Objective: Aripiprazole lauroxil, a long-acting injectable antipsychotic, demonstrated safety and efficacy in treating symptoms of schizophrenia in a double-blind, placebo-controlled trial. Because the metabolic profile of antipsychotics is an important safety feature, the effects of aripiprazole lauroxil on body weight, endocrine and metabolic profiles, and safety were examined in a secondary analysis.
Methods: Patients with schizophrenia (DSM-IV-TR criteria) were randomly assigned to aripiprazole lauroxil 441 mg, aripiprazole lauroxil 882 mg, or placebo intramuscularly once monthly between December 2011 and March 2014. Changes in body weight, body mass index, fasting blood glucose and serum lipids, glycosylated hemoglobin (HbA1c), and prolactin over 12 weeks were assessed. The incidence of treatment-emergent adverse events (AEs) was evaluated.
Results: Among 622 randomized patients, no clinically relevant changes from baseline to week 12 were observed for any serum lipid, lipoprotein, plasma glucose, or HbA1c value with placebo or either dose of aripiprazole lauroxil. Both doses of aripiprazole lauroxil were associated with reductions in mean prolactin levels, whereas placebo treatment was not. The mean (standard deviation) change from baseline for body weight was 0.74 (3.9) kg, 0.86 (3.7) kg, and 0.01 (3.6) kg for aripiprazole lauroxil 441 mg, aripiprazole lauroxil 882 mg, and placebo groups, respectively. AEs related to metabolic parameters were reported in 2.4%, 1.4%, and 2.4% of patients in the aripiprazole lauroxil 441 mg, aripiprazole lauroxil 882 mg, and placebo groups, respectively.
Conclusions: Aripiprazole lauroxil was well tolerated, with a low-risk metabolic profile relative to published data for other antipsychotics. Changes similar to those observed with placebo were observed in the aripiprazole lauroxil groups for metabolic parameters, with modest weight gain in the active treatment groups over the 12-week course.
Trial Registration: ClinicalTrials.gov identifier: NCT01469039
Effect of Aripiprazole Lauroxil on Metabolic and Endocrine Profiles and Related Safety Considerations Among Patients With Acute Schizophrenia

ABSTRACT
Objective: Aripiprazole lauroxil, a long-acting injectable antipsychotic, demonstrated safety and efficacy in treating symptoms of schizophrenia in a double-blind, placebo-controlled trial. Because the metabolic profile of antipsychotics is an important safety feature, the effects of aripiprazole lauroxil on body weight, endocrine and metabolic profiles, and safety were examined in a secondary analysis.
Methods: Patients with schizophrenia (DSM-IV-TR criteria) were randomly assigned to aripiprazole lauroxil 441 mg, aripiprazole lauroxil 882 mg, or placebo intramuscularly once monthly between December 2011 and March 2014. Changes in body weight, body mass index, fasting blood glucose and serum lipids, glycosylated hemoglobin (HbA1c), and prolactin over 12 weeks were assessed. The incidence of treatment-emergent adverse events (AEs) was evaluated.
Results: Among 622 randomized patients, no clinically relevant changes from baseline to week 12 were observed for any serum lipid, lipoprotein, plasma glucose, or HbA1c value with placebo or either dose of aripiprazole lauroxil. Both doses of aripiprazole lauroxil were associated with reductions in mean prolactin levels, whereas placebo treatment was not. The mean (standard deviation) change from baseline for body weight was 0.74 (3.9) kg, 0.86 (3.7) kg, and 0.01 (3.6) kg for aripiprazole lauroxil 441 mg, aripiprazole lauroxil 882 mg, and placebo groups, respectively. AEs related to metabolic parameters were reported in 2.4%, 1.4%, and 2.4% of patients in the aripiprazole lauroxil 441 mg, aripiprazole lauroxil 882 mg, and placebo groups, respectively.
Conclusions: Aripiprazole lauroxil was well tolerated, with a low-risk metabolic profile relative to published data for other antipsychotics. Changes similar to those observed with placebo were observed in the aripiprazole lauroxil groups for metabolic parameters, with modest weight gain in the active treatment groups over the 12-week course.
Trial Registration: ClinicalTrials.gov identifier: NCT01469039
J Clin Psychiatry 2016;77(11):1519-1525
dx.doi.org/10.4088/JCP.15m10467
© Copyright 2016 Physicians Postgraduate Press, Inc.
aSaint Louis University School of Medicine, St Louis, Missouri
bFlorida Atlantic University, Charles E. Schmidt College of Medicine, Boca Raton
cAlkermes, Inc, Waltham, Massachusetts
*Corresponding author: Henry A. Nasrallah, MD, Department of Neurology and Psychiatry, Saint Louis University School of Medicine, 1438 S. Grand Blvd, St Louis, MO 63104 ([email protected]).
Schizophrenia represents a major medical illness, with a prevalence of 0.5%-1.5% in different regions of the globe, imparting a major burden on patients and their families and on health care systems.1 Despite the availability of a number of antipsychotics for controlling symptoms of schizophrenia and achieving a clinical response, a large proportion of patients fail to achieve a satisfactory outcome because of side effects or poor adherence to medication.2,3 Atypical antipsychotics often are preferred to conventional antipsychotics for treating schizophrenia because they provide comparable efficacy with an improved safety and neurologic tolerability profile. However, some atypical antipsychotics are associated with potential adverse metabolic and endocrine effects, including clinically significant weight gain, prolactin abnormalities, lipid abnormalities, insulin resistance, and increased risk for diabetes.4-9 These adverse events (AEs) are associated with increased burden and impact on medication adherence10-12 and potentially contribute to observed increases in cardiovascular risk and morbidity and mortality in this population.13,14
The severity of metabolic- and endocrine-related side effects varies across individual antipsychotic medications, with aripiprazole generally manifesting low levels of risk relative to other antipsychotics.7,15-17 In addition, treatment of schizophrenia with aripiprazole offers comparable therapeutic efficacy and symptom control18-21 and has been associated with reduced risk of cardiovascular morbidity and mortality.22
An underutilized alternative to oral antipsychotics is long-acting injectable antipsychotics, which offer efficacy and tolerability profiles that are comparable to those of oral antipsychotics, with more consistent rates of adherence and greater patient satisfaction.23 In an early clinical study in 40 patients with schizophrenia, single doses of aripiprazole lauroxil 221 mg, 441 mg, and 588 mg were not associated with clinically significant safety concerns.24 Results from a phase 3, randomized, placebo-controlled trial of patients with acute schizophrenia found that aripiprazole lauroxil 441 mg and 882 mg doses were effective and well tolerated.25 More detailed descriptions of metabolic and endocrine outcomes were subsequently conducted from this phase 3 study in order to better characterize the effects of aripiprazole lauroxil in this population. This descriptive analysis from the phase 3 trial reports on the effects of aripiprazole lauroxil on prolactin and metabolic parameters including body weight, plasma lipids, and glycemic control.
METHODS
The detailed methodology for this study was presented previously and is summarized briefly.25 The study was conducted across 8 countries between December 2011 and March 2014 in accordance with the Declaration of Helsinki, 1964, and Good Clinical Practice principles (International Conference on Harmonization, 1997). At each clinical study site, the protocol, amendments, and informed consent were approved by a qualified institutional review board, and all participants completed written informed consent prior to study participation. This study was registered at ClinicalTrials.gov: NCT01469039.
Study Design
This phase 3, randomized, double-blind, placebo-controlled study evaluated 2 doses of aripiprazole lauroxil in patients with schizophrenia (DSM-IV-TR criteria) who were experiencing an acute relapse. After admission to an inpatient setting, all current antipsychotics were discontinued, and a test dose of oral aripiprazole 5 mg was administered daily for 2 days prior to randomization to assess tolerability for those who had not previously taken aripiprazole.

- As the metabolic profile of antipsychotics is an important safety feature, the effects of aripiprazole lauroxil on metabolic parameters were examined.
- Characterization of the risk/benefit profile of newer formulations of antipsychotics is helpful when planning treatment strategies for patients with schizophrenia.
- This analysis may be helpful when assessing treatment options for an individual patient given the currently available long-acting injectable antipsychotics.
Patient Selection
Patients were aged 18 to 70 years with a body mass index (BMI) of 18.5 to 40 kg/m2. Patients were eligible if they were experiencing a current acute exacerbation or relapse of schizophrenia with an onset < 2 months prior to screening (< 2 weeks for the current exacerbation if hospitalized) and ≥ 2 years had elapsed since the initial onset of symptoms. Patients also were required to have a clinically significant beneficial response to treatment with an antipsychotic medication other than clozapine and to have been an outpatient for > 3 months during the past year. At screening and baseline, a Positive and Negative Syndrome Scale (PANSS)26 total score of 70 to 120, a score of ≥ 4 for ≥ 2 of the Positive Scale items (item 1: delusions; item 2: conceptual disorganization; item 3: hallucinatory behavior; item 6: suspiciousness/persecution), and a Clinical Global Impression—Severity of Illness (CGI-S)27 score of ≥ 4 were required.
Patients were excluded who (1) had a poor or inadequate response to aripiprazole; (2) had a history of treatment resistance (failure to respond to 2 adequate trials of different antipsychotics for ≥ 4 weeks at a maximum tolerated dose); (3) had hypersensitivity to aripiprazole, other antipsychotics, or fat emulsion; (4) had any other clinically significant neuropsychiatric disorder, medical illness, or laboratory abnormality that would interfere with the conduct of the study; and (5) were women who were pregnant, lactating, or breastfeeding. In addition, patients using long-acting antipsychotics within 60 days of screening or who were under current involuntary hospitalization or underwent psychiatric hospitalization for > 30 days during the 90 days before screening were excluded.
On day 1, patients were randomized to an intramuscular (IM) dose of aripiprazole lauroxil 441 mg, aripiprazole lauroxil 882 mg, or placebo. In addition to IM study drug, patients randomized to an aripiprazole lauroxil treatment group received oral aripiprazole 15 mg, and patients randomized to placebo received matching oral placebo for 3 weeks after randomization. Patients remained in the inpatient study unit for at least 2 weeks after administration of the first dose of IM study drug. Two subsequent doses of IM study drug were administered on days 29 and 57.
Study Assessments
A blood sample for serum chemistry was obtained at screening and on days 1, 29, 57, and 85 (12 weeks) to measure total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, glycosylated hemoglobin (HbA1c), plasma glucose levels, and prolactin. Body weight was measured at screening and on days 1, 15, 29, 57, and 85, and BMI was calculated. All AEs during the treatment period, including those related to metabolic and endocrine parameters, were recorded. An AE was any untoward medical occurrence, which may or may not have a causal relation to the investigational treatment and may have included any clinical or laboratory change that was considered clinically significant.
Statistical Methods
The safety population included all randomized patients who received at least 1 dose of IM study drug (N = 622). Analyses were descriptive including mean, standard deviation (SD), range, and mean change from baseline to the last postbaseline value obtained during the treatment period. Shifts in total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, HbA1c, and plasma glucose levels from “normal” at baseline to “high” at last postbaseline assessment and “high” at baseline to “normal” at last postbaseline assessment were evaluated (see Supplementary eTable 1 at PSYCHIATRIST.COM.). The proportions of patients with a body weight change of at least 7% and at least 10% were determined. An analysis of the effect of baseline BMI category (< 25 kg/m2, 25 to < 30 kg/m2, and ≥ 30 kg/m2) on PANSS total score was undertaken using analysis of covariance and last observation carried forward.
RESULTS
Of 848 patients who were screened, 623 were randomized, and 622 received at least 1 dose of IM study drug. One patient randomized to placebo was discontinued for a protocol violation prior to receiving IM study drug. Baseline demographics, including body weight and BMI, were comparable among treatment groups (Table 1). More than 60% of patients were either overweight (BMI 25 to < 30 kg/m2) or obese (BMI ≥ 30 kg/m2) at baseline.
Changes in Body Weight and BMI
Mean body weight increased modestly in both aripiprazole lauroxil groups relative to placebo, with similar SD values for all groups (Table 2). Mean (SD) change from baseline to the last postbaseline visit for body weight was 0.7 (3.9) kg, 0.9 (3.7) kg, and 0.01 (3.6) kg for aripiprazole lauroxil 441 mg, aripiprazole lauroxil 882 mg, and placebo. Greater proportions of patients in the aripiprazole lauroxil groups gained ≥ 7% body weight. Of the 50 patients with ≥ 7% increase in body weight, 31 experienced ≥ 10% increase (9 each in the aripiprazole lauroxil 441 mg and placebo groups and 13 in the aripiprazole lauroxil 882 mg group). Of the 50 patients with ≥ 7% increase in body weight, 70% were nonwhite and male, and of the 31 patients with ≥ 10% increase, 77% were nonwhite and male.
The greatest percent increase in body weight in an individual patient within each group was +14.8% (aripiprazole lauroxil 441 mg), +28.5% (aripiprazole lauroxil 882 mg), and +24.3% (placebo). The greatest percent decrease in weight in an individual patient in each group was -28.8% (aripiprazole lauroxil 441 mg), -18.3% (aripiprazole lauroxil 882 mg), and -11.1% (placebo).
An analysis was undertaken of the effect of BMI on PANSS total score. Least squares mean change from baseline to day 85 for PANSS total score was analyzed for patients in 3 BMI subgroups (< 25 kg/m2, 25 to < 30 kg/m2, and ≥ 30 kg/m2). Significant (P < .025) improvement was observed with both aripiprazole lauroxil dose groups vs placebo for all 3 BMI subgroups. The mean decrease in PANSS total score with placebo was 6 in the overweight group (25 to < 30 kg/m2) and 13 in the obese group (≥ 30 kg/m2). In contrast, mean decreases from baseline in PANSS total score for both aripiprazole lauroxil dose groups ranged from 17 to 24.
Glycemic Control
Shifts from normal at baseline to high at last postbaseline assessment were similar across groups for glucose and HbA1c (Table 3). For HbA1c, only minor changes from baseline values to last postbaseline visit of 0.01%-0.03% were observed (Table 2). For glucose, mean values increased from baseline by up to 3% in the placebo group but remained unchanged in the aripiprazole lauroxil 441 mg group and decreased 1% from baseline in the 882 mg group; shifts from high to normal were 47% and 83% vs 55% for aripiprazole lauroxil 441 mg and 882 mg vs placebo, respectively (Table 3). One patient in the placebo group experienced levels of plasma glucose that were at least ≥ 3 times the upper limit of normal (ULN).
Changes in Lipid Parameters
The mean change from baseline for lipid parameters was similar between treatment groups (Table 2). Total cholesterol, LDL cholesterol, and triglyceride levels decreased by 5%-8% from baseline in the aripiprazole lauroxil groups. Shifts from normal levels of total cholesterol, LDL cholesterol, and triglycerides at baseline to high levels at last postbaseline assessment were < 20% among aripiprazole lauroxil 441 mg and 882 mg and placebo groups (Table 3). Shifts from high levels at baseline to normal levels at last postbaseline assessment were similarly comparable among both dose groups and placebo. The majority of patients (80%) who entered the study had normal HDL cholesterol levels, and no patients had shifts in HDL levels from normal to high. More patients shifted from normal to low levels of HDL cholesterol in the placebo group (15.1%) than aripiprazole lauroxil 441 mg (11.3%) and 882 mg (10.3%) groups. No increases to ≥ 3 times ULN were observed for total, HDL, and LDL cholesterol levels. However, 3 (1.6%) patients in the placebo group, 4 (2.2%) in the aripiprazole lauroxil 441 mg group, and 2 (1.1%) in the 882 mg group had shifts or remained with triglyceride levels to ≥ 3 times ULN.
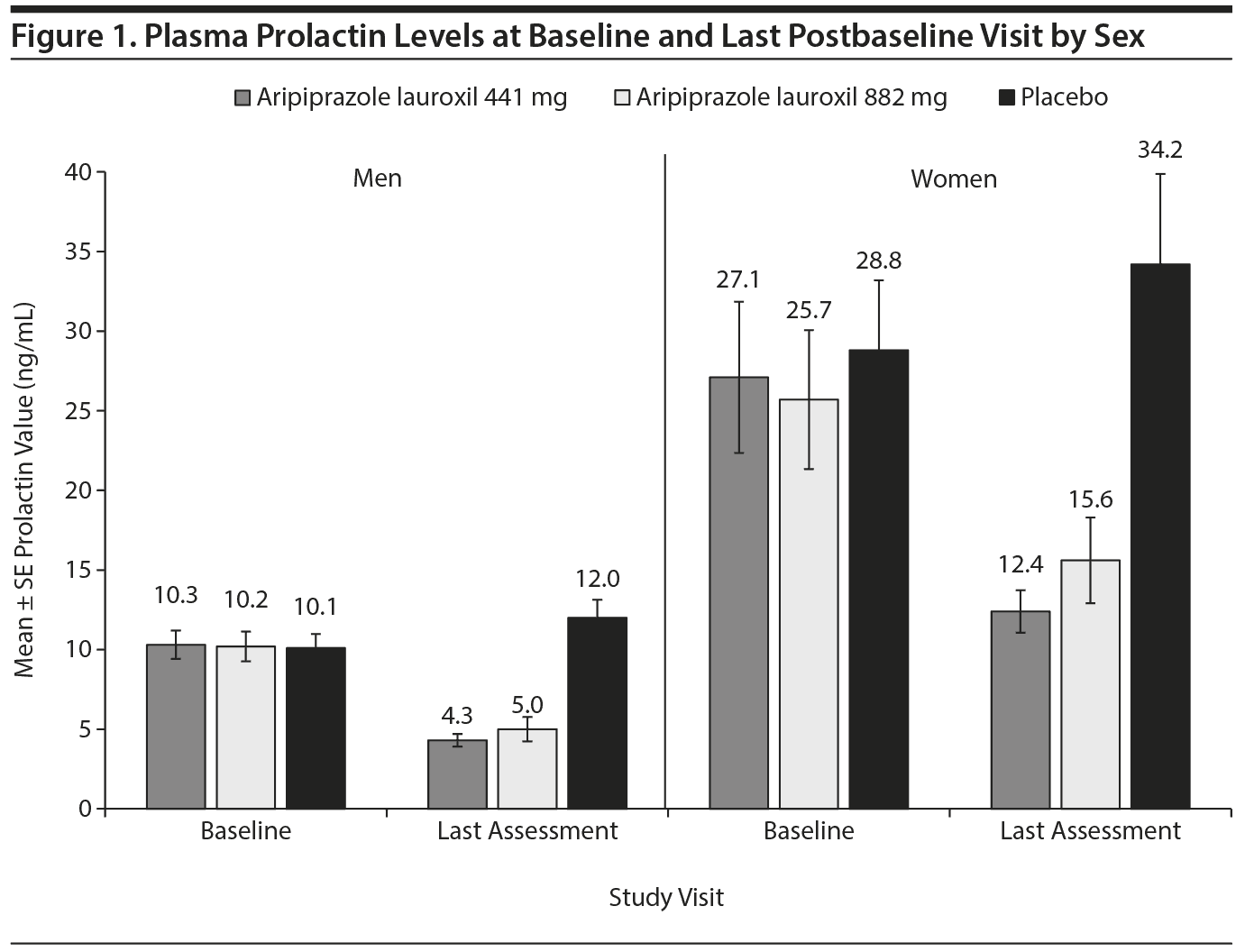
Prolactin
Both aripiprazole lauroxil dose groups were associated with decreases in prolactin levels. When further evaluated by sex, reductions seen in both men and women were clinically meaningful (Figure 1). Mean prolactin levels decreased in both aripiprazole lauroxil dose groups compared to the placebo group starting on day 29 and continued to decrease through day 85. In contrast, decreases in mean prolactin levels were not seen with placebo.
Potentially clinically significant increases in prolactin levels to ≥ 3 times ULN at any postbaseline visit occurred in 0 patients, 1 (1.1%) male and 1 (2.9%) female patient, and 2 (2.7%) male and 5 (14.3%) female patients in the aripiprazole lauroxil 441 mg, aripiprazole lauroxil 882 mg, and placebo groups, respectively (Table 4). The 2 patients in the aripiprazole lauroxil 882 mg group had single elevations of prolactin to 108.8 ng/mL and 268.9 ng/mL, respectively, and both patients completed the study without adverse consequences.
In the aripiprazole lauroxil groups, the proportion of men that had a shift from normal or low prolactin level at baseline to ≥ 3 times ULN at any postbaseline visit (2.9% and 3.6% for aripiprazole lauroxil 441 mg and 882 mg) was much lower than in the placebo group (25.5%). Among patients with elevated prolactin levels at baseline, men in the aripiprazole lauroxil groups with baseline levels ≥ 3 times ULN tended to normalize (86.4% and 70.6% for aripiprazole lauroxil 441 mg and 882 mg). A lesser proportion of women in both aripiprazole lauroxil dose groups shifted from a normal baseline to ≥ 3 times ULN (15.8% and 7.3% for aripiprazole lauroxil 441 mg and 882 mg vs 34% for placebo). The proportion of women with elevations ≥ 3 times ULN at baseline who normalized in each aripiprazole lauroxil group (60.0% and 57.9% for aripiprazole lauroxil 441 mg and 882 mg) was greater than in the placebo group (30.0%) (Supplementary eTable 2).
Adverse Events Related to Metabolic Effects
Four patients in the aripiprazole lauroxil 441 mg group (blood glucose increased [2]; serum triglycerides increased [2]), 2 patients in the aripiprazole lauroxil 882 mg group (hypoglycemia; hyperlipidemia), and 2 patients in the placebo group (serum cholesterol increased; blood glucose increased) experienced AEs related to metabolic effects. All patients had elevated levels at baseline, and none discontinued from the study. One patient in the aripiprazole lauroxil 882 mg group had a serious AE of hypoglycemia after the second injection. The patient had a history of recurrent hypoglycemic episodes, and this event was considered not related to study drug. Three (1.4%) patients in the placebo group and 1 (0.5%) each in the aripiprazole lauroxil groups had AEs of increased prolactin. Prolactin levels were elevated at baseline and did not increase further during treatment in the aripiprazole lauroxil groups.
An AE of weight increased was reported by 6 (2.9%) of patients in the aripiprazole lauroxil 441 mg group, 5 (2.4%) in the aripiprazole lauroxil 882 mg group, and 1 (0.5%) in the placebo group. Of these, 5 patients on aripiprazole lauroxil had a ≥ 7% increase in body weight at any study visit, and 1 patient in the aripiprazole lauroxil 441 mg group had a > 10% increase in weight.
DISCUSSION
Results from the primary analysis of this study revealed that aripiprazole lauroxil was effective and tolerated for the treatment of patients with acute schizophrenia.25 The more detailed descriptive analysis included in the present report demonstrates that aripiprazole lauroxil had few clinically relevant AEs on metabolic parameters among a population of patients with acute schizophrenia.
In contrast to placebo, both doses of aripiprazole lauroxil were associated with decreased mean prolactin below baseline levels toward the normal range. This result of decreased prolactin production toward the normal range is consistent with results from other studies of aripiprazole in patients with schizophrenia.28,29 Thus, it would be expected that aripiprazole lauroxil would have a low rate of hyperprolactinemic effects with treatment. This significant result represents potential advantages of aripiprazole lauroxil treatment.
Results of this 12-week study also indicated that aripiprazole lauroxil succeeded in limiting the pronounced weight gain and other metabolic risks commonly encountered during antipsychotic treatment. Minimal changes from baseline to last postbaseline assessment were observed for lipid parameters, plasma glucose, and HbA1c with aripiprazole lauroxil, consistent with results from other studies of oral aripiprazole.9,30-34 While a clinically meaningful change in HbA1c is not feasible in a short-term study, this outcome was included along with other metabolic parameters because of the well-known AEs of antipsychotics. The change in mean plasma glucose, which is a more immediate measure of glycemic control, with aripiprazole lauroxil was similar to that with placebo. Longer-term studies of aripiprazole lauroxil are needed to fully characterize the effects on HbA1c. Mean changes to last postbaseline assessment for lipid and glycemic parameters were mostly unchanged from baseline for the majority of patients. While mean body weight and BMI increased in both aripiprazole lauroxil dose groups, the number of patients with at least a 7% increase from baseline in body weight was < 10% among all groups. No adverse outcomes were reported from these elevations. In fact, total and LDL cholesterol levels decreased by 6.0% and 8.4% in the aripiprazole lauroxil 882 mg group. Clinically relevant adverse outcomes related to any metabolic abnormality were uncommon.
The results from this study can be compared and contrasted with results from studies of other atypical antipsychotics that report significant AEs on metabolic parameters.7,35 A 12-week study with aripiprazole monohydrate found significant increases in weight and numerically greater fasting glucose vs placebo but no significant effects on lipid profile.28 During a placebo-controlled, 52-week study of aripiprazole monohydrate, no clinically significant metabolic abnormalities of new onset were observed.36 In a case-control study, Olfson et al37 found a significant increase in the risk of hyperlipidemia with all first- and second-generation antipsychotics except for aripiprazole. The risk of diabetes was significantly increased with commonly used doses of olanzapine, quetiapine, and risperidone but not with aripiprazole or ziprasidone in schizophrenic patients.9 A large claims database study found that olanzapine, quetiapine, and risperidone, but not aripiprazole, were associated with elevated HbA1c levels.32
Serum prolactin levels decreased with aripiprazole lauroxil, which is consistent with results from other studies of aripiprazole in patients with schizophrenia.28,29 Effects of some atypical antipsychotics on body weight are varied. A meta-analysis found that mean increases in body weight over a period of 10 weeks were > 4 kg with clozapine and olanzapine and > 2 kg with risperidone, while the increase with ziprasidone was 0.04 kg.38 This compares with < 1 kg increases in our study. Results from a 52-week, randomized, double-blind trial found ≥ 7% increases in body weight among 80% of patients on olanzapine, 50% on quetiapine, and 58% on risperidone.39 In our analysis, 9.2% of patients in either dose group of aripiprazole lauroxil had an increase in body weight of ≥ 7%. Thus, our analysis demonstrated a substantially lower, clinically nonsignificant change in body weight with aripiprazole lauroxil. Using baseline BMI status, we noted that for patients in the categories of normal (< 25 kg/m2), overweight (25 to < 30 kg/m2), and obese (≥ 30 kg/m2), the improvement in PANSS total score from baseline to endpoint (day 85) was statistically significant for both aripiprazole lauroxil doses compared to placebo (data not shown). During treatment with some antipsychotics, positive associations between weight gain and/or increased BMI and therapeutic improvements have also been reported.40,41
Limitations of the present analysis include the 12-week duration of the study; the time course of developing metabolic abnormalities may be longer. A planned long term-extension study will provide additional information on these outcomes. The present study also suggests that further analysis into baseline characteristics including age, race, and weight at baseline, as well as AEs, may be warranted in future studies. The proportion of patients with an increase in body weight of at least 7% was greater in the aripiprazole lauroxil 441 mg group than the 882 mg and placebo groups, providing no clear dose-response relation.
In summary, these findings of a low risk for metabolic abnormalities with aripiprazole lauroxil are important when considering overall patient health in the management of schizophrenia. Aripiprazole lauroxil represents an important option for the treatment of schizophrenia, with demonstrated efficacy and tolerability and a low risk of metabolic side effects that often impede maintenance of long-term antipsychotic treatment and may lead to higher cardiovascular risk.
Submitted: October 17, 2015; accepted March 20, 2016.
Online first: August 30, 2016.
Drug names: aripiprazole monohydrate (Abilify Maintena), aripiprazole lauroxil (Aristada), clozapine (Clozaril, FazaClo, and others), olanzapine (Zyprexa and others), quetiapine (Seroquel and others), risperidone (Risperdal and others), ziprasidone (Geodon and others).
Potential conflicts of interest: Dr Nasrallah has received honoraria and has been a speaker and/or consultant for Alkermes, Acadia, Allergan, Genentech, Forum, Janssen, Merck, Otsuka, Sunovion, and Vanda; has received grant and/or research support from Forest, Genentech, and Forum; and has no stock holdings to disclose. Dr Newcomer has received grant support from the National Institutes of Health, Foundation2Recovery, and Otsuka America and consulting fees from Reviva Pharmaceuticals and has served on a data safety monitoring board for Amgen. All other authors are employees of Alkermes, Inc, Waltham, Massachusetts.
Funding/support: The study was sponsored by Alkermes, Inc.
Role of the sponsor: The sponsor was involved in conducting the 12-week clinical trial and the analysis reported here. All authors were responsible for the interpretation of the analysis and approved the manuscript for submission to The Journal of Clinical Psychiatry.
Previous presentation: The study data were first presented as a poster (#145) at the 15th Annual International Conference on Schizophrenia Research (ICOSR); March 29, 2015; Colorado Springs, Colorado.
Acknowledgments: The authors acknowledge the editorial assistance of Alexandra Corrigan and Richard S. Perry, PharmD, in the preparation of this manuscript, which was supported by Alkermes Inc, Waltham, Massachusetts.
Supplementary material: Available at PSYCHIATRIST.COM.
REFERENCES
1. Lieberman JA. Is schizophrenia a neurodegenerative disorder? a clinical and neurobiological perspective. Biol Psychiatry. 1999;46(6):729-739. PubMed doi:10.1016/S0006-3223(99)00147-X
2. Lindenmayer JP, Liu-Seifert H, Kulkarni PM, et al. Medication nonadherence and treatment outcome in patients with schizophrenia or schizoaffective disorder with suboptimal prior response. J Clin Psychiatry. 2009;70(7):990-996. PubMed doi:10.4088/JCP.08m04221
3. Ascher-Svanum H, Zhu B, Faries DE, et al. Medication adherence levels and differential use of mental-health services in the treatment of schizophrenia. BMC Res Notes. 2009;2(1):6. PubMed doi:10.1186/1756-0500-2-6
4. Correll CU, Manu P, Olshanskiy V, et al. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302(16):1765-1773. PubMed doi:10.1001/jama.2009.1549
5. Kessing LV, Thomsen AF, Mogensen UB, et al. Treatment with antipsychotics and the risk of diabetes in clinical practice. Br J Psychiatry. 2010;197(4):266-271. PubMed doi:10.1192/bjp.bp.109.076935
6. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223. PubMed doi:10.1056/NEJMoa051688
7. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(suppl 1):1-93. PubMed doi:10.2165/00023210-200519001-00001
8. Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123(2-3):225-233. PubMed doi:10.1016/j.schres.2010.07.012
9. Ulcickas Yood M, Delorenze GN, Quesenberry CP Jr, et al. Association between second-generation antipsychotics and newly diagnosed treated diabetes mellitus: does the effect differ by dose? BMC Psychiatry. 2011;11(1):197. PubMed doi:10.1186/1471-244X-11-197
10. Al-Zoairy R, Ress C, Tschoner A, et al. The effects of psychotropic drugs on the regulation of glucose metabolism. Curr Diabetes Rev. 2013;9(5):362-370. PubMed doi:10.2174/15733998113099990067
11. Tschoner A, Engl J, Laimer M, et al. Metabolic side effects of antipsychotic medication. Int J Clin Pract. 2007;61(8):1356-1370. PubMed doi:10.1111/j.1742-1241.2007.01416.x
12. McIntyre RS, McCann SM, Kennedy SH. Antipsychotic metabolic effects: weight gain, diabetes mellitus, and lipid abnormalities. Can J Psychiatry. 2001;46(3):273-281. PubMed
13. Daumit GL, Goff DC, Meyer JM, et al. Antipsychotic effects on estimated 10-year coronary heart disease risk in the CATIE schizophrenia study. Schizophr Res. 2008;105(1-3):175-187. PubMed doi:10.1016/j.schres.2008.07.006
14. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80(1):19-32. PubMed doi:10.1016/j.schres.2005.07.014
15. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, et al. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601. PubMed doi:10.2337/diacare.27.2.596
16. Balf G, Stewart TD, Whitehead R, et al. Metabolic adverse events in patients with mental illness treated with antipsychotics: a primary care perspective. Prim Care Companion J Clin Psychiatry. 2008;10(1):15-24. PubMed doi:10.4088/PCC.v10n0104
17. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161(8):1334-1349. PubMed doi:10.1176/appi.ajp.161.8.1334
18. Citrome L, Kalsekar I, Baker RA, et al. A review of real-world data on the effects of aripiprazole on weight and metabolic outcomes in adults. Curr Med Res Opin. 2014;30(8):1629-1641. PubMed doi:10.1185/03007995.2014.908280
19. Khanna P, Suo T, Komossa K, et al. Aripiprazole versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2014;1(1):CD006569. PubMed
20. Newcomer JW, Meyer JM, Baker RA, et al. Changes in non-high-density lipoprotein cholesterol levels and triglyceride/high-density lipoprotein cholesterol ratios among patients randomized to aripiprazole versus olanzapine. Schizophr Res. 2008;106(2-3):300-307. PubMed doi:10.1016/j.schres.2008.09.002
21. Newcomer JW, Campos JA, Marcus RN, et al. A multicenter, randomized, double-blind study of the effects of aripiprazole in overweight subjects with schizophrenia or schizoaffective disorder switched from olanzapine. J Clin Psychiatry. 2008;69(7):1046-1056. PubMed doi:10.4088/JCP.v69n0702
22. Kasteng F, Eriksson J, Sennfפlt K, et al. Metabolic effects and cost-effectiveness of aripiprazole versus olanzapine in schizophrenia and bipolar disorder. Acta Psychiatr Scand. 2011;124(3):214-225. PubMed doi:10.1111/j.1600-0447.2011.01716.x
23. Zhornitsky S, Stip E. Oral versus long-acting injectable antipsychotics in the treatment of schizophrenia and special populations at risk for treatment nonadherence: a systematic review. Schizophr Res Treatment. 2012;2012:407171. PubMed
24. Turncliff R, Hard M, Brown D, et al. Aripiprazole lauroxil (ALKS 9070), a novel once-monthly prodrug of aripiprazole, achieves therapeutically relevant levels and is well-tolerated in adult patients with schizophrenia following deltoid administration. Presented at the American College of Neuropsychopharmacology Annual Meeting. Hollywood, Florida; December 8-12, 2013.
25. Meltzer HY, Risinger R, Nasrallah HA, et al. A randomized, double-blind, placebo-controlled trial of aripiprazole lauroxil in acute exacerbation of schizophrenia. J Clin Psychiatry. 2015;76(8):1085-1090. PubMed doi:10.4088/JCP.14m09741
26. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276. PubMed doi:10.1093/schbul/13.2.261
27. Guy W. ECDEU Assessment Manual for Psychopharmacology: Revised Edition (DHEW publication number ADM 76-338). Rockville, MD, US Department of Health, Education and Welfare, Public Health Service, Alcohol, Drug Abuse and Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research Programs, 1976:534-537.
28. Kane JM, Peters-Strickland T, Baker RA, et al. Aripiprazole once-monthly in the acute treatment of schizophrenia: findings from a 12-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(11):1254-1260. PubMed doi:10.4088/JCP.14m09168
29. Hanssens L, L’ Italien G, Loze JY, et al. The effect of antipsychotic medication on sexual function and serum prolactin levels in community-treated schizophrenic patients: results from the Schizophrenia Trial of Aripiprazole (STAR) study (NCT00237913). BMC Psychiatry. 2008;8(1):95. PubMed doi:10.1186/1471-244X-8-95
30. Baker RA, Pikalov A, Tran QV, et al. Atypical antipsychotic drugs and diabetes mellitus in the US Food and Drug Administration Adverse Event database: a systematic Bayesian signal detection analysis. Psychopharmacol Bull. 2009;42(1):11-31. PubMed
31. Cutler AJ, Marcus RN, Hardy SA, et al. The efficacy and safety of lower doses of aripiprazole for the treatment of patients with acute exacerbation of schizophrenia. CNS Spectr. 2006;11(9):691-702, quiz 719. PubMed
32. Guo Z, L’ italien GJ, Jing Y, et al. A real-world data analysis of dose effect of second-generation antipsychotic therapy on hemoglobin A1C level. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(5):1326-1332. PubMed doi:10.1016/j.pnpbp.2011.03.020
33. Kane JM, Carson WH, Saha AR, et al. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2002;63(9):763-771. PubMed doi:10.4088/JCP.v63n0903
34. Potkin SG, Raoufinia A, Mallikaarjun S, et al. Safety and tolerability of once monthly aripiprazole treatment initiation in adults with schizophrenia stabilized on selected atypical oral antipsychotics other than aripiprazole. Curr Med Res Opin. 2013;29(10):1241-1251. PubMed doi:10.1185/03007995.2013.821973
35. Nielsen J, Skadhede S, Correll CU. Antipsychotics associated with the development of type 2 diabetes in antipsychotic-naׯve schizophrenia patients. Neuropsychopharmacology. 2010;35(9):1997-2004. PubMed doi:10.1038/npp.2010.78
36. Kane JM, Sanchez R, Perry PP, et al. Aripiprazole intramuscular depot as maintenance treatment in patients with schizophrenia: a 52-week, multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2012;73(5):617-624. PubMed doi:10.4088/JCP.11m07530
37. Olfson M, Marcus SC, Corey-Lisle P, et al. Hyperlipidemia following treatment with antipsychotic medications. Am J Psychiatry. 2006;163(10):1821-1825. PubMed doi:10.1176/ajp.2006.163.10.1821
38. Allison DB, Casey DE. Antipsychotic-induced weight gain: a review of the literature. J Clin Psychiatry. 2001;62(suppl 7):22-31. PubMed
39. McEvoy JP, Lieberman JA, Perkins DO, et al. Efficacy and tolerability of olanzapine, quetiapine, and risperidone in the treatment of early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry. 2007;164(7):1050-1060. PubMed doi:10.1176/ajp.2007.164.7.1050
40. Czobor P, Volavka J, Sheitman B, et al. Antipsychotic-induced weight gain and therapeutic response: a differential association. J Clin Psychopharmacol. 2002;22(3):244-251. PubMed doi:10.1097/00004714-200206000-00003
41. Ascher-Svanum H, Stensland M, Zhao Z, et al. Acute weight gain, gender, and therapeutic response to antipsychotics in the treatment of patients with schizophrenia. BMC Psychiatry. 2005;5(1):3. PubMed doi:10.1186/1471-244X-5-3
This PDF is free for all visitors!
Save
Cite