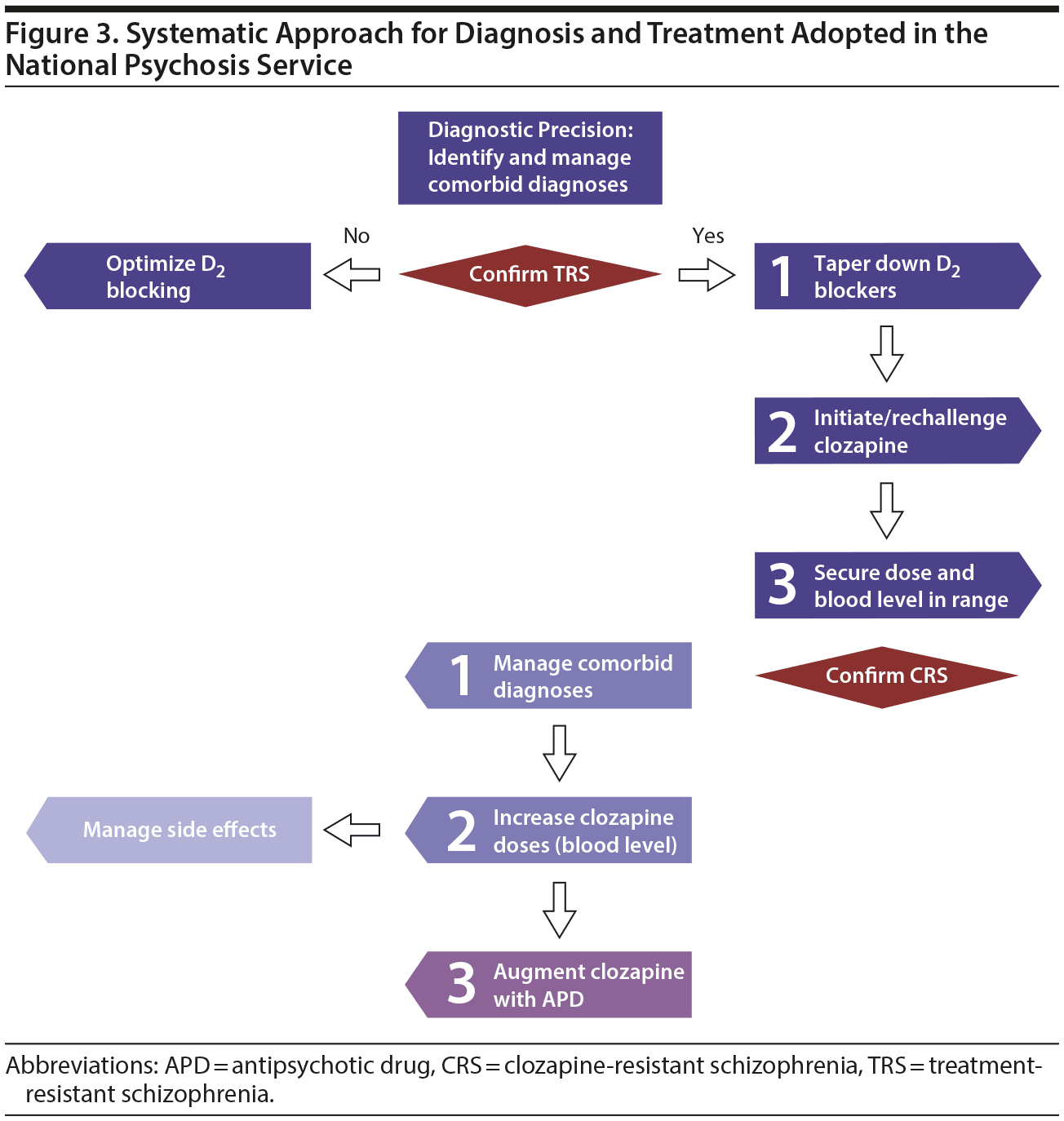
There are obvious issues with rater, indication, and sampling bias in the use of retrospective cohort data compared with prospective blinded data from clinical trials. Moreover, real-world data are usually more confounded than data derived from randomized controlled trials. However, given the lack of adequately powered studies of multiple sequential pharmacologic intervention in treatment-resistant schizophrenia, this study offers the advantage of assembling treatment data from a large group of patients with relatively homogenous clinical presentation. The analysis is predicated on the assumption that the clinical presentation and medication upon discharge reflected the optimal choice for each patient journey. Furthermore, data are mainly descriptive and some groups are rather small, so inferential conclusions are difficult to make. Another limitation is that the numbers for each treatment approach do not take into account those who had time-limited exposures to medications that were discontinued before discharge. Moreover, we did not analyze the effect of combined augmentation strategies, such as augmentation with both a mood stabilizer and an antipsychotic. One could conceptualize this approach as the NPS operating as a “black box” in which the patient goes through a clinical process designed to achieve optimal outcomes through optimizing his or her pharmacologic treatment—enabling a step-down to less restrictive accommodation.17 This process is predicated on multidisciplinary decision making and clinical work, operating as a common thread in all patients (see Figure 3 for description of the systematic approach adopted in the NPS).
In summary, there is potential for positive symptomatic change allied to rationalization of medication in patients with a diagnosis of refractory psychosis. A positive hopeful approach34 is at the core of the NPS philosophy. The optimal strategy is to initiate clozapine treatment whenever possible—a practice that is well supported by an extensive literature. Doing so may require clinicians to enhance their expertise in the use of clozapine, particularly in the monitoring for, and proactive management of, any adverse effects. This strategy would then lead to increased confidence in safe clozapine rechallenge when necessary. The clozapine dose is optimized, using plasma level monitoring, and then augmented with either amisulpride or mood stabilizers in the first instance. In specific circumstances, augmentation of clozapine with aripiprazole could be considered for control of metabolic dysregulation.
Submitted: December 23, 2018; accepted May 2, 2019.
Published online: September 24, 2019.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, clozapine is the only medication approved by the US Food and Drug Administration for treatment-resistant schizophrenia.
Financial disclosure: Dr Gaughran has received honoraria for advisory work and lectures or Continuing Medical Education activity support from Roche, Bristol-Myers Squibb, Lundbeck, Otsuka, Janssen, and Sunovion; is a collaborator on a National Health Service (NHS) Innovations project co-funded by Janssen; and has a family member with professional links, including shares, to Lilly and GlaxoSmithKline. Dr Shergill is supported by a European Research Council Consolidator Award (#311686). This study represents independent research partly funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London and a joint infrastructure grant from Guy’s and St Thomas’ Charity and the Maudsley Charity. Drs Krivoy, Joyce, Tracy, MacCabe, Lally, and Sarkar and Mr Whiskey have no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
Funding/support: No direct funding was provided for this research.
Disclaimer: The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
This CME activity is expired. For more CME activities, visit cme.psychiatrist.com.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCES
1.Levine SZ, Rabinowitz J, Case M, et al. Treatment response trajectories and their antecedents in recent-onset psychosis: a 2-year prospective study. J Clin Psychopharmacol. 2010;30(4):446-449. PubMed CrossRef
2.Lally J, Ajnakina O, Stubbs B, et al. Remission and recovery from first-episode psychosis in adults: systematic review and meta-analysis of long-term outcome studies. Br J Psychiatry. 2017;211(6):350-358. PubMed CrossRef
3.Demjaha A, Lappin JM, Stahl D, et al. Antipsychotic treatment resistance in first-episode psychosis: prevalence, subtypes and predictors. Psychol Med. 2017;47(11):1981-1989. PubMed CrossRef
4.Siskind D, McCartney L, Goldschlager R, et al. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209(5):385-392. PubMed CrossRef
5.Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796. PubMed CrossRef
6.Bachmann CJ, Aagaard L, Bernardo M, et al. International trends in clozapine use: a study in 17 countries. Acta Psychiatr Scand. 2017;136(1):37-51. PubMed CrossRef
7.Lieberman JA, Safferman AZ, Pollack S, et al. Clinical effects of clozapine in chronic schizophrenia: response to treatment and predictors of outcome. Am J Psychiatry. 1994;151(12):1744-1752. PubMed CrossRef
8.Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry. 2017;62(11):772-777. PubMed CrossRef
9.Kennedy JL, Altar CA, Taylor DL, et al. The social and economic burden of treatment-resistant schizophrenia: a systematic literature review. Int Clin Psychopharmacol. 2014;29(2):63-76. PubMed CrossRef
10.Royal Australian and New Zealand College of Psychiatrists Clinical Practice Guidelines Team for the Treatment of Schizophrenia and Related Disorders. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of schizophrenia and related disorders. Aust N Z J Psychiatry. 2005;39(1-2):1-30. PubMed
11.Remington G, Addington D, Honer W, et al. Guidelines for the pharmacotherapy of schizophrenia in adults. Can J Psychiatry. 2017;62(9):604-616. PubMed CrossRef
12.Beck K, McCutcheon R, Bloomfield MA, et al. The practical management of refractory schizophrenia—the Maudsley Treatment REview and Assessment Team service approach. Acta Psychiatr Scand. 2014;130(6):427-438. PubMed CrossRef
13.Tracy DK, Joyce DW, Sarkar SN, et al. Skating on thin ice: pragmatic prescribing for medication refractory schizophrenia. BMC Psychiatry. 2015;15(1):174. PubMed CrossRef
14.Taylor DM, Smith L, Gee SH, et al. Augmentation of clozapine with a second antipsychotic—a meta-analysis. Acta Psychiatr Scand. 2012;125(1):15-24. PubMed CrossRef
15.Siskind DJ, Lee M, Ravindran A, et al. Augmentation strategies for clozapine refractory schizophrenia: a systematic review and meta-analysis. Aust N Z J Psychiatry. 2018;52(8):751-767. PubMed CrossRef
16.Barber S, Olotu U, Corsi M, et al. Clozapine combined with different antipsychotic drugs for treatment-resistant schizophrenia. Cochrane Database Syst Rev. 2017;3:CD006324. PubMed
17.Sarkar SN, Tracy DK, Fernandez MJ, et al. Unheard voices: outcomes of tertiary care for treatment-refractory psychosis. Psychiatr Bull (2014). 2014;38(2):71-74. PubMed CrossRef
18.Brittain PJ, Stahl D, Rucker J, et al. A review of the reliability and validity of OPCRIT in relation to its use for the routine clinical assessment of mental health patients. Int J Methods Psychiatr Res. 2013;22(2):110-137. PubMed CrossRef
19.McGuffin P, Farmer A, Harvey I. A polydiagnostic application of operational criteria in studies of psychotic illness: development and reliability of the OPCRIT system. Arch Gen Psychiatry. 1991;48(8):764-770. PubMed CrossRef
20.Howes OD, Vergunst F, Gee S, et al. Adherence to treatment guidelines in clinical practice: study of antipsychotic treatment prior to clozapine initiation. Br J Psychiatry. 2012;201(6):481-485. PubMed CrossRef
21.Kahn RS, Winter van Rossum I, Leucht S, et al; OPTiMiSE study group. Amisulpride and olanzapine followed by open-label treatment with clozapine in first-episode schizophrenia and schizophreniform disorder (OPTiMiSE): a three-phase switching study. Lancet Psychiatry. 2018;5(10):797-807. PubMed CrossRef
22.Warnez S, Alessi-Severini S. Clozapine: a review of clinical practice guidelines and prescribing trends. BMC Psychiatry. 2014;14(1):102. PubMed CrossRef
23.Subramanian S, Völlm BA, Huband N. Clozapine dose for schizophrenia. Cochrane Database Syst Rev. 2017;6:CD009555. PubMed
24.Couchman L, Morgan PE, Spencer EP, et al. Plasma clozapine, norclozapine, and the clozapine:norclozapine ratio in relation to prescribed dose and other factors: data from a therapeutic drug monitoring service, 1993-2007. Ther Drug Monit. 2010;32(4):438-447. PubMed CrossRef
25.Sommer IE, Begemann MJH, Temmerman A, et al. Pharmacological augmentation strategies for schizophrenia patients with insufficient response to clozapine: a quantitative literature review. Schizophr Bull. 2012;38(5):1003-1011. PubMed CrossRef
26.Fan X, Borba CPC, Copeland P, et al. Metabolic effects of adjunctive aripiprazole in clozapine-treated patients with schizophrenia. Acta Psychiatr Scand. 2013;127(3):217-226. PubMed CrossRef
27.Shiloh R, Zemishlany Z, Aizenberg D, et al. Sulpiride augmentation in people with schizophrenia partially responsive to clozapine: a double-blind, placebo-controlled study. Br J Psychiatry. 1997;171(6):569-573. PubMed CrossRef
28.Barnes TRE, Leeson V, Paton C, et al. Amisulpride augmentation of clozapine for treatment-refractory schizophrenia: a double-blind, placebo-controlled trial. Ther Adv Psychopharmacol. 2018;8(7):185-197. PubMed CrossRef
29.Genç Y, Taner E, Candansayar S. Comparison of clozapine-amisulpride and clozapine-quetiapine combinations for patients with schizophrenia who are partially responsive to clozapine: a single-blind randomized study. Adv Ther. 2007;24(1):1-13. PubMed CrossRef
30.Senn S. Sample size considerations for n-of-1 trials. Stat Methods Med Res. 2019;28(2):372-383. PubMed CrossRef
31.Srisurapanont M, Suttajit S, Maneeton N, et al. Efficacy and safety of aripiprazole augmentation of clozapine in schizophrenia: a systematic review and meta-analysis of randomized-controlled trials. J Psychiatr Res. 2015;62:38-47. PubMed CrossRef
32.Zheng W, Xiang Y-T, Yang X-H, et al. Clozapine augmentation with antiepileptic drugs for treatment-resistant schizophrenia: a meta-analysis of randomized controlled trials. J Clin Psychiatry. 2017;78(5):e498-e505. PubMed CrossRef
33.Porcelli S, Balzarro B, Serretti A. Clozapine resistance: augmentation strategies. Eur Neuropsychopharmacol. 2012;22(3):165-182. PubMed CrossRef
34.Bressan RA, Grohs GEM, Matos G, et al. Hope or hype in the treatment of schizophrenia—what’s the role of the physician? Br J Psychiatry. 2018;212(1):1-3. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top