Excessive daytime sleepiness (EDS) negatively affects patients’ safety and quality of life. Clinicians can assess EDS using a thorough clinical interview, patient-report scales, informant reports, and objective tests. Patients should be encouraged to develop good sleep hygiene and avoid insufficient sleep time. Clinicians must recognize conditions that may be causing EDS, such as sleep disorders, medical or neurologic conditions, or psychiatric disorders. Behavioral and pharmacologic strategies are available to help clinicians treat both EDS and associated conditions.
From the Henry Ford Hospital Sleep Center, Detroit, Michigan (Dr Roth) and NeuroTrials Research and the Atlanta School of Sleep Medicine and Technology, Georgia (Dr Rosenberg).
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
This Commentary section of The Journal of Clinical Psychiatry presents the highlights of the planning teleconference series “Recognizing and Treating Excessive Daytime Sleepiness and Narcolepsy,” which was held in January 2015. This report was prepared and independently developed by the CME Institute of Physicians Postgraduate Press, Inc., and was supported by an educational grant from Jazz Pharmaceuticals.
The teleconference was chaired by Thomas Roth, PhD, Henry Ford Hospital Sleep Center, Detroit, Michigan. The faculty was Russell P. Rosenberg, PhD, NeuroTrials Research and Atlanta School of Sleep Medicine and Technology, Georgia.
Financial disclosure: Dr Roth is a consultant for Jazz, Pfizer, Merck, Pernix, Sunovion, and Flamel; has received grant/research support from Sunovion; and is a member of the speakers/advisory board for Merck. Dr Rosenberg is a consultant for Pfizer; has received grant/research support from Merck, Jazz, Shire, Respironics, Cereve, Eisai, Sunovion, and Pfizer; and is a member of the speakers/advisory board for Merck.
The opinions expressed herein are those of the faculty and do not necessarily reflect the opinions of the CME provider and publisher or the commercial supporter.
J Clin Psychiatry 2015;76(11):1518-1521
dx.doi.org/10.4088/JCP.14019co5c
© Copyright 2015 Physicians Postgraduate Press, Inc.
Excessive daytime sleepiness (EDS) is common in clinical practice, with the National Sleep Foundation’s Sleep in America poll1 finding that about 18% of respondents qualified as excessively sleepy. Negative effects of EDS include decreased quality of life, cognitive impairment, poor work performance, poor medical health, and motor vehicle accidents.2,3 To avoid these problems, clinicians must assess patients for EDS and associated conditions and provide appropriate treatment.
Assessment of EDS
Due to the negative effects of EDS, clinicians should routinely assess patients’ sleepiness. In the clinical interview, questions must be worded carefully. Simply asking patients if they are tired during the day is not informative because most people experience some daytime tiredness. Better questions to identify clinically relevant sleepiness are “Do you ever unintentionally fall asleep,” “Do you feel fully alert throughout the day,” and “Do you persistently have to fight off sleep at work or school?” To evaluate the severity of patients’ EDS, clinicians may also use patient-report scales and objective tests.
Patient-Report Scales
The Epworth Sleepiness Scale (ESS)4 is the mostly widely used self-report measure of sleepiness.3 When completing the ESS, patients are asked to rate the likelihood that they would fall asleep in 8 situations such as reading or waiting in traffic.4 A score of 10 or above indicates pathological daytime sleepiness.
Other patient-rated scales include the Karolinska Sleepiness Scale, the Stanford Sleepiness Scale, and the Sleep-Wake Activity Inventory.5 While these tests are not as well validated as the ESS for assessing sleepiness, they can be used to track symptoms over time.5 Validated patient questionnaires are generally efficient for clinical practice but are subject to errors of patient bias as well as cognitive or memory impairments. These problems can be offset by questioning patients’ family members or friends to obtain another perspective.
Objective Measures
A number of objective measures can be useful for assessing causes of EDS. Polysomnography tests patients for sleep-disordered breathing or abnormal movements during sleep. It typically requires an overnight stay to monitor neurophysiologic and cardiorespiratory variables.5 Actigraphy is a cost-effective measure of movement and sleep/wake patterns over days or weeks, rather than just overnight, and is useful for diagnosing insomnia, circadian rhythm disorders, or EDS due to insufficient sleep.6 If obstructive sleep apnea (OSA) is thought to be contributing to EDS, home sleep apnea testing devices can be used to measure breathing during sleep, but they do not provide an objective measure of sleepiness.7

- Patients may be experiencing negative effects from EDS that influence their safety, work performance, and overall health.
- Clinicians should collect a thorough history during the patient interview and use patient-report and objective tests to assess for EDS and associated conditions.
- Treatment for EDS varies depending on the underlying cause, but behavioral strategies and pharmacologic treatments are available to improve patients’ symptoms.
The gold standard objective measure of excessive sleepiness is the Multiple Sleep Latency Test (MSLT).5 The MSLT is conducted in a sleep laboratory and determines the amount of time until sleep initiation and whether rapid eye movement sleep is achieved.8 Falling asleep in less than 10 minutes is generally considered abnormal, and a sleep latency under 5 minutes indicates severe sleepiness.8 Another objective test is the Maintenance of Wakefulness Test (MWT), which is used to assess patients’ ability to stay awake while seated in a quiet, dim room.8 Unlike the MSLT, the MWT cannot be used to make a diagnosis. Neither test should be used in the absence of clinical history and polysomnography to diagnose a disorder involving EDS.5
Disorders Associated With EDS
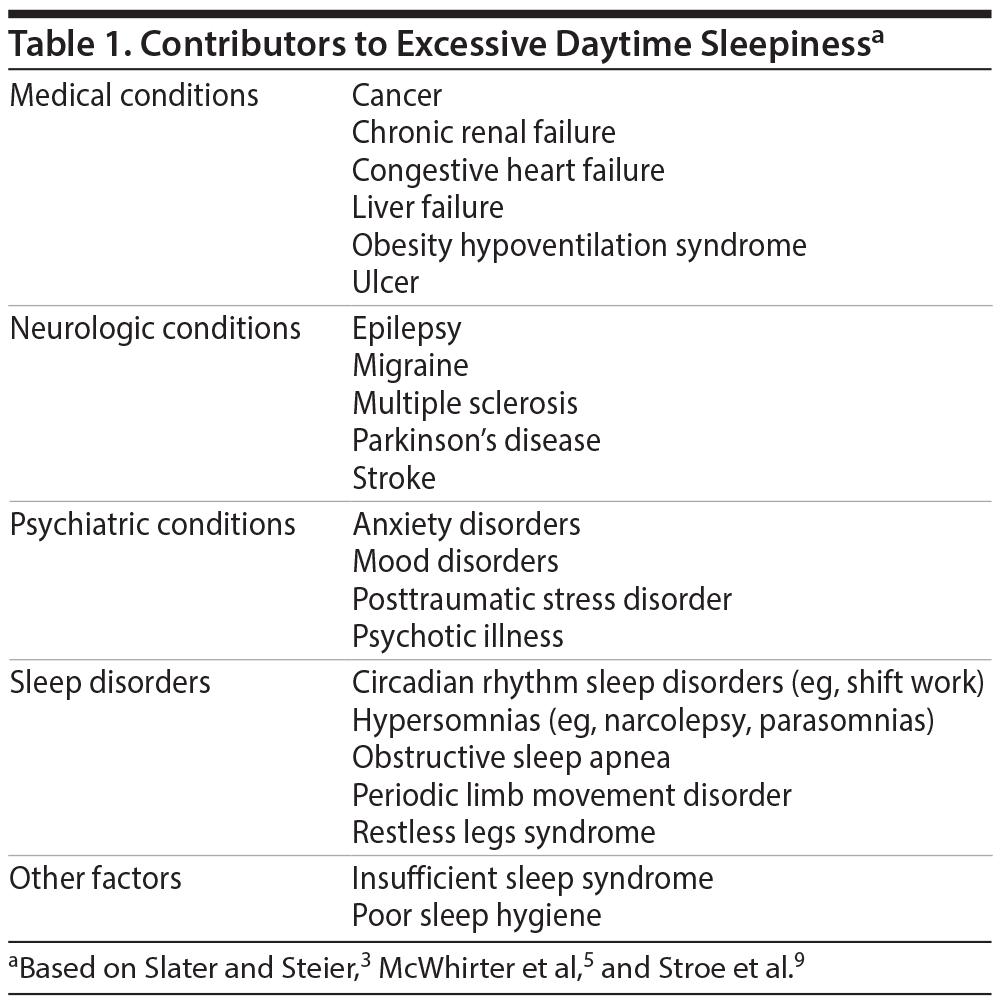
EDS is associated with a variety of sleep disorders, medical and neurologic conditions, and psychiatric disorders (Table 1).3,5,9 By considering these conditions and ruling out medication side effects and behaviors that cause sleep deprivation, clinicians can differentiate what is causing or contributing to EDS, which may be more than one condition.
Sleep Disorders
If the patient’s EDS is not caused by poor sleep hygiene, medication side effects, or insufficient sleep time, it may be a symptom of sleep-related breathing disorders, hypersomnias, sleep-related movement disorders, or circadian rhythm sleep disorders.3,10
Medical and Neurologic Conditions
Medical and neurologic disorders are a common cause of EDS. In a community sample of 2,612 individuals without sleep-disordered breathing, narcolepsy, or shift work disorder,9 67% reported a medical disorder, and over 30% of those had EDS. Ulcers, migraines, and depression were independent predictors of EDS. Prevalence rates for EDS were 50% in those with ulcers; close to 40% in those with neurologic disorders, migraines, depression, and heart disease; and 24% and 19% for those with thyroid disorders and cancer, respectively.9 Other conditions associated with EDS are rheumatoid arthritis, fibromyalgia,11 head trauma, encephalitis, stroke,2 multiple sclerosis, epilepsy, Parkinson’s disease, and Alzheimer’s disease.8
Psychiatric Disorders
Psychiatric disorders are also often associated with EDS.8 An epidemiologic study12 found psychiatric disorders in 47% of individuals with hypersomnia but in only 16% of those without sleep complaints. Depression, bipolar disorder, posttraumatic stress disorder, and alcohol dependency are all associated with sleep/wake impairments, and their treatments may also produce iatrogenic effects on sleep.2,13 Conversely, many sleep disorders can lead to psychiatric symptoms.13 For example, OSA is associated with depression3 and recurrent hypersomnia with hallucinations, binge eating, and irritability.14 Because sleep disturbances affect outcomes in psychiatric disorders, measuring and treating EDS is a critical part of psychiatric treatment.13
Treatment of EDS and Related Conditions
The goals of treatment should be to reduce EDS and increase alertness, thereby improving psychosocial and occupational functioning and safety. Effective management of EDS typically involves both behavioral and pharmacologic interventions.
Behavioral Strategies
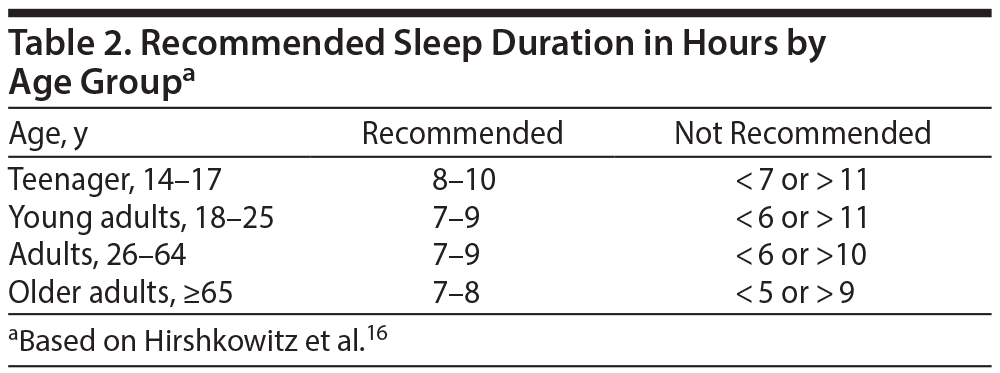
Focusing on patients’ sleep hygiene is an important first step to improving EDS. Patients should adhere to a consistent sleep/wake schedule, exercise regularly (but not within 3 hours of bedtime), maintain a comfortable bedroom environment, and avoid heavy meals, alcohol, caffeine, and smoking before bedtime.15 Individuals must also ensure they are allowing themselves enough sleep time within the recommended range for their age group (Table 2).16
Disorder-Specific Pharmacologic Treatments
Practice guidelines and US Food and Drug Administration (FDA)-approved treatments are available for many, but not all, of the disorders associated with EDS. Best practices for managing EDS vary depending on the underlying disorder.
Narcolepsy. In most cases, individuals with narcolepsy, particularly those who experience cataplexy, require lifelong treatment because the orexin deficiency underlying this disorder is permanent.17 In addition to adhering to good sleep hygiene, patients with narcolepsy require pharmacotherapy. Several agents are available for the treatment of EDS and cataplexy associated with narcolepsy.
Sodium oxybate may be considered a first-line treatment option because it is the only agent with strong evidence of efficacy for both EDS and cataplexy and is FDA-approved for these indications.17,18 It also may improve disturbed nocturnal sleep, sleep paralysis, and hypnagogic hallucinations.17,18 However, this agent has a short half-life and brief duration of action, requiring the patient to take it at bedtime and to set an alarm for a second dose.17 Sodium oxybate also can cause severe withdrawal symptoms and be fatal in overdose, but usually this occurs only when other agents are also being used.17 Symptoms of anxiety or depression may occur,19 and all patients should be questioned about current or past suicidal ideation or behavior.20 Finally, sodium oxybate has been abused because of its rapid sedative properties, and therefore its use is restricted.17 Both prescribers and patients must go through registration and training,20 and all prescriptions are filled through a central pharmacy.17 Despite these limitations, sodium oxybate is a safe and effective agent for narcolepsy when prescribed and taken correctly.17
The wakefulness-promoting agents modafinil and armodafinil are also approved for treating EDS associated with narcolepsy. When cataplexy is not present, modafinil or armodafinil can be considered a first-line option.19 Both modafinil and armodafinil are well tolerated, but clinicians should educate female patients about potentially reduced effectiveness of some oral contraceptives.19 Patients should also be warned to immediately report rashes or allergic reactions.21,22 The stimulant methylphenidate and several amphetamines are FDA-approved for treating EDS, but because of their abuse potential, side effect profiles, and limited evidence, they should be considered only if sodium oxybate or modafinil/armodafinil have not resulted in desired outcomes.18,19
Treatments for cataplexy include not only sodium oxybate but also off-label antidepressants. The greatest evidence exists for the use of norepinephrine reuptake inhibitors, particularly venlafaxine and duloxetine, but selective serotonin reuptake inhibitors and tricyclic antidepressants are sometimes used.17-19 These agents may improve cataplexy, but whether they also improve sleep paralysis and hypnagogic hallucinations is uncertain,17-19 and they do not improve EDS. Individuals taking antidepressants may experience sexual dysfunction, gastrointestinal upset, and dry mouth.17,19 Atomoxetine, a norepinephrine reuptake inhibitor that is used to treat attention-deficit/hyperactivity disorder, is also used for cataplexy and may alleviate EDS via stimulant effects.17,23
Idiopathic hypersomnia. No FDA-approved treatments are available for idiopathic hypersomnia, but the treatment of EDS associated with idiopathic hypersomnia is similar to that of narcolepsy. Practice guidelines18 suggest the use of modafinil and methylphenidate or other stimulants. Most patients are able to benefit from monotherapy with one of these drugs.24 A novel approach to idiopathic hypersomnia is to administer drugs that inhibit γ-aminobutyric acid (GABA) production.25 Studies examining these compounds in the population are underway.
Obstructive sleep apnea. Combating EDS associated with OSA should begin with attempts to improve the quality of nighttime sleep by addressing the upper airway obstruction. In some individuals, weight loss and position training can lead to improvement.26 Other individuals will require treatment with a positive airway pressure device or an oral appliance.27 Some patients continue to experience EDS despite receiving treatment for OSA and may benefit from an adjunctive agent during the day28; modafinil and armodafinil are approved for treating EDS associated with OSA.
Shift work disorder. Shift work disorder occurs in individuals who must be awake and alert when their body’s circadian clock says they should be asleep, and vice versa.29 Night shift workers are vulnerable to this disorder as well as individuals who rotate shifts frequently. Behavioral interventions include improving sleep hygiene, using timed light exposure, and scheduling naps.30 Sedative hypnotics or melatonin may be used to combat insomnia and ensure individuals get a sufficient amount of sleep.30 If sleepiness persists during working hours, stimulants may be used. Modafinil and armodafinil are both approved for the treatment of EDS associated with shift work disorder. Amphetamines can increase alertness but are not recommended because of their abuse potential.30
Kleine-Levin syndrome and Parkinson’s disease. No FDA-approved treatments are available for EDS associated with Kleine-Levin syndrome or Parkinson’s disease, but modafinil may be effective.8
Conclusion
Unrecognized and untreated EDS can endanger patients’ safety, reduce their function at work or school, and negatively impact their health. Clinicians can assess EDS using appropriate questions during the clinical interview, patient-report questionnaires such as the ESS, and objective tests such as polysomnography and the MSLT. Patients should be encouraged to develop good sleep habits and get sleep within the recommended range for their age. By recognizing conditions that may be causing EDS, clinicians can develop a treatment plan to target EDS and associated conditions. Pharmacologic treatments are available to improve EDS in sleep disorders such as narcolepsy and OSA, while other conditions are not as well researched. Using a combination of behavioral and pharmacologic strategies will enable clinicians to improve the safety and quality of life for patients with EDS.
Drug names: armodafinil (Nuvigil), atomoxetine (Strattera), duloxetine (Cymbalta and others), methylphenidate (Daytrana, Concerta, and others), modafinil (Provigil and others), sodium oxybate (Xyrem), venlafaxine (Effexor and others)
Disclosure of off-label usage: Dr Roth has determined that, to the best of his knowledge, no investigational information about pharmaceutical agents that is outside US Food and Drug Administration-approved labeling has been presented in this activity.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
References
1. Swanson LM, Arnedt JT, Rosekind MR, et al. Sleep disorders and work performance: findings from the 2008 National Sleep Foundation Sleep in America poll. J Sleep Res. 2011;20(3):487-494. PubMed doi:10.1111/j.1365-2869.2010.00890.x
2. Pagel JF. Excessive daytime sleepiness. Am Fam Physician. 2009;79(5):391-396. PubMed
3. Slater G, Steier J. Excessive daytime sleepiness in sleep disorders. J Thorac Dis. 2012;4(6):608-616. PubMed
4. Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14(6):540-545. PubMed
5. McWhirter D, Bae C, Budur K. The assessment, diagnosis, and treatment of excessive sleepiness: practical considerations for the psychiatrist. Psychiatry (Edgmont). 2007;4(9):26-35. PubMed
6. Ancoli-Israel S, Cole R, Alessi C, et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342-392. PubMed
7. American Academy of Sleep Medicine. Home sleep apnea testing: overview. http://www.sleepeducation.com/essentials-in-sleep/home-sleep-apnea-testing. 2014. Accessed September 10, 2015.
8. Boulos MI, Murray BJ. Current evaluation and management of excessive daytime sleepiness. Can J Neurol Sci. 2010;37(2):167-176. PubMed doi:10.1017/S0317167100009896
9. Stroe AF, Roth T, Jefferson C, et al. Comparative levels of excessive daytime sleepiness in common medical disorders. Sleep Med. 2010;11(9):890-896. PubMed doi:10.1016/j.sleep.2010.04.010
10. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014.
11. Roehrs T, Diederichs C, Gillis M, et al. Nocturnal sleep, daytime sleepiness and fatigue in fibromyalgia patients compared to rheumatoid arthritis patients and healthy controls: a preliminary study. Sleep Med. 2013;14(1):109-115. PubMed doi:10.1016/j.sleep.2012.09.020
12. Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479-1484. PubMed
13. Sateia MJ. Update on sleep and psychiatric disorders. Chest. 2009;135(5):1370-1379. PubMed doi:10.1378/chest.08-1834
14. Wise MS, Arand DL, Auger RR, et al, for the American Academy of Sleep Medicine. Treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30(12):1712-1727. PubMed
15. Schwartz JR, Roth T, Hirshkowitz M, et al. Recognition and management of excessive sleepiness in the primary care setting. Prim Care Companion J Clin Psychiatry. 2009;11(5):197-204. PubMed doi:10.4088/PCC.07r00545
16. Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1(1):40-43. doi:10.1016/j.sleh.2014.12.010
17. Mignot EJ. A practical guide to the therapy of narcolepsy and hypersomnia syndromes. Neurotherapeutics. 2012;9(4):739-752. PubMed doi:10.1007/s13311-012-0150-9
18. Morgenthaler TI, Kapur VK, Brown T, et al, for the Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30(12):1705-1711. PubMed
19. Thorpy MJ. Update on therapy for narcolepsy. Curr Treat Options Neurol. 2015;17(5):347. PubMed doi:10.1007/s11940-015-0347-4
20. Xyrem (sodium oxybate)[package insert]. Palo Alto, CA: Jazz Pharmaceuticals, Inc; 2014.
21. Provigil (modafinil)[package insert]. North Wales, PA: Teva Pharmaceuticals USA, Inc; Revised 2015.
22. Nuvigil (armodafinil)[package insert]. North Wales, PA: Teva Pharmaceuticals USA, Inc; Revised 2015.
23. De la Herrán-Arita AK, Garc×a-Garc×a F. Current and emerging options for the drug treatment of narcolepsy. Drugs. 2013;73(16):1771-1781. PubMed doi:10.1007/s40265-013-0127-y
24. Ali M, Auger RR, Slocumb NL, et al. Idiopathic hypersomnia: clinical features and response to treatment. J Clin Sleep Med. 2009;5(6):562-568. PubMed
25. Rye DB, Bliwise DL, Parker K, et al. Modulation of vigilance in the primary hypersomnias by endogenous enhancement of GABAA receptors. Sci Transl Med. 2012;4(161):161ra151.
26. Morgenthaler TI, Kapen S, Lee-Chiong T, et al, for the Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep. 2006;29(8):1031-1035. PubMed
27. Kushida CA, Morgenthaler TI, Littner MR, et al, for the American Academy of Sleep. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep. 2006;29(2):240-243. PubMed
28. Roth T, White D, Schmidt-Nowara W, et al. Effects of armodafinil in the treatment of residual excessive sleepiness associated with obstructive sleep apnea/hypopnea syndrome: a 12-week, multicenter, double-blind, randomized, placebo-controlled study in nCPAP-adherent adults. Clin Ther. 2006;28(5):689-706. doi:10.1016/j.clinthera.2006.05.013 PubMed
29. Rosenberg R, Doghramji PP. Is shift work making your patient sick? emerging theories and therapies for treating shift work disorder. Postgrad Med. 2011;123(5):106-115. PubMed doi:10.3810/pgm.2011.09.2465
30. Roth T. Appropriate therapeutic selection for patients with shift work disorder. Sleep Med. 2012;13(4):335-341. PubMed doi:10.1016/j.sleep.2011.11.006