Click to enlarge page
Cognition is a renewed area of clinical focus in major depressive disorder (MDD). Cognitive problems such as difficulty concentrating and making decisions are common symptoms of MDD, and while these symptoms improve as depressive symptoms improve in some patients, a substantial portion of patients experience cognitive impairment even while euthymic. In turn, impaired cognition hinders restoration of psychosocial functioning, allowing for continued difficulty at work, at home, and in social settings. Therefore, clinicians must routinely measure and manage cognitive functioning in patients with MDD.
This CME activity is expired. For more CME activities, visit CMEInstitute.com.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders

This Academic Highlights section of The Journal of Clinical Psychiatry presents the highlights of the planning teleconference series “Cognition in the Depressed Patient,” which was held in September and October 2014. This report was prepared and independently developed by the CME Institute of Physicians Postgraduate Press, Inc., and was supported by an educational grant from Takeda International, Inc., U.S. Region and Lundbeck.
The teleconference was chaired by George I. Papakostas, MD, from the Clinical Trials Network and Institute, Massachusetts General Hospital, Boston. The faculty member was Larry Culpepper, MD, MPH, from the Department of Family Medicine, Boston University School of Medicine, Boston, Massachusetts.
Financial disclosure: Dr Papakostas has served as a consultant for Abbott, AstraZeneca, Avanir, Brainsway, Bristol-Myers Squibb, Cephalon, Dey, Eli Lilly, GlaxoSmithKline, Evotec, Lundbeck, Inflabloc, Jannsen, Jazz, Johnson & Johnson, Novartis, Otsuka, Pamlab, Pfizer, Pierre Fabre, Ridge Diagnostics, Shire, Sunovion, Takeda, Theracos, and Wyeth; has received honoraria from Abbott, AstraZeneca, Avanir, Bristol-Myers Squibb, Brainsway, Cephalon, Dey, Eli Lilly, Evotec, GlaxoSmithKline, Inflabloc, Jazz, Lundbeck, Novartis, Otsuka, Pamlab, Pfizer, Pierre Fabre, Ridge Diagnostics, Shire, Sunovion, Takeda, Theracos, Titan, and Wyeth; has received research support from AstraZeneca, Bristol-Myers Squibb, Forest, NIMH, Pamlab, Pfizer, Ridge Diagnostics, Sunovion, and Theracos; and has served on the speaker’s bureau for Bristol-Myers Squibb and Pfizer. Dr Culpepper is a consultant for AstraZeneca, Forest, Jazz, Lundbeck, Merck, Shire, Sunovion, Takeda, and Elsevier Press. He owns stock in M3 (My Mood Monitor) and receives royalties from UpToDate, Oxford University Press.
The opinions expressed herein are those of the faculty and do not necessarily reflect the opinions of the CME provider and publisher or the commercial supporters.
J Clin Psychiatry 2015;76(4):418-425 (doi:10.4088/JCP.13086ah1c)
© Copyright 2015 Physicians Postgraduate Press, Inc.
Cognition is a renewed area of clinical focus in major depressive disorder (MDD). Cognitive problems such as difficulty concentrating and making decisions are common symptoms of MDD, and while these symptoms improve as depressive symptoms improve in some patients,1,2 a substantial portion of patients experience cognitive impairment even while euthymic. In turn, impaired cognition hinders restoration of psychosocial functioning,3 allowing for continued difficulty at work, at home, and in social settings.4 Therefore, clinicians must routinely measure and manage cognitive functioning in patients with MDD.
General Components of Cognitive Functioning
The basic components of cognitive functioning are broadly defined as follows3:
- Attention: the ability to focus on a stimulus of interest against a background of stimuli considered irrelevant and potentially distracting
- Immediate memory: the ability to remember something a short time after it was presented
- Delayed memory: the ability to recall something presented in the past
- Cognitive speed: the rate at which different mental processes and tasks happen
- Executive functioning: the ability to integrate sensory input and memory in order to complete a task.
Executive functioning is a multidimensional process with several requirements. First, it requires focusing motivational input or positive affect to complete a task or achieve a goal.5 Second, executive functioning requires being able to ignore negative affective stimuli. For example, fear and anxiety can overpower problem-solving skills and organized action, increasing the chance of errors or even inactivity. Finally, executive functioning also requires the ability to ignore irrelevant stimuli, allowing the person to focus on a task and to create a plan for accomplishing it, as well as the ability to quickly and accurately access memory.
Cognitive Deficits in MDD
Cognition can be divided into cold cognition (where the task is, for the most part, emotion-independent) and hot cognition (where the task is emotion-laden), even though both cold and hot cognition have affective requirements, noted above.6 For patients with MDD, the most replicated cold cognition deficits occur in the domains of executive function, attention, working memory, and general and psychomotor processing speed.7 Between 20% and 30% of individuals with MDD have executive function deficits.7
Problems in hot cognition associated with MDD include misinterpretation of social cues and negative bias.6 Negative bias is a negative perception of and response to sensory inputs and experiences, with increased focus on negative information compared with positive information.8 Negative bias in patients with MDD may contribute to difficulties in social interactions as well as feelings of sadness, frustration, and low self-esteem, while normalized perception can develop with antidepressant treatment.9,10
Neuroanatomy and Physiology of Cognition
Research on the neuroanatomy and physiology of cognition is rapidly expanding, with applications for the treatment of MDD and other psychiatric conditions.8 Cognitive processes once considered to be based in specific brain areas are now understood to result from interconnections involving multiple areas of the brain.11
A key recent advance is in understanding the functions and interrelationships between 3 brain networks: the central executive network (CEN), the salience network (SN), and the default mode network (DMN).12,13 Alterations in these networks manifest not only in symptoms that contribute to psychiatric disorders such as MDD14 but also in cognitive deficits that may be less evident to the clinician. For many patients, these deficits and the underlying network alterations are present before symptoms develop and persist between episodes, suggesting that they are core processes in the development of the illness.

- Cognition is both an important symptom and outcome measure in patients with MDD, and clinicians should consider cognition when developing a treatment plan.
- Cognitive dysfunction often exists in patients with MDD before, during, and after treatment, and understanding the relationship between MDD and cognition will aid clinicians in creating effective treatment strategies.
- The efficacy of various antidepressant agents on treating cognition in MDD should be a key factor in treatment selection for patients with cognitive symptoms.
Cognitive Processes
The brain inputs sensory information to perceive the environment and monitor it for changes. Memory enables comparison of the present with past environments. When environmental changes are perceived, the brain evaluates their significance and prepares responses to minimize threats and maximize rewards, as needed. This cognitive activity involves changes that impact central and peripheral functioning. The central change involves a switch from the DMN, which is engaged when nothing unusual is occurring, to the engagement of the CEN and activation of executive function. Peripheral changes involve activation of connections from the CEN, SN, and DMN and other cortical and higher brain regions to the basal ganglia, hypothalamus, midbrain, and other regions that enable communication with and control of organs and body systems.
Core Networks
The 2 cognitive control networks, the CEN and SN, work with the DMN to control much of the interaction with the environment.15,16 The DMN, normally active when no response to the environment is needed, is also active with introspection and autobiographical memory activity.12 In depressed individuals, overactivity of this network may play a role in the perseverance of negative thought content and interference with executive functioning. The SN and its connections to the DMN and CEN provide the mechanism by which the brain evaluates the salience of events, identifies those requiring nonautomatic response, and activates the CEN to provide executive control to the organism’s response.13 With engagement of the SN and CEN, the DMN deactivates. Insufficient switching of networks has been thought to result in anhedonia, decreased concentration, executive dysfunction, and other cognitive problems seen often in patients with MDD. This executive dysfunction in MDD often persists in some patients between episodes.17
Other Brain Areas Interacting With Cognitive Processes
Cognitive impairments associated with depression are influenced by bottom-up and top-down neural connections, where “top” refers to the cortex,6 with the hippocampus and the amygdala serving as critical intermediary regions. The prefrontal cortex signals directly down to the basal ganglia and lower regions as well as to the hippocampus and amygdala. These lower brain regions similarly have strong connections to the hippocampus and the amygdala and project directly up to cortical regions involved in cognition.6
The hippocampus is critical to learning, memory, and the integration of cognition and memory. Reduced hippocampal activity in those with MDD compared with never-depressed control subjects is thought to contribute to negative bias and reduced incentive salience.18,19
The amygdala is another key hub which is involved in the integration of emotion and memory, affective perception, and it has extensive connectivity with other brain regions central to interactions between cognition and emotion.8,20 Emotional regulation includes both explicit and implicit regulation. Explicit regulation requires deliberate and effortful cognitive processing and involves activation of the CEN and SN along with decreasing activity in the amygdala.21 Implicit regulation does not require activation of the 2 cognitive control networks but does involve decreasing amygdala activity. Failure to inhibit amygdala activity during implicit regulation is thought to contribute to negative bias.8,22
Altered Neurotransmitters and Other Alterations Influencing Cognition in Depression
Multiple neurotransmitter systems are involved in signaling within and between brain regions involved in cognition.23 Prefrontal cortex regions involved in the cognitive control networks also have top-down connections to the cell bodies in the brain stem from which monoamines originate.24 In depressed individuals, deficient top-down cognitive control and increased bottom-up signaling resulting from altered monoamine transmission and can lead to negative patterns of thought and behavior.6 Serotonin, which originates mostly in the raphe nuclei, affects the function of many upper cognitive brain regions. Norepinephrine originates in the locus ceruleus and has widespread projections to higher regions that alter forebrain activities such as attention, perception, and memory25,26 while dopamine neurons located in the ventral tegmental area with substantia nigra project to the nucleus accumbens and prefrontal cortex and alter reward response.27 In parallel, glutamate activity is thought to be involved in the activation and switching of the SN and CEN networks and in the deactivation of the DMN.28
Depression is also thought to be associated with decreased neurogenesis and reduced neuron and glia production and dendritic and synaptic proliferation. These processes may interact with other alterations that contribute to cognitive changes, such as hormonal or inflammatory changes, oxidative stress, and production of brain-derived neurotrophic factor.29,30 Finally, cognition may also be influenced by genetic and epigenetic changes, including those related to early life experiences and trauma.31
Occurrence and Impact of Cognitive Symptoms
A study32 of subjective experience found that about 27% of participants with MDD felt that they were moderately cognitively impaired, while only about 2% of healthy control participants perceived this level of impairment.32 While negative bias may contribute to patients’ perception of impaired cognition, studies that measure cognition objectively have demonstrated deficits.
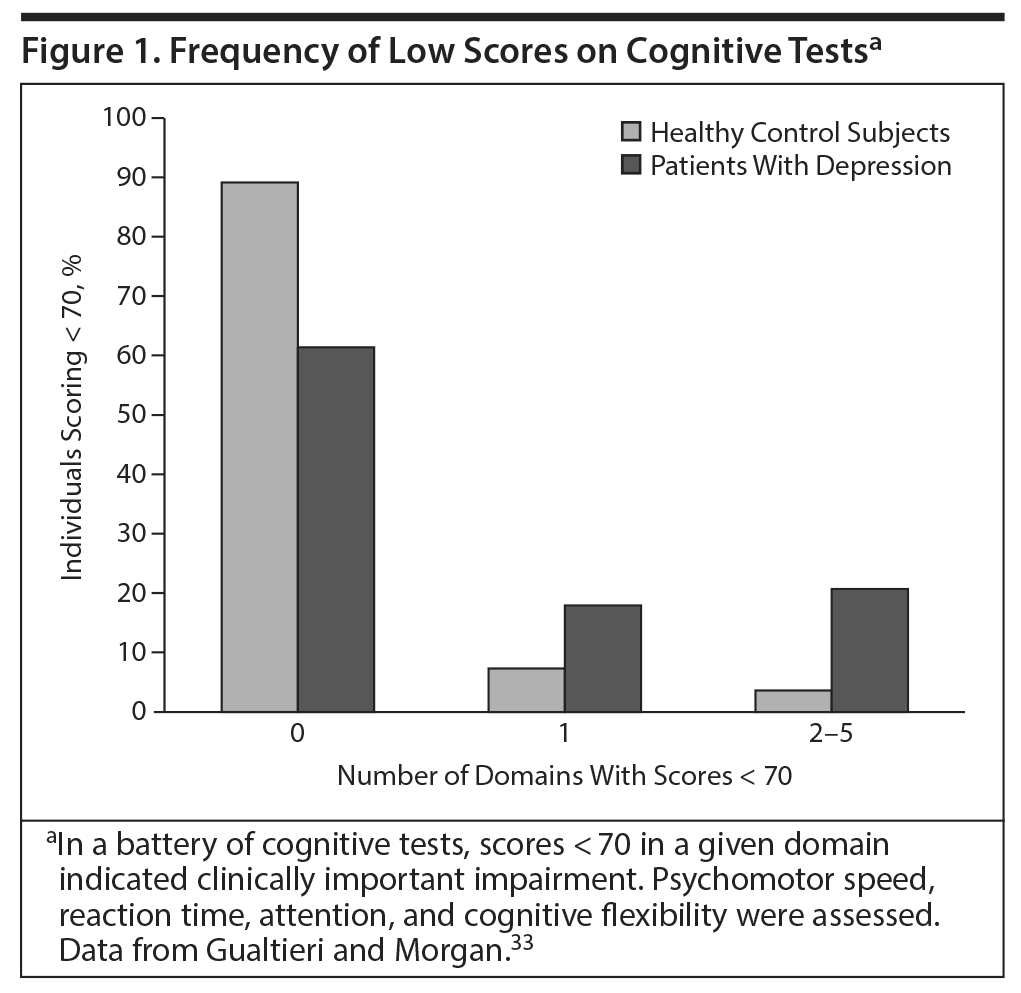
A separate study33 measured performance on tests measuring 5 cognitive domains in patients with MDD and healthy control participants (Figure 1). Patients had no other conditions that might affect cognition. Only 4% of healthy controls scored more than 2 SDs below the mean (which is clinically meaningful) on 2 or more cognitive domains, but 21% of patients with depression did so.33
Timing of Cognitive Deficits
Cognitive impairment may occur in individuals before onset of the illness, during the first and subsequent depressive episodes, and between episodes. A population-based study34 assessed 708 nondepressed adults. At 3-year follow up including the majority with no previous history of MDD, the presence of MDD was associated with baseline impairments in episodic memory, indicating that low episodic memory performance may be a premorbid indicator of depression.
A 3-year prospective study35 of 267 patients found that cognitive problems were present 94% of the time during depressive episodes. In patients experiencing subsequent depressive episodes, cognitive impairment sometimes became more severe. Patients with more depressive episodes have shown a more severe level of psychomotor retardation on neurocognitive tests.36
Cognitive impairments may not only be present before and during depressive episodes but also persist after remission of mood symptoms. A meta-analysis37 of cognitive impairment in depression found moderate deficits in executive function and attention and small-to-moderate deficits in memory that persisted in remitted patients. According to the 3-year prospective study,35 cognitive symptoms are present 44% of the time during remission.
Impact of Cognitive Deficits on Daily Function
As Judd and colleagues38 reported almost 20 years ago, individuals with depressive symptoms have a higher risk of household and financial strain, social irritability, physical and job limitations, and health problems. More recently, connections have been made between functional deficits and cognitive impairments associated with depression.39 For severely depressed patients, cognitive deficits were better predictors of daily impairments, including managing finances and medication, shopping and preparing meals, using transportation, and housework than age or MDD severity.40 However, this study did not account for physical health.
McIntyre and colleagues41 assessed workplace adjustment in adults with depression. Workplace performance was explained to a greater extent by subjective measures of cognitive dysfunction than by depression symptom severity, although the latter was important to global measures of disability. A recent systematic review42 of the relationship between cognitive and psychosocial functioning concluded that neurocognitive deficits are clinically important factors related to the quality of life and social and occupational functioning of individuals with MDD.
A caveat about the literature is that most studies report comparisons of the means of depressed and nondepressed group scores. While this method demonstrates that a large portion of depressed patients have ongoing moderate cognitive impairments, it may obscure the fact that some subgroups of patients have large depression-related deficits. Nonetheless, the prevalence of cognitive symptoms in patients with MDD and the link between cognition and psychosocial functioning should motivate clinicians to not only monitor mood symptoms but also assess and manage cognitive symptoms.
Measurement of Cognitive Functioning
A recent international survey43 of 61 psychiatrists who routinely assessed cognition in their patients found little consensus regarding what constitutes proper assessment of cognitive deficits in MDD. Most respondents (61%) indicated they relied on patient history alone rather than objective formal tests of cognition. Among clinicians who did use cognitive tests, no consensus emerged on which was the optimal instrument, underscoring the need for further research and education on the topic.
Clinician-Administered Scales
Several such tools are available for measuring various components of cognition (Table 1). While these tools are reliable, they are not always easily administered in clinical practice since some require time and trained personnel to administer. However, clinicians should become familiar with these tests in order to better understand the results of published studies that report on their use.
Mini-Mental Status Exam (MMSE). The MMSE is widely used to test overall cognitive function, excluding executive functioning. Possible scores range from 0 to 30; a score of 24 or higher indicates no impairment.44 Scoring of the MMSE has shown an association with education level, which is problematic for those who would like to use the tool across a diverse population. Also, the MMSE often is not sensitive enough to detect subtle cognitive deficits in patients without dementia or psychosis.
Digit Symbol Substitution Test (DSST). In the DSST, participants match symbols with the corresponding number based on a 9-digit coding table. A higher score, based on the number of symbols coded in 90 seconds, indicates better performance.45
Stroop Test. The Stroop Test presents a series of words, printed in color, that may name another color. Participants must name the color of the text instead of reading the word. Several versions of the test exist, but scores are typically based on the number of items completed correctly in the time limit.46
Trail Making Test (TMT). The TMT consists of 2 timed parts. In Part A, a test of attention and cognitive speed, participants draw lines connecting numbered circles in ascending order from 1 to 25. In Part B, a test of executive function, participants draw lines connecting circles in an ascending pattern, alternating between numbers and letters. The number of seconds required to complete the task determines the score; a higher score indicates greater impairment.47,48
Rey Auditory Verbal Learning Test (RAVLT). In the RAVLT, participants are read a list of 15 words, are asked to repeat as many as possible immediately after hearing them and then again after a period of time. Score is determined simply by the number of words recalled.49,50
Simple Reaction Time (SRT). The SRT test involves the delivery of a stimulus at irregular intervals. Participants are asked to press a button following each stimulus; timing is the only uncertainty. The SRT test score is based on reaction time.51
Choice Reaction Time (CRT). The CRT test is similar to the SRT test, but participants are given 2 possible stimuli with 2 possible responses. Participants are asked to press button A following one stimulus, and button B following the second. The score is based on reaction time, with deductions for errors.51
Letter-Number Sequencing Test (LNST). The LNST, like the DSST, is a component of the Wechsler Adult Intelligence Scale.45 The test administrator reads numbers and letters aloud, then patients sequence them by number value and alphabetical order. The sequence length is increased from 2 to 8, until 3 trials have failed.
Two-Digit Cancellation Test (TDCT). In the TDCT, the patient has 45 seconds to search for 2 specific digits among rows of numbers on a page. The number of digits found, minus the number of errors and the number of times a subject needs reminders, is the score.52
Patient-Rated Scales
In routine practice, clinicians require easy-to-administer, validated scales that are sensitive to change. Presently, 2 such scales are available for MDD. Their limitation is that they report patient perception of functioning rather than actual functioning, and they do not directly assess executive functioning, making that limitation particularly pronounced.
Massachusetts General Hospital Cognitive and Physical Functioning Questionnaire (MGH CPFQ). The MGH CPFQ53 is a 7-item questionnaire that asks patients to compare their functioning with their best level of functioning. Administering this scale in patients with MDD after antidepressant therapy has shown that cognitive impairment remains in many patients despite successful treatment.53
Perceived Deficits Questionnaire (PDQ). The PDQ is a 20-item scale validated for use in MDD.54 A brief 5-question version (PDQ-5) is also available. The questions assess attention and concentration, prospective memory, retrospective memory, and planning and organization.
Efficacy of Antidepressants on Cognitive Dysfunction in MDD
Randomized, placebo-controlled clinical trials investigating the effects of antidepressants on cognitive functioning in general and executive functioning in particular have been scarce55,56 and, until recently, focused exclusively on older adults despite the fact that such symptoms are prevalent across all age groups affected by MDD. A growing body of literature suggests that cognitive dysfunction and, in particular, executive dysfunction in MDD are difficult to treat with traditional antidepressants.56,57 For instance, Culang-Reinlieb and colleagues58 compared the selective serotonin-reuptake inhibitor (SSRI) sertraline and the tricyclic antidepressant nortriptyline for 12 weeks in 63 adults over the age of 45 years with MDD. The cognitive battery comprised 6 tests. Aside from improved scores on 1 test of memory and verbal learning in those taking sertraline (P = .001), no statistically significant improvement was found in either the sertraline- or nortriptyline-treated groups, a surprising finding in a disease state with a substantial placebo response.3
Duloxetine
In a 2007 study,59 Raskin and colleagues measured the effects of the serotonin-norepinephrine reuptake inhibitor duloxetine on cognitive functioning in 311 elderly outpatients with MDD (aged 65-90 years), using the RAVLT, the DSST, the TDCT, and the LNST. This 8-week study, the first placebo-controlled trial in MDD to use cognitive functioning as the primary outcome measure, found duloxetine to be superior to placebo in improving a composite cognitive test score (P < .02). However, when the cognitive scales were viewed individually, duloxetine was significantly superior to placebo in improving only working memory (P = .003) and delayed recall (P = .02), as reflected by the RAVLT. The other 3 cognitive tests, which measured executive functioning and other domains, showed no significant differences between duloxetine and placebo.
Citalopram
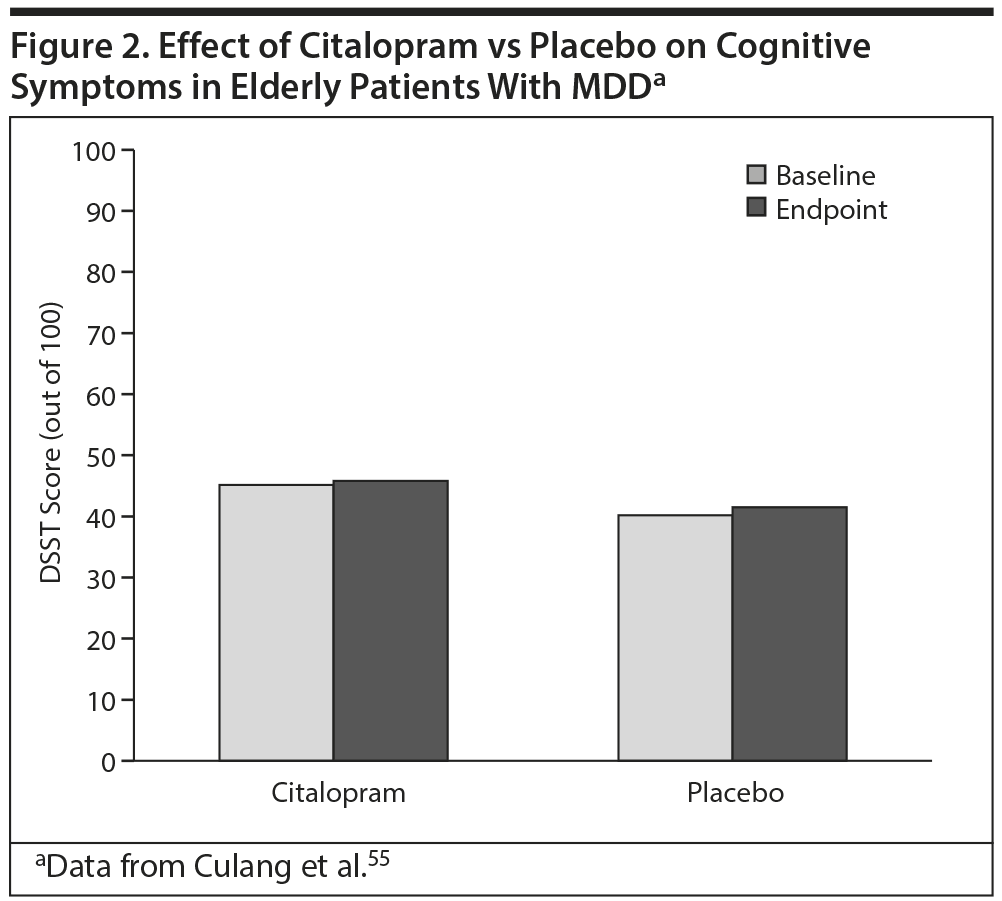
The effects of the SSRI citalopram on cognitive symptoms in elderly patients (aged ≥ 75 years, N = 174) with MDD were studied in 2009 by Culang and colleagues.55 In that 8-week study, no numerical or statistical differences in DSST scores (reflecting global and executive functioning) were found between citalopram- and placebo-treated patients (Figure 2).
Vortioxetine
Vortioxetine is a multimodal antidepressant with affinity for the serotonin transporter as well as several serotonin receptors. Katona and colleagues60 compared the effects of vortioxetine, duloxetine, and placebo for 8 weeks in 452 outpatients with MDD aged ≥ 65 years. Both drugs improved performance on the RAVLT measures of immediate and delayed recall, but only vortioxetine was superior to placebo in improving DSST performance. Although cognition was not the primary outcome measure, but rather a key secondary one, this study was the first randomized controlled trial showing an antidepressant to be significantly superior to placebo in improving a measure of global and executive functioning (ie, DSST). Path analysis confirmed that 83% of the effect of vortioxetine on DSST scores was a direct effect, independent of improvement in depressive symptoms, versus 26% direct effect for duloxetine.
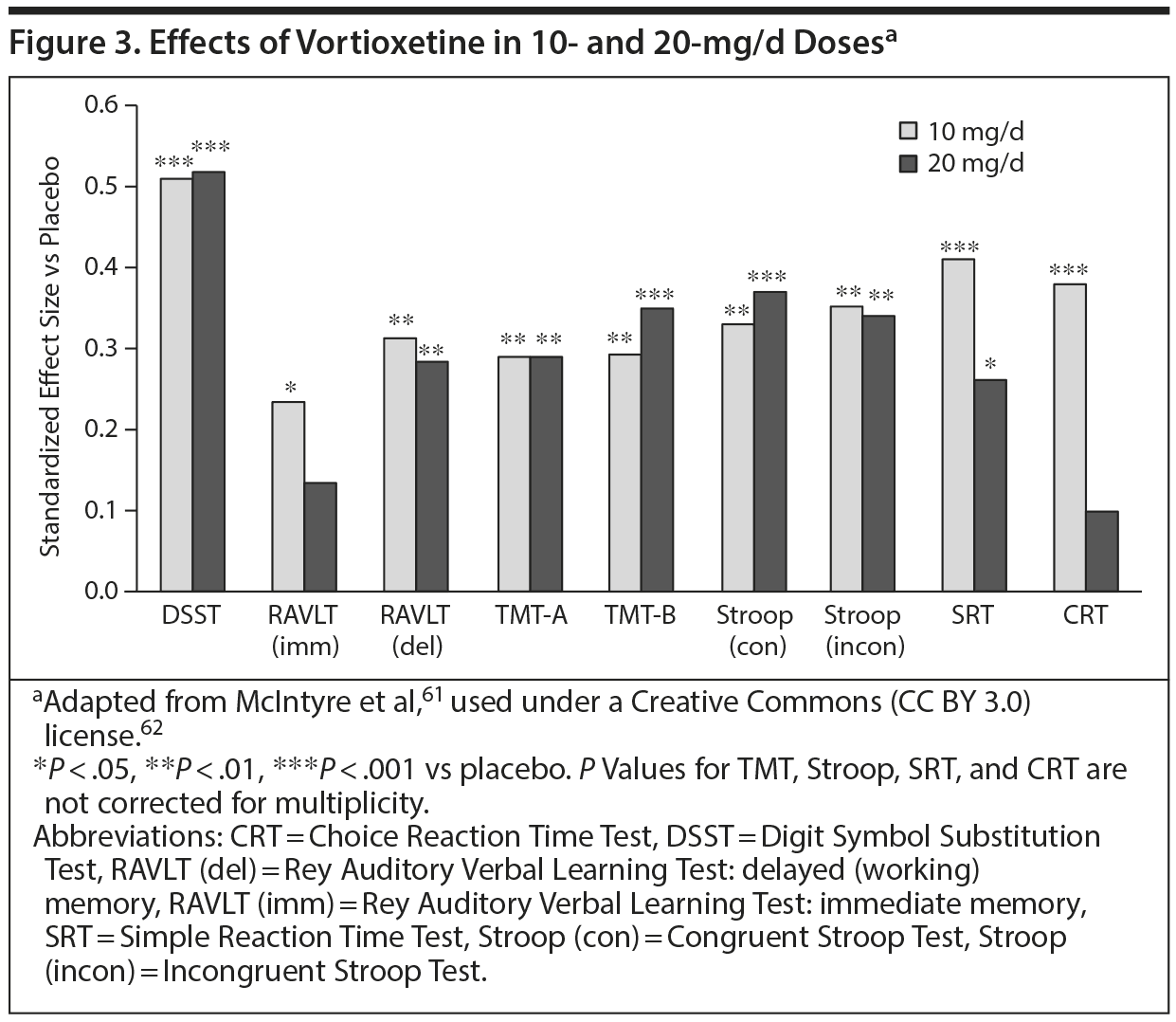
McIntyre and colleages61 reported the first placebo-controlled trial to examine cognition in patients with MDD between the ages of 18 and 65 years. A composite cognitive functioning score employing the DSST and RAVLT was the primary outcome measure. In this 8-week study, 602 patients were randomly assigned to receive placebo, 10 mg/d of vortioxetine, or 20 mg/d of vortioxetine. Both doses were significantly superior to placebo in improving overall cognitive functioning (P < .001 for both doses). Both doses produced significantly greater effect sizes versus placebo on the DSST (P < .001 for both). Patients’ cognitive functioning was also measured with numerous other tests, all of which showed the benefits of active treatment (Figure 3). Patients also reported improved cognitive functioning via the PDQ (P < .001 for both doses).
Most recently, an 8-week study63 evaluated 10 to 20 mg/d of vortioxetine versus 60 mg/d of duloxetine and placebo in 602 outpatients with MDD aged 18 to 65 years; global and executive functioning was the primary outcome measure, assessed using the DSST. Vortioxetine was significantly more effective than placebo at improving DSST scores over the course of treatment (P < .05), but duloxetine was not. Replicating the results of the 2012 study by Katona and colleagues,60 path analysis confirmed that the cognitive benefit of vortioxetine was mainly a direct effect and largely independent of improvement in depressive symptoms.
Conclusion
To date, the efficacy of most antidepressants in treating cognitive dysfunction in MDD has not been compared with that of placebo. Though all 3 agents discussed (citalopram, duloxetine, and vortioxetine) were effective at reducing depressive symptoms and memory in older patients, only vortioxetine has shown efficacy in improving global cognition and executive functioning and has done so in tests of younger and older patients. Given that cognitive impairment is a common residual symptom of depression, is a predictor of poor antidepressant treatment outcomes, and is linked to poor restoration of psychosocial functioning, cognitive functioning should be routinely assessed in both research trials and in clinical practice and should be addressed when managing patients with MDD.
Drug names: citalopram (Celexa and others), duloxetine (Cymbalta and others), nortriptyline (Aventyl, Pamelor, and others), sertraline (Zoloft and others), vortioxetine (Brintellix).
Disclosure of off-label usage: Dr Papakostas has determined that citalopram, duloxetine, nortriptyline, sertraline, and vortioxetine are not approved for the treatment of cognitive function in depression.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
References
1. Herrera-Guzmán I, Herrera-Abarca JE, Gudayol-Ferré E, et al. Effects of selective serotonin reuptake and dual serotonergic-noradrenergic reuptake treatments on attention and executive functions in patients with major depressive disorder. Psychiatry Res. 2010;177(3):323-329. PubMed doi:10.1016/j.psychres.2010.03.006
2. Pehrson AL, Leiser SC, Gulinello M, et al. Treatment of cognitive dysfunction in major depressive disorder—a review of the preclinical evidence for efficacy of selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors and the multimodal-acting antidepressant vortioxetine (published online ahead of print August 5, 2014). Eur J Pharmacol. 2014. PubMed doi:10.1016/j.ejphar.2014.07.044
3. Papakostas GI. Cognitive symptoms in patients with major depressive disorder and their implications for clinical practice. J Clin Psychiatry. 2014;75(1):8-14. PubMed doi:10.4088/JCP.13r08710
4. Godard J, Baruch P, Grondin S, et al. Psychosocial and neurocognitive functioning in unipolar and bipolar depression: a 12-month prospective study. Psychiatry Res. 2012;196(1):145-153. PubMed doi:10.1016/j.psychres.2011.09.013
5. Soskin DP, Holt DJ, Sacco GR, et al. Incentive salience: novel treatment strategies for major depression. CNS Spectr. 2013;18(6):307-314. PubMed doi:10.1017/S1092852913000345
6. Roiser JP, Sahakian BJ. Hot and cold cognition in depression. CNS Spectr. 2013;18(3):139-149. PubMed doi:10.1017/S1092852913000072
7. McIntyre RS, Cha DS, Soczynska JK, et al. Cognitive deficits and functional outcomes in major depressive disorder: determinants, substrates, and treatment interventions. Depress Anxiety. 2013;30(6):515-527. PubMed doi:10.1002/da.22063
8. Roiser JP, Elliott R, Sahakian BJ. Cognitive mechanisms of treatment in depression. Neuropsychopharmacology. 2012;37(1):117-136. PubMed doi:10.1038/npp.2011.183
9. Victor TA, Furey ML, Fromm SJ, et al. Relationship between amygdala responses to masked faces and mood state and treatment in MDD. Arch Gen Psychiatry. 2010;67(11):1128-1138. PubMed doi:10.1001/archgenpsychiatry.2010.144
10. Harmer CJ, O’ Sullivan U, Favaron E, et al. Effect of acute antidepressant administration on negative affective bias in depressed patients. Am J Psychiatry. 2009;166(10):1178-1184. PubMed doi:10.1176/appi.ajp.2009.09020149
11. Lindquist KA, Wager TD, Kober H, et al. The brain basis of emotion: a meta-analytic review. Behav Brain Sci. 2012;35(3):121-143. PubMed doi:10.1017/S0140525X11000446
12. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124(1-38):351-358.
13. Goulden N, Khusnulina A, Davis NJ, et al. The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. Neuroimage. 2014;99:180-190. PubMed doi:10.1016/j.neuroimage.2014.05.052
14. Etkin A, Gyurak A, O’ Hara R. A neurobiological approach to the cognitive deficits of psychiatric disorders. Dialogues Clin Neurosci. 2013;15(4):419-429. PubMed
15. Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15(10):483-506. PubMed doi:10.1016/j.tics.2011.08.003
16. Chen AC, Oathes DJ, Chang C, et al. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proc Natl Acad Sci U S A. 2013;110(49):19944-19949. PubMed doi:10.1073/pnas.1311772110
17. Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull. 2013;139(1):81-132. PubMed doi:10.1037/a0028727
18. Milne AM, MacQueen GM, Hall GB. Abnormal hippocampal activation in patients with extensive history of major depression: an fMRI study. J Psychiatry Neurosci. 2012;37(1):28-36. PubMed doi:10.1503/jpn.110004
19. Toki S, Okamoto Y, Onoda K, et al. Hippocampal activation during associative encoding of word pairs and its relation to symptomatic improvement in depression: a functional and volumetric MRI study. J Affect Disord. 2014;152-154:462-467. PubMed doi:10.1016/j.jad.2013.07.021
20. Pessoa L. Emotion and cognition and the amygdala: from “what is it?” to “what’s to be done?” Neuropsychologia. 2010;48(12):3416-3429. PubMed doi:10.1016/j.neuropsychologia.2010.06.038
21. Gyurak A, Gross JJ, Etkin A. Explicit and implicit emotion regulation: a dual-process framework. Cogn Emotion. 2011;25(3):400-412. PubMed doi:10.1080/02699931.2010.544160
22. Kong L, Chen K, Tang Y, et al. Functional connectivity between the amygdala and prefrontal cortex in medication-naive individuals with major depressive disorder. J Psychiatry Neurosci. 2013;38(6):417-422. PubMed doi:10.1503/jpn.120117
23. Trivedi MH, Greer TL. Cognitive dysfunction in unipolar depression: implications for treatment. J Affect Disord. 2014;152-154:19-27. PubMed doi:10.1016/j.jad.2013.09.012
24. Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32(1):267-287. PubMed doi:10.1146/annurev.neuro.051508.135535
25. Chamberlain SR, Robbins TW. Noradrenergic modulation of cognition: therapeutic implications. J Psychopharmacol. 2013;27(8):694-718. PubMed doi:10.1177/0269881113480988
26. Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10(3):211-223. PubMed doi:10.1038/nrn2573
27. Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30(1):259-288. PubMed doi:10.1146/annurev.neuro.28.061604.135722
28. Walter M, Henning A, Grimm S, et al. The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch Gen Psychiatry. 2009;66(5):478-486. PubMed doi:10.1001/archgenpsychiatry.2009.39
29. Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732-741. PubMed doi:10.1016/j.biopsych.2008.11.029
30. Lee BH, Kim YK. The roles of BDNF in the pathophysiology of major depression and in antidepressant treatment. Psychiatry Investig. 2010;7(4):231-235. PubMed doi:10.4306/pi.2010.7.4.231
31. Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301(21):2252-2259. PubMed doi:10.1001/jama.2009.754
32. Iverson GL, Lam RW. Rapid screening for perceived cognitive impairment in major depressive disorder. Ann Clin Psychiatry. 2013;25(2):135-140. PubMed
33. Gualtieri CT, Morgan DW. The frequency of cognitive impairment in patients with anxiety, depression, and bipolar disorder: an unaccounted source of variance in clinical trials. J Clin Psychiatry. 2008;69(7):1122-1130. PubMed doi:10.4088/JCP.v69n0712
34. Airaksinen E, Wahlin A, Forsell Y, et al. Low episodic memory performance as a premorbid marker of depression: evidence from a 3-year follow-up. Acta Psychiatr Scand. 2007;115(6):458-465. PubMed doi:10.1111/j.1600-0447.2006.00932.x
35. Conradi HJ, Ormel J, de Jonge P. Presence of individual (residual) symptoms during depressive episodes and periods of remission: a 3-year prospective study. Psychol Med. 2011;41(6):1165-1174. PubMed doi:10.1017/S0033291710001911
36. Gorwood P, Richard-Devantoy S, Baylé F, et al. Psychomotor retardation is a scar of past depressive episodes, revealed by simple cognitive tests. Eur Neuropsychopharmacol. 2014;24(10):1630-1640. PubMed doi:10.1016/j.euroneuro.2014.07.013
37. Rock PL, Roiser JP, Riedel WJ, et al. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. 2013;44(10):1-12. PubMed
38. Judd LL, Paulus MP, Wells KB, et al. Socioeconomic burden of subsyndromal depressive symptoms and major depression in a sample of the general population. Am J Psychiatry. 1996;153(11):1411-1417. PubMed doi:10.1176/ajp.153.11.1411
39. Withall A, Harris LM, Cumming SR. The relationship between cognitive function and clinical and functional outcomes in MDD. Psychol Med. 2009;39(3):393-402. PubMed doi:10.1017/S0033291708003620
40. McCall WV, Dunn AG. Cognitive deficits are associated with functional impairment in severely depressed patients. Psychiatry Res. 2003;121(2):179-184. PubMed doi:10.1016/j.psychres.2003.09.003
41. McIntyre RS, Soczynska JZ, Woldeyohannes HO, et al. The impact of cognitive impairment on perceived workforce performance: results from the International Mood Disorders Collaborative Project. Compr Psychiatry. 2015;56:279-282. PubMed doi:10.1016/j.comppsych.2014.08.051
42. Evans VC, Iverson GL, Yatham LN, et al. The relationship between neurocognitive and psychosocial functioning in major depressive disorder: a systematic review. J Clin Psychiatry. 2014;75(12):1359-1370. PubMed doi:10.4088/JCP.13r08939
43. El Hammi E, Samp J, Rémuzat C, et al. Difference of perceptions and evaluation of cognitive dysfunction in major depressive disorder patients across psychiatrists internationally. Ther Adv Psychopharmacol. 2014;4(1):22-29. PubMed doi:10.1177/2045125313507946
44. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. PubMed doi:10.1016/0022-3956(75)90026-6
45. Kaplan RM, Saccuzzo DP. Psychological Testing: Principles, Applications, and Issues. 7th ed. Belmont, CA: Wadsworth; 2009.
46. Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Oxford, England: Oxford University Press; 2006.
47. Division of Motor Vehicles DoASoA. Trail Making Test (TMT Parts A & B). http://doa.alaska.gov/dmv/akol/pdfs/uiowa_trailmaking.pdf. Published March 15, 2012. Accessed October 30, 2014.
48. Gaudino EA, Geisler MW, Squires NK. Construct validity in the Trail Making Test: what makes Part B harder? J Clin Exp Neuropsychol. 1995;17(4):529-535. PubMed doi:10.1080/01688639508405143
49. Rey A. Examen Clinique En Psychologie. Paris, France: Press Universitaire de France; 1964. [Clinical Tests in Psychology].
50. Schmidt M. Rey Auditory Verbal Learning Test: A Handbook. Los Angeles, CA: Western Psychological Services; 1996.
51. Deary IJ, Liewald D, Nissan J. A free, easy-to-use, computer-based simple and four-choice reaction time programme: the Deary-Liewald reaction time task. Behav Res Methods. 2011;43(1):258-268. PubMed doi:10.3758/s13428-010-0024-1
52. Neisser U. Visual search. Sci Am. 1964;210(210):94-102. PubMed doi:10.1038/scientificamerican0664-94
53. Fava M, Iosifescu DV, Pedrelli P, et al. Reliability and validity of the Massachusetts general hospital cognitive and physical functioning questionnaire. Psychother Psychosom. 2009;78(2):91-97. PubMed doi:10.1159/000201934
54. Lam RW, Saragoussi D, Danchenko N, et al. Psychometric validation of Perceived Deficits Questionnaire-Depression (PDQ-D) in patients with major depressive disorder (MDD). Value Health. 2013;16(7):A330. doi:10.1016/j.jval.2013.08.046
55. Culang ME, Sneed JR, Keilp JG, et al. Change in cognitive functioning following acute antidepressant treatment in late-life depression. Am J Geriatr Psychiatry. 2009;17(10):881-888. PubMed doi:10.1097/JGP.0b013e3181b4bf4a
56. Keefe RSE, McClintock SM, Roth RM, et al. Cognitive effects of pharmacotherapy for major depressive disorder: a systematic review. J Clin Psychiatry. 2014;75(8):864-876. PubMed doi:10.4088/JCP.13r08609
57. Fava M, Graves LM, Benazzi F, et al. A cross-sectional study of the prevalence of cognitive and physical symptoms during long-term antidepressant treatment. J Clin Psychiatry. 2006;67(11):1754-1759. PubMed doi:10.4088/JCP.v67n1113
58. Culang-Reinlieb ME, Sneed JR, Keilp JG, et al. Change in cognitive functioning in depressed older adults following treatment with sertraline or nortriptyline. Int J Geriatr Psychiatry. 2012;27(8):777-784. PubMed doi:10.1002/gps.2783
59. Raskin J, Wiltse CG, Siegal A, et al. Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: an 8-week, double-blind, placebo-controlled trial. Am J Psychiatry. 2007;164(6):900-909. PubMed doi:10.1176/ajp.2007.164.6.900
60. Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol. 2012;27(4):215-223. PubMed doi:10.1097/YIC.0b013e3283542457
61. McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol. 2014;17(10):1557-1567. PubMed doi:10.1017/S1461145714000546
62. Creative Commons. Attribution 3.0 Unported. http://creativecommons.org/licenses/by/3.0/. Accessed February 13, 2015.
63. Mahableshwarkar A, Zajecka J, Jacobson W, et al. Efficacy of vortioxetine on cognitive function in adult patients with major depressive disorder: results of a randomized, double-blind, active-referenced, placebo-controlled trial. In: 29th CINP World Congress of Neuropsychopharmacology; June 24, 2014; Vancouver, British Columbia, Canada.