![]()
ABSTRACT
Objective: To determine the efficacy of measurement-based care (MBC), defined as routinely administered outcome measures with practitioner and patient review to inform clinical decision-making, for adults with depressive disorders.
Data Sources: Embase, MEDLINE, PsycINFO, ClinicalTrials.gov, CNKI, and Wanfang Data were searched through July 1, 2020, using search terms for measurement-based care, depression, antidepressant or pharmacotherapy, and randomized controlled trials (RCTs), without language restriction.
Study Selection: Of 8,879 articles retrieved, 7 RCTs (2,019 participants) evaluating MBC for depressive disorders, all involving pharmacotherapy, were included.
Data Extraction: Two independent reviewers extracted data. The primary outcome was response rate (≥ 50% improvement from baseline to endpoint on a depression scale). Secondary clinical outcomes were remission rate (endpoint score in remission range), difference in endpoint severity, and medication adherence.
Results: Meta-analysis with random-effects models found no significant difference between MBC and comparison groups in response rates (3 studies; odds ratio [OR] = 1.66; 95% CI, 0.66–4.17; P = .279). MBC was associated with significantly greater remission rates (5 studies; OR = 1.83; 95% CI, 1.12–2.97; P = .015), lower endpoint severity (5 studies; standardized mean difference = 0.53; 95% CI, 0.06–0.99; P = .026), and greater medication adherence (3 studies; OR = 1.68; 95% CI, 1.22–2.30; P = .001).
Conclusions: Although benefits for clinical response are unclear, MBC is effective in decreasing depression severity, promoting remission, and improving medication adherence in patients with depressive disorders treated with pharmacotherapy. The results are limited by the small number of included trials, high risk of bias, and significant study heterogeneity.
J Clin Psychiatry 2021;82(5):21r14034
To cite: Zhu M, Hong RH, Yang T, et al. The efficacy of measurement-based care for depressive disorders: a systematic review and meta-analysis of randomized controlled trials. J Clin Psychiatry. 2021;82(5):21r14034.
To share: https://doi.org/10.4088/JCP.21r14034
© Copyright 2021 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, University of British Columbia, Vancouver, Canada
bShanghai Mental Health Center and Shanghai Jiao Tong University School of Medicine, Shanghai, China
cHongkou Mental Health Center, Shanghai, China
*Corresponding author: Raymond W. Lam, MD, 2255 Wesbrook Mall, Vancouver, BC, Canada V6T 2A1 ([email protected]).
CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation. This activity is free.
CME Objective
After studying this article, you should be able to:
- Use measurement-based care for patients with depressive disorders treated with pharmacotherapy
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Nurses Credentialing Center (ANCC) and the American Academy of Physician Assistants (AAPA) accept certificates of participation for educational activities certified for AMA PRA Category 1 Credit™ from organizations accredited by the ACCME.
Release, Expiration, and Review Dates
This educational activity was published in September 2021 and is eligible for AMA PRA Category 1 Credit™ through October 31, 2023. The latest review of this material was September 2021.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past 3 years, Marlene P. Freeman, MD, Editor in Chief, has received research funding from JayMac and Sage; has been a member of the Independent Data Safety and Monitoring Committee for Janssen (Johnson & Johnson) and Novartis; and has served on advisory boards for Eliem and Sage. As an employee of Massachusetts General Hospital (MGH), Dr Freeman works with the MGH National Pregnancy Registry, which receives funding from Alkermes, Aurobindo, AuroMedics, Johnson & Johnson/Janssen, Otsuka, Sage, Sunovion, Supernus, and Teva, and works with the MGH Clinical Trials Network and Institute, which receives research funding from multiple pharmaceutical companies and the National Institute of Mental Health. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
Depressive disorders, including major depressive disorder (MDD), are common psychiatric disorders worldwide and a leading contributor to the global burden of disease.1 Despite many evidence-based pharmacologic and psychosocial interventions, treatment outcomes for depression are relatively poor in clinical practice.2–5 A key contributor to poor outcomes may be the unstructured approaches used by clinicians in assessing patient progress that make therapeutic effects difficult to quantify.6 Clinicians may also have difficulty detecting deterioration of patient symptoms, leading to delay of needed treatment adjustments.7 Thus, relying on clinical judgment alone to assess progress may lead to an inappropriate treatment regimen, poor treatment adherence, and failure to treat to full symptom remission.7,8 Therefore, a standardized approach involving routine monitoring of patient outcomes may help to improve treatment adjustments and outcomes for MDD.
Measurement-based care (MBC) is an evidence-based practice that provides a systematic framework for routine outcome monitoring and has demonstrated benefit in treating a range of psychiatric disorders.6,9,10 MBC includes (1) routine administration of validated rating scales, either by clinician-rated or patient-reported outcomes (PROs); (2) review of scores by practitioners and patients; and (3) using scores to inform shared clinical decision-making.11,12 MBC has specifically been recommended for the management of MDD by the American Psychiatric Association (APA),13 Canadian Network for Mood and Anxiety Treatments (CANMAT),14 and UK National Institute for Health and Care Excellence (NICE)15 clinical practice guidelines.
MBC offers several potential benefits for depression management. For example, MBC enables clinicians to individualize depression treatment based on up-to-date information about patient symptoms and severity. Quantifiable patient data can be readily incorporated into medication algorithms, facilitating standardized care.16,17 MBC may also help to identify treatment nonresponders, detect residual symptoms, and increase treatment adherence by encouraging patient participation.18–21 Although there are recognized barriers and facilitators to implementation and scalability of MBC,22,23 its feasibility for depression treatment in clinical settings and propensity to improve patient outcomes were demonstrated in several large trials and projects.11,24,25 Despite these benefits, MBC continues to be underutilized in clinical practice.26–28
A number of reviews and meta-analyses6,9,29,30 have examined the efficacy of MBC for mental health outcomes, but these have focused on broad diagnoses and varied outcome measures. To our knowledge, there are no quantitative syntheses of the most rigorous studies investigating effects of MBC with depressive disorders as the primary diagnosis. Therefore, our aim was to conduct a systematic review and meta-analysis of randomized controlled trials (RCTs) to determine the efficacy of MBC for depression management. We sought to compare MBC to comparison interventions in adults with depressive disorders receiving antidepressant treatment, psychotherapy, or both for improvement on clinician-rated and PRO measures.
METHODS
Search Strategy and Selection Criteria
The systematic review was registered with PROSPERO (https://www.crd.york.ac.uk/prospero/), number CRD#42019147274. The electronic databases Embase (OVID), MEDLINE (OVID), PsycINFO, ClinicalTrials.gov, CNKI, and Wanfang Data were searched from their inception until July 1, 2020. The search was conducted by combining search terms from 4 categories: (1) measurement-based care, (2) depressive disorder, (3) antidepressant OR psychotherapy, (4) randomized controlled trial (see Supplementary Appendix 1 for complete search strategy used for each database). Medical subject heading (MeSH) terms for these 4 categories of search terms were included when available. Reference mining was performed by searching through bibliographies of relevant articles to identify any studies that were missed through the database search.
Studies were included if they were (1) RCTs involving (2) adults ≥ 18 years of age (3) currently diagnosed with a depressive disorder based on validated criteria (ie, DSM-IV, DSM-5, or ICD-10) and were given (4) MBC as an intervention. To be considered MBC, the intervention had to include a routinely administered validated symptom, outcome, or process measure that involved practitioner review of data and use of data to inform clinical decisions. There was no restriction on language.
Studies were excluded if the participants had other comorbidities as a primary diagnosis or were children or adolescents. Review articles, abstracts, commentaries, case reports, and case series were also excluded.
Two independent reviewers (M.Z., X.Y.) screened titles and abstracts of retrieved articles for inclusion. Full-text reviews were subsequently conducted for potentially eligible articles. Discrepancies were resolved by consensus or through consultation with an independent third reviewer (R.W.L.) when consensus could not be reached.
Data Extraction
Two independent reviewers extracted data using a data extraction form designed for the study. Any discrepancies were resolved through consensus or consultation with a third reviewer (R.W.L.). Study authors were contacted if additional information was required. The following data were extracted: study setting, study duration, study design, participant characteristics (eg, age, sex, primary diagnosis), outcome measures and scores at baseline and post-intervention, and details of the interventions and comparison conditions.
Data Analysis
The primary outcome was efficacy based on clinical response rate (50% or greater improvement from baseline score at end of treatment), which was originally defined from a clinician-administered depression rating scale. However, after the literature search, we found that only 2 studies reported clinician-rated scales; hence, we expanded our definition to include either clinician-rated or patient-rated depression scales. Secondary outcomes included (1) clinical response rate on patient-rated depression measures, (2) clinical remission rate (endpoint score in the remission range, eg, 17-item Hamilton Depression Rating Scale [HDRS] score ≤ 7) by either clinician- or patient-rated depression measures, (3) depression severity at endpoint (endpoint scores on a clinician- or patient-rated depression rating scale) or change scores (endpoint scores minus the baseline scores) if endpoint scores were not available, (4) change in measures of quality of life or functioning, (5) attrition rate (dropouts for any reason), and (6) medication adherence.
All outcomes were analyzed with the intent-to-treat (ITT) samples if available. To protect for inflation of effect size for studies with more than one intervention arm, the results of active arms were pooled as one intervention, as recommended by the Cochrane Handbook for Systematic Reviews of Interventions.31 Risk of bias was assessed using version 2 of the Cochrane Risk of Bias tool for RCTs.32 Meta-analysis was performed using the Comprehensive Meta-Analysis Version 2.0 software (Biostat, USA). Categorical outcomes such as response and remission rates were analyzed using odds ratios (ORs). Continuous outcomes were analyzed using the standardized mean difference (SMD) as a measure of effect size. Outcome data were taken at the end of treatment for each study unless otherwise specified.
A random-effects model was used to account for expected study heterogeneity, including variations in depression severity, the type of depression (eg, major depression, chronic depression, treatment-resistant depression), study duration, clinician-rated versus self-rated measures, and how MBC was delivered. Statistical heterogeneity was assessed using Q χ2 statistics and I2; the I2 can be interpreted as 50%–70% indicating substantial heterogeneity and 75%–100% indicating considerable heterogeneity.33 Publication bias was examined using funnel plots of outcomes plotted against their standard error (with asymmetric distribution of data points suggesting bias), the Rosenthal fail-safe N (the number of unidentified null studies that would need to exist to change the result),34 and the Egger regression intercept (a statistical test for asymmetry in the funnel plot).35 If bias was suggested, the trim-and-fill procedure with a random-effects model was used to impute potential missing studies.36
RESULTS
Study Selection
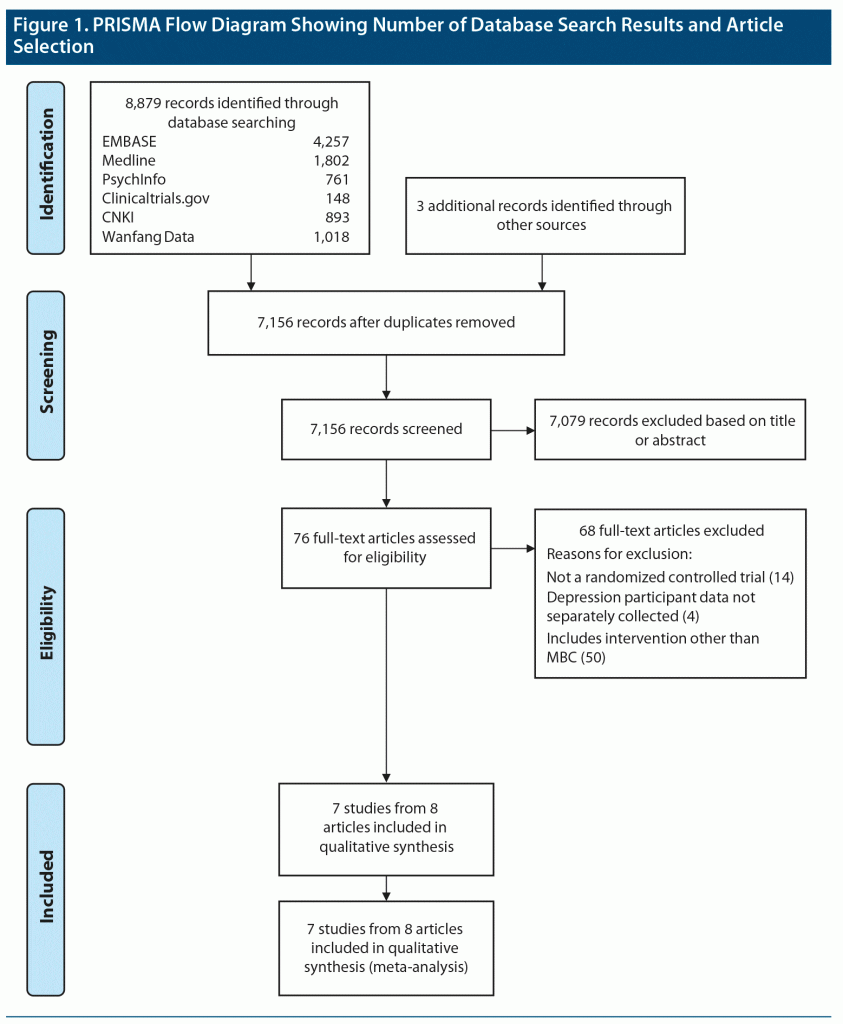
The literature search and selection are outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram (Figure 1). The search yielded 8,879 articles and 3 that were identified through other sources, such as hand-searching. After duplicate removal, 7,156 remaining articles were assessed through title and abstract review and 76 articles were assessed for eligibility through full-text review. The final 8 articles yielded outcome data from 7 studies for the meta-analysis. Two articles, Chang et al19 and Yeung et al,37 reported different outcomes from a single study, the Clinical Outcomes in Measurement‐based Treatment (COMET) trial. For the meta-analysis, clinical response, remission, and endpoint PHQ-9 scores were retrieved from Yeung et al,37 while medication adherence data was taken from Chang et al.19 Additionally, the COMET trial used a quasi-randomized cluster design in which physician sites were alternately assigned to study arms. The remaining 6 studies were parallel-group RCTs. Therefore, data from a total of 7 studies were included in the meta-analysis.
Study Characteristics
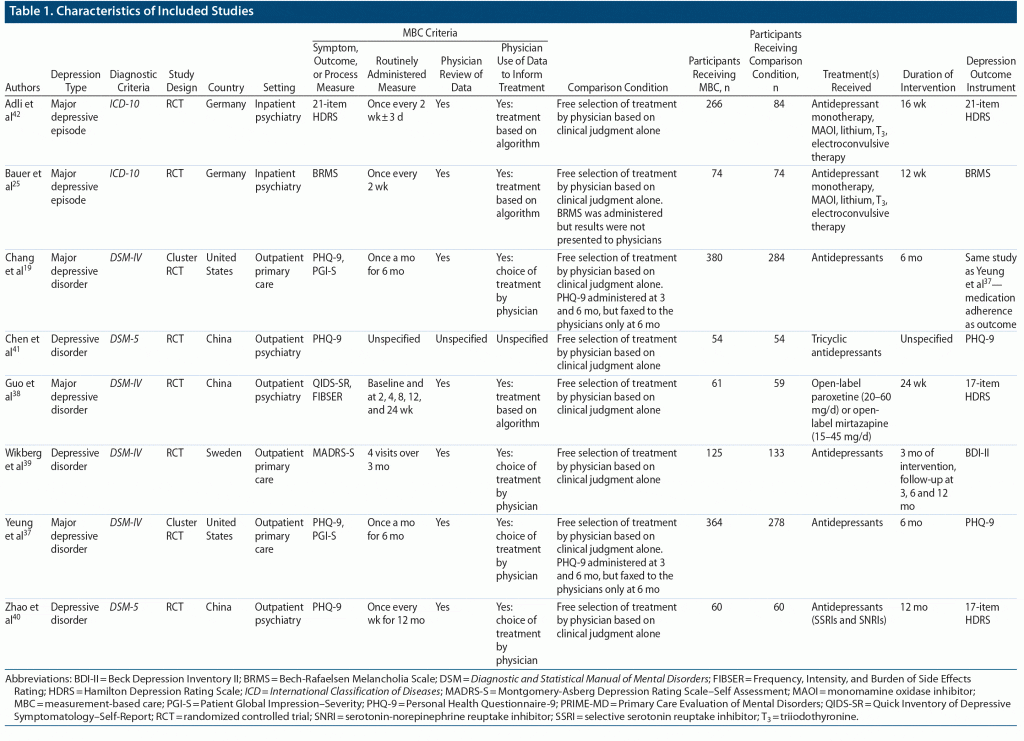
Table 1 shows the main characteristics of the 7 included RCTs. All the included studies involved pharmacotherapy management. Identified studies of psychotherapy were excluded because they were not RCTs or did not involve depressive disorders separately from other psychiatric diagnoses. Individual study sample sizes ranged from 108 to 915 participants with a total of 2,019 participants in the 7 studies. The participants in all studies were diagnosed with a depressive disorder or a major depressive episode based on DSM-IV, DSM-5, or ICD-10 criteria, with severity of depression ranging from mild to severe. Three studies were conducted in outpatient psychiatry settings, 2 studies in outpatient primary care settings, and 2 studies in inpatient psychiatry settings.
The studies used various outcome measures for depression severity. Clinician-rated depression measures included the HDRS and Bech-Rafaelsen Melancholia Scale (BRMS). Patient-rated depression measures included the Patient Health Questionnaire-9 (PHQ-9), and Beck Depression Inventory-II (BDI-II).
The routinely administered outcome measure for MBC varied across all studies and included the QIDS-SR,38 MADRS-S,39 PHQ-9,37,40,41 21-item HDRS,42 and the BRMS.25 In keeping with the criteria for MBC, all studies involved physician review of the measures to inform clinical decisions. Physicians in 3 studies used algorithms to further guide clinical decision-making,25,38,42 while the physicians in the other 4 studies were not guided by algorithms. The physicians assigned to the comparison groups were able to freely choose the treatments for the patients without routine depression measures as feedback. Three studies used antidepressant monotherapy as the chosen form of treatment, whereas the Adli et al42 and Bauer et al25 studies used a complex stepwise approach that included antidepressant monotherapy, monoamine oxidase inhibitors, lithium, and triiodothyronine augmentation, sleep deprivation, and electroconvulsive therapy. None of the studies explicitly reported psychotherapy as a form of treatment. Frequency of MBC measures ranged from once every week to once a month. The study durations ranged from 3 months to 12 months.
Risk of Bias Assessment
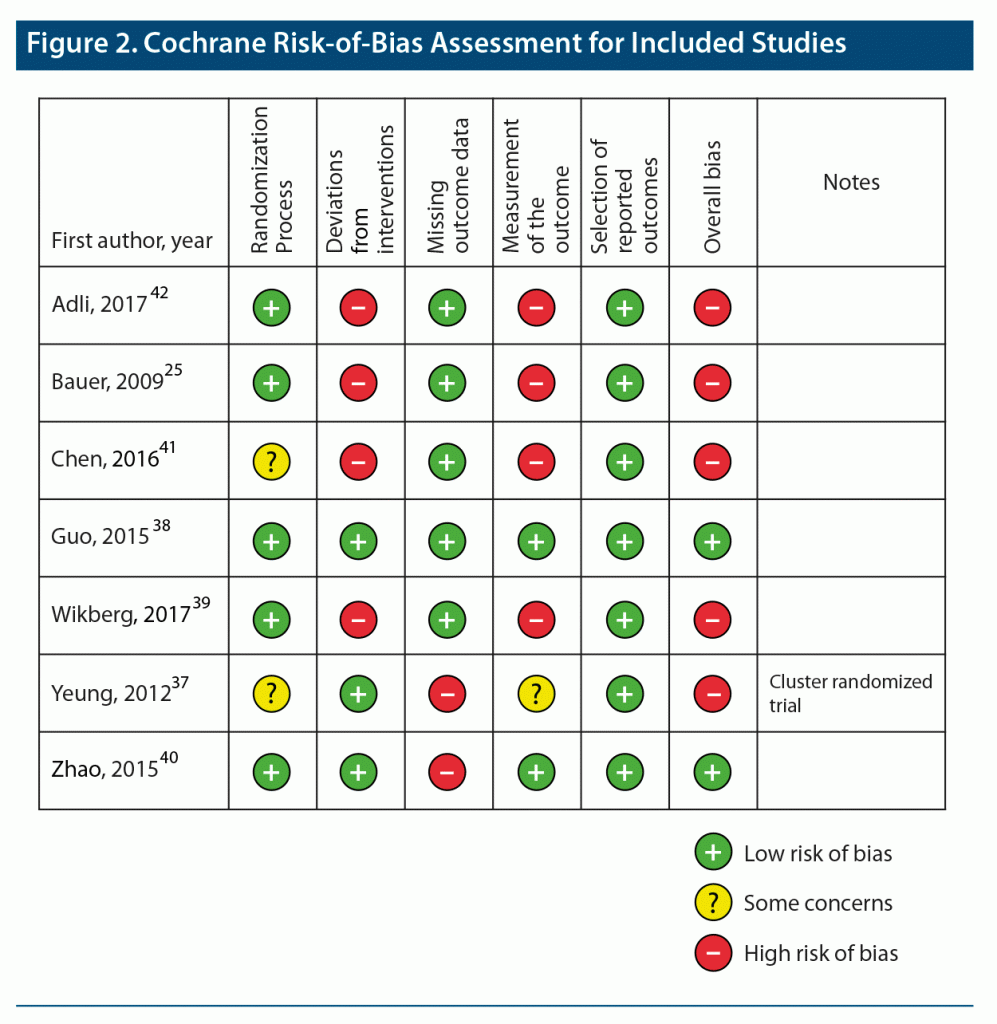
Figure 2 shows the summary of the risk of bias assessment from the Cochrane Risk of Bias tool. A study was judged to be high risk if greater than one domain received an assessment of high risk of bias. Two studies were assessed to have an overall low risk of bias,38,40 whereas the remaining 5 studies were judged to have a high risk of bias.25,37,39,41,42 For the randomization process, Yeung et al37 used a pseudorandomized cluster design, and the randomization process was unclear for Chen et al.41 Four studies either lacked or did not report blinding of participants, physicians, and assessors, and were assessed to have either uncertain or high risk of bias due to potential deviations from intended interventions. Two studies had missing outcome data due to incomplete data on dropouts; ie, the COMET study19,37 and the Zhao et al study40 excluded data from dropouts in their final analyses. Five studies had either high or uncertain risk of bias for measurement of the outcome. None of the studies had high risk of bias in the selection of reported outcomes.
Meta-Analysis
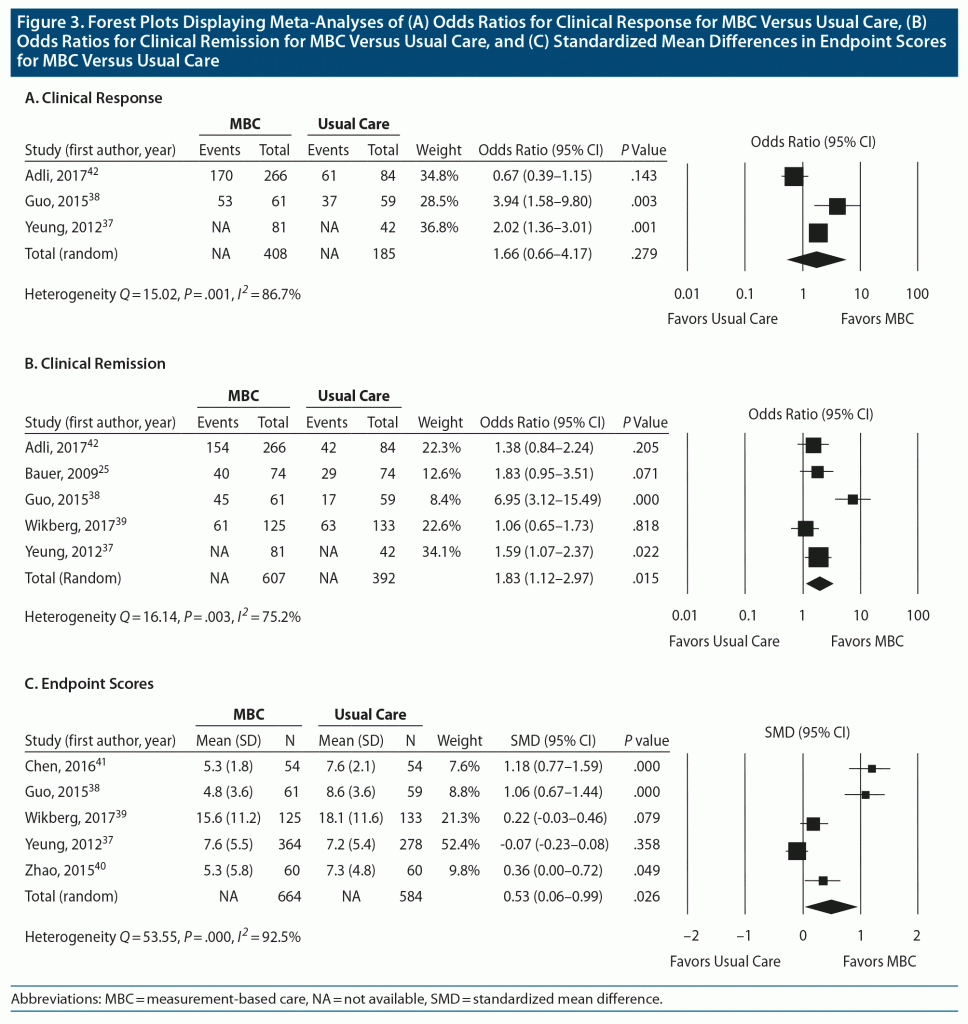
Efficacy outcomes. The original primary outcome was clinical response rate using clinician-rated depression scales. As only 2 studies reported clinician-rated scales, we expanded our definition to include either clinician-rated or patient-rated depression scales. Overall, 3 studies (1,112 participants) provided clinical response rates, with the Guo et al38 and Adli et al42 studies using versions of the clinician-rated HDRS and the Yeung et al37 study using the patient-rated PHQ-9. The Adli et al study42 had 3 intervention arms that were consistent with MBC, so the data from these arms were pooled into 1 intervention group.
With a random-effects model, there was no significant difference in clinical response between MBC and comparison conditions (OR 1.66; 95% CI, 0.66–4.17; P = .279) (Figure 3A). There was significant heterogeneity between these trials, with Q statistic of 15.02 and I2 of 86.7% (degrees of freedom [df] = 2; P = .001). The funnel plot of standard errors by effect size estimates was broadly symmetrical (Supplementary Figure 1), the fail-safe N was 4, and the Egger intercept had a 1-tailed P value of .45, suggesting there is little probability of publication bias (see Supplementary Appendix 2). The unadjusted rates for clinical response were 67.6% and 62.7%, respectively, for active MBC versus comparison conditions.
Secondary efficacy outcomes included clinical remission rate and endpoint depression severity. Five studies (1,518 participants) reported remission rates. Three studies used clinician-rated scales, and 2 studies used patient-rated scales. For clinician-rated scales, remission was defined as 17-item HDRS score ≤ 7,38 21-item HDRS score ≤ 9,42 and BRMS score ≤ 7.25 For patient-rated scales, remission was defined as BDI-II score ≤ 13 at 3-month follow-up39 and PHQ-9 score < 5 at 6 months.37 MBC was associated with a significantly higher clinical remission rate compared to the comparison condition (OR 1.83; 95% CI, 1.12–2.97; P = .015) (Figure 3B). There was significant heterogeneity between these trials, with Q statistic of 16.14 and I2 of 75.2% (df = 4, P = .003). The funnel plot of standard errors by effect size estimates was broadly symmetrical (Supplementary Figure 2), the fail-safe N was 23, and the Egger intercept had a 1-tailed P value of .08, suggesting a low probability of publication bias (see Supplementary Appendix 2). The unadjusted rates for clinical remission were 52.8% for MBC and 43.0% for comparison conditions.
Difference in endpoint depression scores were reported for 5 studies (1,248 participants). For endpoint depression scores, 3 studies used the PHQ-9,37,40,41 1 study used the 17-item HDRS,38 and 1 study used the BDI-II.39 Overall, the MBC condition had significantly lower endpoint depression scores versus the comparison condition (SMD 0.53; 95% CI, 0.06–0.99; P = .026), with the SMD representing a medium effect size (Figure 3C). There was significant heterogeneity between these trials, with Q statistic of 53.55 and I2 of 92.5% (df = 4; P < .001). The funnel plot of standard errors by effect size estimates showed some asymmetry (Supplementary Figure 3) and, although the fail-safe N was 45, the Egger intercept was 8.10 (95% CI, 2.26–13.95, t3 = 4.4, 1-tailed P = .01), suggesting probability of publication bias (see Supplementary Appendix 2). The trim-and-fill procedure, however, suggested that there were no missing studies, and the random-effects model did not change the SMD point estimate.
Because all 3 efficacy outcomes showed significant statistical heterogeneity, we conducted post hoc sensitivity analyses to explore potential sources of heterogeneity, segregating by methodology variables that might differ between studies. For each outcome, we examined (1) studies that used antidepressants only (ie, excluding those with multitreatment algorithms), (2) studies conducted in psychiatric settings (ie, excluding those in primary care settings), and (3) studies conducted outside of China (ie, because of potential differences in health care systems) (Supplementary Table 1). For clinical response, the studies using antidepressants only did not have significant heterogeneity (Q=1.73, df = 1, P = .19, I2=42.2); the OR was 2.48 (95% CI, 1.36–4.51; P = .003) in favor of MBC. For clinical remission, the 4 studies conducted outside of China did not show significant heterogeneity Q=2.28, df = 3, P = .52, I2=0.00); the OR was 1.42 (95% CI, 1.11–1.80; P = .005) in favor of MBC. All of the remaining analyses continued to show significant heterogeneity in the results.
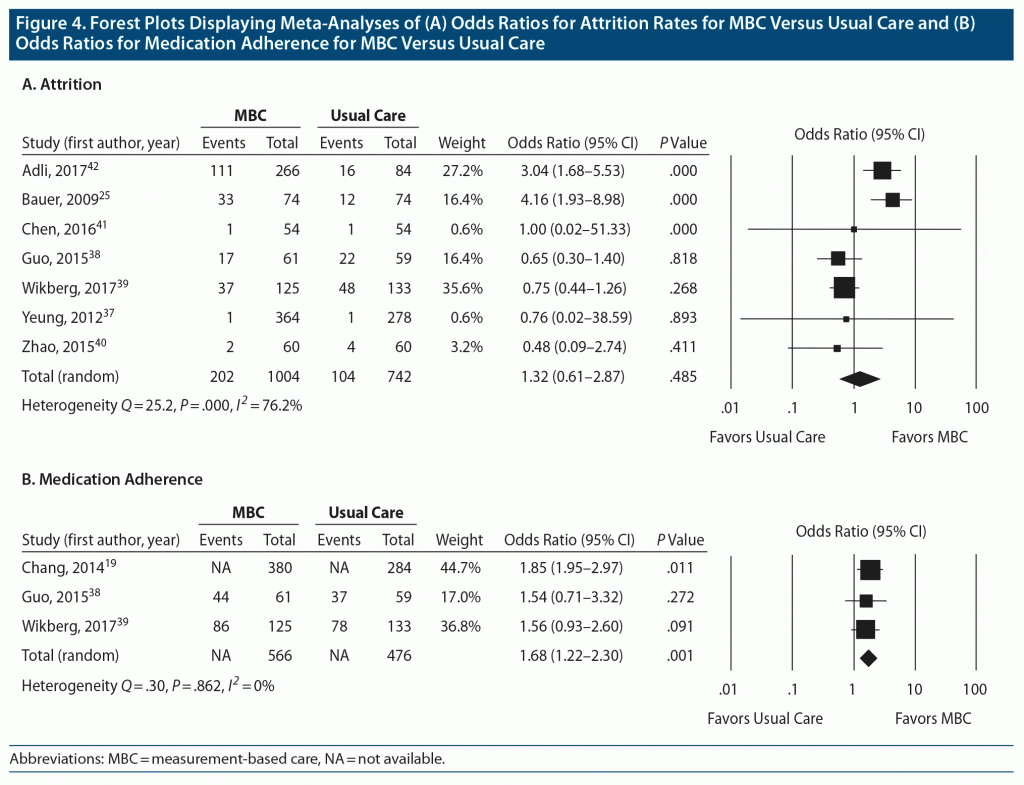
Attrition rate and adherence to medication. Data on attrition rates were available for all 7 studies (1,746 participants). There was no significant difference in attrition rates between MBC and comparison conditions (OR 1.32; 95% CI, 0.61–2.87; P = .49) (Figure 4A). There was significant heterogeneity between these trials, with Q statistic of 25.2 and I2 of 76.2% (df = 6, P < .001). The funnel plot of standard errors by effect size estimates was broadly symmetrical (Supplementary Figure 4), the fail-safe N could not be calculated because it was not relevant to the observed lack of significant difference, and the Egger intercept had a 1-tailed P value of .42, indicating a low probability of publication bias (see Supplementary Appendix 2).
Three studies (1,042 participants) reported on medication adherence,19,38,40 defined as the proportion of participants who continued their medication by study end. Medication adherence data were taken at the end of the trial for 2 studies19,38 and 6 months following the end of the trial for 1 study.39 Results using random-effects analysis revealed that medication adherence was more likely in the MBC group (OR 1.68; 95% CI, 1.22–2.30; P = .001) (Figure 4B). There was no significant heterogeneity between these trials, with Q statistic of 0.30 and I2 of 0% (df = 1, P = .862). The funnel plot of standard errors by effect size estimates was broadly symmetrical (Supplementary Figure 5), the fail-safe N was 6, and the Egger intercept had a 1-tailed P value of .48, suggesting a low probability of publication bias (see Supplementary Appendix 2). Overall unadjusted rates of medication adherence for the 3 studies were 76.1% for the MBC conditions and 63.9% for the comparison conditions.
There was an insufficient number of studies that reported quality of life and functional level measures for meta-analysis. Only 2 studies reported quality of life findings: Wikberg et al39 found no significant differences in EuroQoL-5D (EQ-5D)43 scores between the MBC and treatment-as-usual groups at the 12-month follow-up, while Zhao et al40 found significant differences in scores on the World Health Organization Quality of Life assessment (WHOQOL-BREF)44 in favor of MBC at 12-month follow-up. No studies reported functional outcomes, so the category was not further assessed.
DISCUSSION
Our objective was to determine the efficacy of MBC for depression from a meta-analysis of RCTs. We identified 8 articles reporting on 7 studies of MBC with pharmacologic treatment that met the inclusion criteria. The findings from our overall meta-analysis showed no significant difference between MBC and comparison groups in clinical response rates based on clinician- and patient-rated depression scales. The nonsignificant finding may be a result of Type II error, as only 3 studies were pooled for the clinical response outcome, which may have led to nonrejection of a false null hypothesis. In contrast, MBC was associated with a significant increase in clinical remission rates and decrease in endpoint depression severity, with clinically relevant effect size, compared to comparison conditions. Clinical remission is an important outcome because it is associated with lower risk of relapse45,46 and better long-term outcomes47 compared with achieving clinical response without remission. Additionally, MBC was associated with significantly greater medication adherence. Moreover, there was no significant difference in attrition rates between the two groups. Overall, these results support the benefit of MBC for improving outcomes in patients with depressive disorders treated with pharmacotherapy. However, a caution for these results is the high risk of bias in the majority of studies (5 of 7), although both trials with low overall risk of bias, Guo et al38 and Zhao et al,40 found positive effects in favor of MBC.
MBC has shown benefits in the management of a range of psychiatric disorders.6,9,10 Despite the benefits of MBC described in implementation and non-randomized studies, a 2016 Cochrane review of 17 RCTs30 concluded that there was insufficient evidence to support the routine use of PROs due to low quality studies with high risk of bias. However, the evidence then was limited to use of multidomain PRO measures instead of more specific measures from a single domain such as depression. In addition, a broad range of mental health disorders were included in populations with multiple comorbidities. Interestingly, most of the included studies focused on psychotherapy as a treatment modality, whereas none of the included RCTs in our current review used psychotherapy. Studies of MBC with psychotherapy have focused on broader groups of diagnoses and hence did not meet the depressive disorder diagnosis criterion for inclusion in our meta-analysis.
MBC may be particularly relevant to antidepressant prescribing, in which simple algorithms can guide stepwise changes to the dosage or medications depending on measurement outcome. In this regard, favorable outcomes with MBC may in part be due to increased adherence to medication. Our results support findings from other studies, such as the Combining Medications to Enhance Depression Outcomes (CO-MED) study,48 which incorporated MBC into treatment delivery and found higher medication adherence among adults with chronic or recurrent MDD compared to rates reported by prior studies that did not use MBC. On the other hand, a meta-analysis of psychotherapy studies49 found that MBC with clinical decision support tools offering specific treatment recommendations (analogous to medication algorithms) were also associated with larger positive effect sizes.
Unfortunately, there were too few studies that included quality of life and functional outcomes to synthesize. The 2 studies with quality of life measures found discrepant findings, with MBC showing significant benefit on the WHOQOL-BREF40 but no significant improvement on the EQ-5D.39 The EQ-5D has fewer items than the WHOQOL-BREF (6 items versus 26 items, respectively) and may be less responsive to change for mental health conditions.
A limitation of our meta-analysis was the heterogeneity in methodologies of the included studies. Currently, there is no consensus on the most effective measures or the optimal frequency of measurement for MBC. The MBC measures used in the included RCTs varied across all studies, with frequency of MBC assessment ranging from once a week to once a month. Similarly, there was substantial variation among studies in the nature, fidelity, and reporting of other elements of MBC, such as reviewing scores with patients, how scores were used to make clinical decisions, and the degree of shared decision making between clinicians and patients. For example, the type and complexity of medication algorithms used in the studies varied considerably: 4 studies did not use a treatment algorithm, Guo et al38 used a simple medication dosing algorithm, and Adli et al42 and Bauer et al25 used a complex algorithm that included medication and somatic treatments such as electroconvulsive therapy. Other potential sources of heterogeneity in the studies included variability in depression severity, treatment setting, outcome assessments, duration of treatment, and country of study.
We anticipated heterogeneity in the studies and hence a priori set a random-effects model for all outcomes. We did find significant statistical heterogeneity in the efficacy outcomes, with I2 ranging from 75.2% to 92.5%, regarded as considerable heterogeneity. The sensitivity analyses for potential methodology sources of study heterogeneity (studies of antidepressants only, studies in psychiatric settings, studies conducted outside China) were limited by small sample sizes, and the results were not consistent. There was suggestion that the studies of antidepressant-only treatment and studies conducted outside of China had less heterogeneity for clinical response and remission; these sensitivity analyses still showed increased ORs in favor of MBC. Future research should attempt to standardize the methodologies to reduce heterogeneity in outcomes.
MBC may also be more effective in specific patient subgroups, but we were unable to conduct subgroup analyses for the included studies because of lack of segregation of outcomes. Previous meta-analyses evaluating MBC in patients undergoing psychotherapy18,50 showed that the effect of MBC was greater in treatment nonresponders compared to those who showed an initial improvement. The reasoning is that MBC provides information on change or lack of change in patient outcomes that cannot be reliably assessed by clinical judgment alone. Hence, future studies should examine the effects of MBC for treatment-resistant depression.
In summary, the findings from this systematic review and meta-analysis of RCTs support the use of MBC in the management of depression, particularly with pharmacotherapy. MBC allows clinicians to individualize therapeutic decisions based on up-to-date information regarding patient outcomes. MBC may also improve medication adherence. A limitation is that, because we did not identify any eligible psychotherapy studies, our results do not address efficacy of MBC for psychotherapy alone or psychotherapy combined with pharmacotherapy. Future RCTs of MBC should examine the effects of MBC for psychotherapy and for depression subgroups (including treatment-resistant depression), and optimization of algorithm-guided MBC. MBC studies should include outcome measures assessing functioning and quality of life to complement standard symptom measures. Further investigation is also necessary to standardize the type and frequency of routine outcome measures used for MBC in depression management.
Submitted: April 12, 2021; accepted June 28, 2021.
Published online: September 28, 2021.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, no investigational information about pharmaceutical agents or device therapies that is outside US Food and Drug Administration–approved labeling has been presented in this article.
Financial disclosure: Dr Michalak has received funding to support patient education initiatives from Otsuka. Dr Yatham has been on speaker/advisory boards for, or has received research grants from Alkermes, AstraZeneca, Bristol-Myers Squibb, the Canadian Network for Mood and Anxiety Treatments (CANMAT), the Canadian Institutes of Health Research (CIHR), Sumitomo Dainippon Pharma (DSP), Eli Lilly, GlaxoSmithKline, Janssen, the Michael Smith Foundation for Health Research, Pfizer, Servier, Sunovion, and the Stanley Foundation. Dr Chen has received honoraria for ad hoc speaking for DSP, Eli Lilly, Pfizer, Lundbeck, Janssen, and Sunovion. Dr Lam has received honoraria for ad hoc speaking or advising/consulting, or received research funds, from Allergan, Asia-Pacific Economic Cooperation, BC Leading Edge Foundation, CIHR, CANMAT, Healthy Minds Canada, Janssen, Lundbeck, Lundbeck Institute, Michael Smith Foundation for Health Research, Mitacs, Myriad Neuroscience, Ontario Brain Institute, Otsuka, Pfizer, Unity Health, and VGH-UBC Hospital Foundation. Ms Zhu, Ms Hong, Ms Liu, Dr T. Yang, Dr X. Yang, Dr X. Wang, Dr Murphy, and Dr Z. Wang have no disclosures.
Funding/support: This study was supported by the Enhanced Measurement-Based Care Effectiveness for Depression (EMBED) study, funded by the CIHR and the National Natural Sciences Foundation of China (81761128032) through the Global Alliance on Chronic Disease [CIHR Team Grant: GACD Mental Health, GAC-154985; Agreement #01709-000]. Ms Zhu was supported by a Summer Student Research Program (SSRP) award from the UBC Faculty of Medicine. Ms Hong was supported by a Mach-Gaensslen Foundation of Canada Award through the UBC Faculty of Medicine SSRP.
Role of the sponsor: The sponsors had no role in the design, conduct, interpretation, or publication of this study.
Supplementary material: Available at Psychiatrist.com.
CLINICAL POINTS
- Measurement-based care (MBC) is an evidence-based practice that utilizes routine outcome monitoring to inform therapeutic decisions; however, MBC continues to be underutilized in clinical settings.
- For patients with depressive disorders treated with pharmacotherapy, MBC showed higher remission rates, reduced depression severity, and improved medication adherence compared to usual care.
References (50)

- Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10(11):e1001547. PubMed CrossRef
- Richards D. Prevalence and clinical course of depression: a review. Clin Psychol Rev. 2011;31(7):1117–1125. PubMed CrossRef
- Kupfer DJ, Frank E, Phillips ML. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet. 2012;379(9820):1045–1055. PubMed CrossRef
- Eaton WW, Shao H, Nestadt G, et al. Population-based study of first onset and chronicity in major depressive disorder. Arch Gen Psychiatry. 2008;65(5):513–520. PubMed CrossRef
- Steinert C, Hofmann M, Kruse J, et al. The prospective long-term course of adult depression in general practice and the community: a systematic literature review. J Affect Disord. 2014;152–154(1):65–75. PubMed CrossRef
- Fortney JC, Unützer J, Wrenn G, et al. A tipping point for measurement-based care. Psychiatr Serv. 2017;68(2):179–188. PubMed CrossRef
- Hatfield D, McCullough L, Frantz SHB, et al. Do we know when our clients get worse? an investigation of therapists’ ability to detect negative client change. Clin Psychol Psychother. 2010;17(1):25–32. PubMed. CrossRef
- Henke RM, Zaslavsky AM, McGuire TG, et al. Clinical inertia in depression treatment. Med Care. 2009;47(9):959–967. PubMed CrossRef
- Knaup C, Koesters M, Schoefer D, et al. Effect of feedback of treatment outcome in specialist mental healthcare: meta-analysis. Br J Psychiatry. 2009;195(1):15–22. PubMed CrossRef
- Brodey BB, Cuffel B, McCulloch J, et al. The acceptability and effectiveness of patient-reported assessments and feedback in a managed behavioral healthcare setting. Am J Manag Care. 2005;11(12):774–780. PubMed
- Trivedi MH, Rush AJ, Wisniewski SR, et al; STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. PubMed CrossRef
- Hong RH, Murphy JK, Michalak EE, et al. Implementing measurement-based care for depression: practical solutions for psychiatrists and primary care physicians. Neuropsychiatr Dis Treat. 2021;17:79–90. PubMed CrossRef
- Gelenberg AJ, Freeman MP, Markowitz JC, et al; Work Group on Major Depressive Disorder. Practice Guideline for the Treatment of Patients With Major Depressive Disorder. 3rd ed. Arlington, VA: American Psychiatric Association; 2010.
- Lam RW, McIntosh D, Wang J, et al; CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 1, disease burden and principles of care. Can J Psychiatry. 2016;61(9):510–523. PubMed CrossRef
- Depression in adults: recognition and management (Clinical Guideline 90). National Institute for Health and Care Excellence. Published 2009. Accessed March 7, 2021. https://www.nice.org.uk/guidance/cg90
- Crismon ML, Trivedi M, Pigott TA, et al. The Texas Medication Algorithm Project: report of the Texas Consensus Conference Panel on medication treatment of major depressive disorder. J Clin Psychiatry. 1999;60(3):142–156. PubMed CrossRef
- Valenstein M, Adler DA, Berlant J, et al. Implementing standardized assessments in clinical care: now’s the time. Psychiatr Serv. 2009;60(10):1372–1375. PubMed CrossRef
- Lambert MJ, Whipple JL, Hawkins EJ, et al. Is it time for clinicians to routinely track patient outcome? a meta-analysis. Clin Psychol Sci Pract. 2003;10(3):288–301. CrossRef
- Chang TE, Jing Y, Yeung AS, et al. Depression monitoring and patient behavior in the Clinical Outcomes in MEasurement-Based Treatment (COMET) trial. Psychiatr Serv. 2014;65(8):1058–1061. PubMed CrossRef
- Jeon SW, Han C, Ko YH, et al. Measurement-based treatment of residual symptoms using clinically useful depression outcome scale: Korean validation study. Clin Psychopharmacol Neurosci. 2017;15(1):28–34. PubMed CrossRef
- Trivedi MH. How can measurement-based care help improve treatment outcomes for major depressive disorder in primary care? J Clin Psychiatry. 2020;81(2):UT17042BR2C. PubMed CrossRef
- Lewis CC, Boyd M, Puspitasari A, et al. Implementing measurement-based care in behavioral health: a review. JAMA Psychiatry. 2019;76(3):324–335. PubMed CrossRef
- Van Wert MJ, Malik M, Memel B, et al. Provider perceived barriers and facilitators to integrating routine outcome monitoring into practice in an urban community psychiatry clinic: a mixed-methods quality improvement project. J Eval Clin Pract. 2021;27(4):767–775. PubMed CrossRef
- Trivedi MH, Rush AJ, Crismon ML, et al. Clinical results for patients with major depressive disorder in the Texas Medication Algorithm Project. Arch Gen Psychiatry. 2004;61(7):669–680. PubMed CrossRef
- Bauer M, Pfennig A, Linden M, et al. Efficacy of an algorithm-guided treatment compared with treatment as usual: a randomized, controlled study of inpatients with depression. J Clin Psychopharmacol. 2009;29(4):327–333. PubMed CrossRef
- Zimmerman M, McGlinchey JB. Why don’t psychiatrists use scales to measure outcome when treating depressed patients? J Clin Psychiatry. 2008;69(12):1916–1919. PubMed CrossRef
- Hatfield DR, Ogles BM. Why some clinicians use outcome measures and others do not. Adm Policy Ment Health. 2007;34(3):283–291. PubMed CrossRef
- Jensen-Doss A, Haimes EMB, Smith AM, et al. Monitoring treatment progress and providing feedback is viewed favorably but rarely used in practice. Adm Policy Ment Health. 2018;45(1):48–61. PubMed CrossRef
- Morris DW, Trivedi MH. Measurement-based care for unipolar depression. Curr Psychiatry Rep. 2011;13(6):446–458. PubMed CrossRef
- Kendrick T, El-Gohary M, Stuart B, et al. Routine use of patient reported outcome measures (PROMs) for improving treatment of common mental health disorders in adults. Cochrane Database Syst Rev. 2016;7(7):CD011119. PubMed CrossRef
- Higgins J, Thomas J, Chandler J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane Database of Systematic Reviews. Published 2020. https://www.training.cochrane.org/handbook
- Higgins JPT, Savović J, Page MJ, et al. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated February 2020). Cochrane Database of Systematic Reviews. Published 2020. https://www.training.cochrane.org/handbook
- Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. PubMed CrossRef
- Rosenthal R. The file drawer problem” and tolerance for null results. Psychol Bull. 1979;86(3):638–641. CrossRef
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. PubMed CrossRef
- Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. PubMed CrossRef
- Yeung AS, Jing Y, Brenneman SK, et al. Clinical Outcomes in Measurement-based Treatment (Comet): a trial of depression monitoring and feedback to primary care physicians. Depress Anxiety. 2012;29(10):865–873. PubMed CrossRef
- Guo T, Xiang YT, Xiao L, et al. Measurement-based care versus standard care for major depression: a randomized controlled trial with blind raters. Am J Psychiatry. 2015;172(10):1004–1013. PubMed CrossRef
- Wikberg C, Westman J, Petersson E-L, et al. Use of a self-rating scale to monitor depression severity in recurrent GP consultations in primary care - does it really make a difference? a randomised controlled study. BMC Fam Pract. 2017;18(1):6. PubMed CrossRef
- Zhao S, Qin G, Chen J, et al. A study on the effect of measurement-based care using Patient Health Questionnaire Depression Scale (PHQ-9) on patients with depression. Zhejiang Prev Med. 2015;28(5):469–472.
- Chen P, Yang X. A study on the effect of measurement-based care on patients with depression in clinical practice. Psychol Dr. 2016;22(10):1007–8231.
- Adli M, Wiethoff K, Baghai TC, et al. How effective is algorithm-guided treatment for depressed inpatients? results from the randomized controlled multicenter German algorithm project 3 trial. Int J Neuropsychopharmacol. 2017;20(9):721–730. PubMed CrossRef
- EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. PubMed CrossRef
- WHOQOL Group. Development of the WHOQOL: rationale and current status. Int J Ment Health. 1994;23(3):24–56. CrossRef
- Lin EHB, Katon WJ, VonKorff M, et al. Relapse of depression in primary care: rate and clinical predictors. Arch Fam Med. 1998;7(5):443–449. PubMed CrossRef
- Paykel ES, Ramana R, Cooper Z, et al. Residual symptoms after partial remission: an important outcome in depression. Psychol Med. 1995;25(6):1171–1180. PubMed CrossRef
- Keller MB. Past, present, and future directions for defining optimal treatment outcome in depression: remission and beyond. JAMA. 2003;289(23):3152–3160. PubMed CrossRef
- Warden D, Trivedi MH, Carmody T, et al. Adherence to antidepressant combinations and monotherapy for major depressive disorder: a CO-MED report of measurement-based care. J Psychiatr Pract. 2014;20(2):118–132. PubMed CrossRef
- Lambert MJ, Whipple JL, Kleinstäuber M. Collecting and delivering progress feedback: a meta-analysis of routine outcome monitoring. Psychotherapy (Chic). 2018;55(4):520–537. PubMed CrossRef
- Shimokawa K, Lambert MJ, Smart DW. Enhancing treatment outcome of patients at risk of treatment failure: meta-analytic and mega-analytic review of a psychotherapy quality assurance system. J Consult Clin Psychol. 2010;78(3):298–311. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top