Objective: People with psychotic disorders have an increased metabolic risk and a shortened life expectancy compared to the general population. Two large studies showed that metabolic disorders were untreated in a majority of the patients. Since then, guidelines have urged monitoring of metabolic health. This study examined the course of metabolic disorders over time in people with psychotic disorders and investigated current treatment rates.
Methods: A total of 1,259 patients with psychotic disorders, as defined by the DSM-IV, from 4 Dutch mental health institutions participated in 3 yearly assessments of the Pharmacotherapy Monitoring and Outcome Survey (PHAMOUS) between 2006 and 2014. Patients’ metabolic parameters were measured, and the use of pharmacologic treatment for hypertension (systolic blood pressure ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg), dyslipidemia (5% ≤ Systematic COronary Risk Evaluation [SCORE] risk < 10% and low-density lipoprotein [LDL] cholesterol level ≥ 2.5 mmol/L or SCORE risk ≥ 10% and LDL cholesterol level ≥ 1.8 mmol/L and/or triglycerides ≥ 2.3 mmol/L), and hyperglycemia (hemoglobin A1c concentration > 7% and/or fasting glucose concentration ≥ 7.2 mmol/L) was recorded.
Results: Prevalence of the metabolic syndrome, as defined by the National Cholesterol Education Program criteria, was > 50% at each assessment. On the basis of the European Society of Cardiology guidelines, pharmacotherapy for metabolic disorders was recommended for 52%-59% of the patients at each assessment. Treatment rates with antihypertensive (from 31% to 38%, P < .001) pharmacotherapy increased throughout the assessments. However, half of the patients were not treated for their metabolic risk factors while being monitored for 3 years or longer. Older patients were more likely to receive treatment, and patients who received treatment had lower blood pressure and lower cholesterol and triglyceride concentrations than patients not receiving the recommended treatment.
Conclusions: Metabolic risk factors are still seriously undertreated in people with psychotic disorders. Better adherence to and better implementation of guidelines about monitoring and treating metabolic disorders in psychiatry are crucial.
Persistent Low Rates of Treatment of Metabolic Risk Factors in People With Psychotic Disorders:
A PHAMOUS Study

ABSTRACT
Objective: People with psychotic disorders have an increased metabolic risk and a shortened life expectancy compared to the general population. Two large studies showed that metabolic disorders were untreated in a majority of the patients. Since then, guidelines have urged monitoring of metabolic health. This study examined the course of metabolic disorders over time in people with psychotic disorders and investigated current treatment rates.
Methods: A total of 1,259 patients with psychotic disorders, as defined by the DSM-IV, from 4 Dutch mental health institutions participated in 3 yearly assessments of the Pharmacotherapy Monitoring and Outcome Survey (PHAMOUS) between 2006 and 2014. Patients’ metabolic parameters were measured, and the use of pharmacologic treatment for hypertension (systolic blood pressure ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg), dyslipidemia (5% ≤ Systematic COronary Risk Evaluation [SCORE] risk < 10% and low-density lipoprotein [LDL] cholesterol level ≥ 2.5 mmol/L or SCORE risk ≥ 10% and LDL cholesterol level ≥ 1.8 mmol/L and/or triglycerides ≥ 2.3 mmol/L), and hyperglycemia (hemoglobin A1c concentration > 7% and/or fasting glucose concentration ≥ 7.2 mmol/L) was recorded.
Results: Prevalence of the metabolic syndrome, as defined by the National Cholesterol Education Program criteria, was > 50% at each assessment. On the basis of the European Society of Cardiology guidelines, pharmacotherapy for metabolic disorders was recommended for 52%-59% of the patients at each assessment. Treatment rates with antihypertensive (from 31% to 38%, P < .001) pharmacotherapy increased throughout the assessments. However, half of the patients were not treated for their metabolic risk factors while being monitored for 3 years or longer. Older patients were more likely to receive treatment, and patients who received treatment had lower blood pressure and lower cholesterol and triglyceride concentrations than patients not receiving the recommended treatment.
Conclusions: Metabolic risk factors are still seriously undertreated in people with psychotic disorders. Better adherence to and better implementation of guidelines about monitoring and treating metabolic disorders in psychiatry are crucial.
J Clin Psychiatry 2017;78(8):1117-1125
https://doi.org/10.4088/JCP.16m10831
© Copyright 2017 Physicians Postgraduate Press, Inc.
aRob Giel Research Center, University of Groningen, University Medical Center Groningen, University Center Psychiatry, Groningen, The Netherlands
bLentis Mental Health Organization, Groningen, The Netherlands
cDepartment of Psychology, University of Groningen, Groningen, The Netherlands
dDepartment of Psychotic Disorders, GGZ Drenthe, Assen, Drenthe, The Netherlands
eDepartment of Epidemiology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands
fDepartment of Mathematics and Computer Science, Eindhoven University of Technology, Eindhoven, The Netherlands
gResearch Department, Friesland Mental Health Services, Leeuwarden, Friesland, The Netherlands
*Corresponding author: Jojanneke Bruins, PhD, University of Groningen, University Medical Center Groningen, University Center of Psychiatry, Rob Giel Research Center, Hanzeplein 1, CC72, 9713 GZ Groningen, The Netherlands ([email protected]).
Compared to the general population, people with psychotic disorders have an increased metabolic risk and a shortened mean life expectancy.1,2 They are also more likely to suffer from metabolic syndrome (MetS),3 a constellation of interrelated risk factors for cardiovascular diseases: increased waist circumference, hypertension, decreased high-density lipoprotein (HDL) cholesterol, hypertriglyceridemia, and hyperglycemia.4
A recent meta-analysis5 suggests that lifestyle interventions effectively reduce metabolic disorders in people with severe mental illness. However, when hypertension and dyslipidemia are too severe or when changes in lifestyle and type of antipsychotics are not sufficient, pharmacotherapy with antihypertensive and lipid-lowering drugs is recommended.6 Treatment with antihyperglycemic drugs may also be recommended when patients are diagnosed with diabetes mellitus.4
During the last 10 years, 2 large studies7,8 investigated treatment rates for metabolic disorders in US patients with psychotic disorders. They showed that only 38%-54% of the patients with hypertension received adequate antihypertensive treatment, 11%-41% of the patients with dyslipidemia received treatment with lipid-lowering drugs, and 55%-60% of the diabetes mellitus patients received antihyperglycemic drug treatment.7,8 In the meantime, alarming reports9-15 on the physical health of this population have accumulated.
Aim
In this study, we first investigated the prevalence, incidence, and reversal rates of MetS in a longitudinal cohort of people with psychotic disorders. Second, we examined for how many patients treatment with antihypertensive, lipid-lowering, and antihyperglycemic drugs was recommended according to guidelines and the rates of patients receiving treatment according to these guidelines. Third, we explored which demographic and disease variables predict receipt of treatment for metabolic disorders. Last, we investigated whether patients receiving recommended treatment have less-severe metabolic risk factors than patients not receiving treatment.
METHODS
Sample and Procedure
Data for this study were extracted from a large ongoing Dutch observational cohort study, the Pharmacotherapy Monitoring and Outcome Survey (PHAMOUS).16 Mental and physical health of patients with psychotic disorders from 4 mental health institutions in the northern Netherlands are assessed yearly using Routine Outcome Monitoring (ROM), which is part of the regular clinical practice of the participating organizations. ROM procedures are fully explained to participants, after which they are free to opt out of having their anonymized data used in the research database. The procedures are in accordance with local and international rules, as confirmed by the local ethics committee of the University Medical Center of Groningen. Assessments were carried out between 2006 and 2014. By 2014, 8,372 patients were included in the PHAMOUS cohort, and the following screening rates were recorded throughout the years: 1.2% (2006), 4.7% (2007), 11.9% (2008), 19.2% (2009), 34.8% (2010), 42.8% (2011), 42.2% (2012), 53.1% (2013), and 65.7% (2014).
Participants
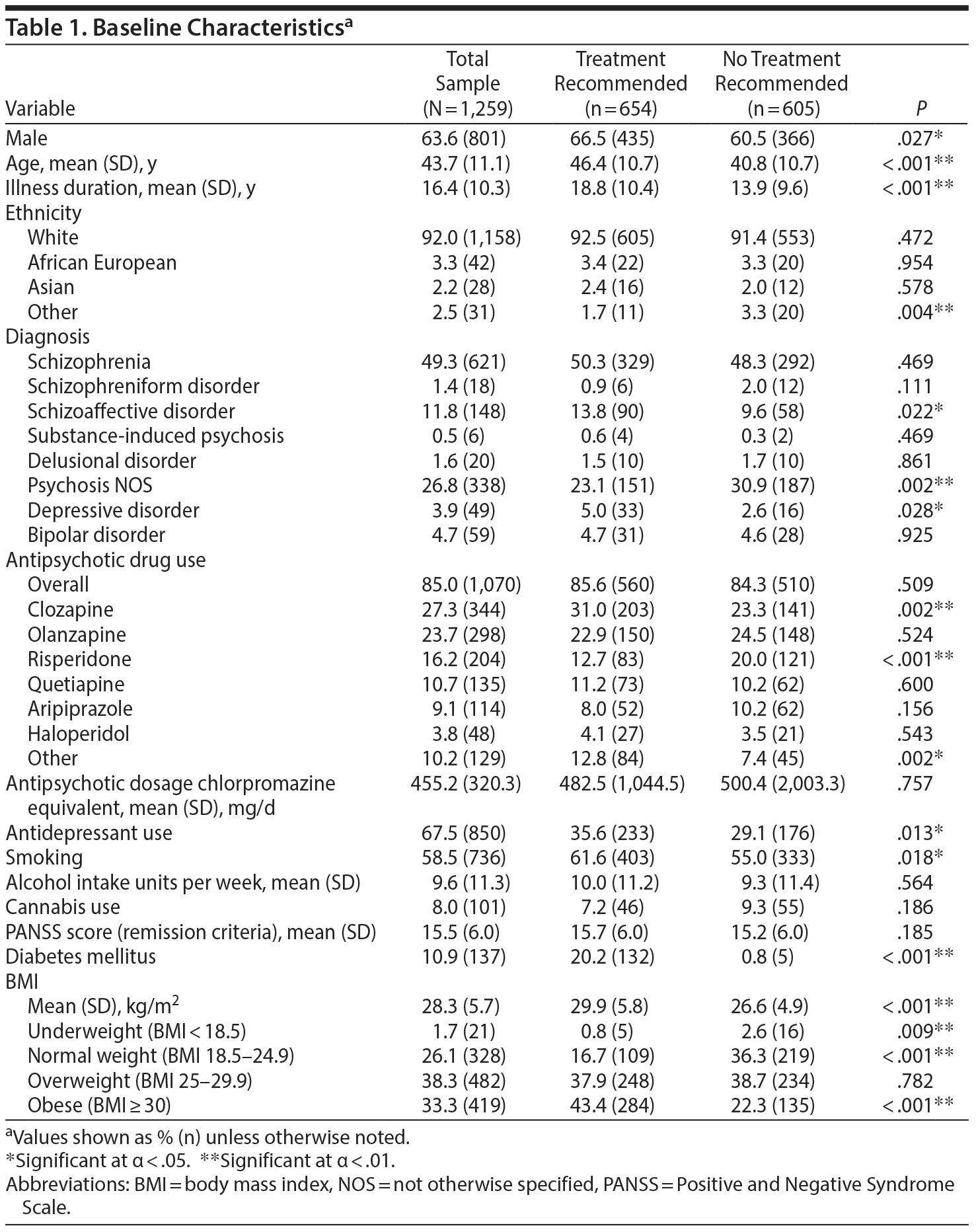
Inclusion criteria were (1) diagnosis of a psychotic disorder or mood disorder with psychotic features as defined by the DSM-IV and (2) participation in at least 3 assessments with data on all 5 MetS criteria. Consecutive assessments had intervals between 9 and 24 months. An overview of the sample demographics is provided in Table 1.
Measurements
Assessments were performed by a trained nurse and included (a) body mass index (BMI, kg/m2); (b) waist circumference (centimeters) measured with a flexible measuring tape between the lower rib and the upper edge of the hip bone; (c) systolic and diastolic blood pressure (mm Hg), measured twice with an interval of 15 seconds using a manometer (reported systolic blood pressure and diastolic blood pressure scores are the mean values of the 2 measurements); (d) a blood sample to establish total cholesterol (mmol/L), HDL cholesterol (mmol/L), low-density lipoprotein (LDL) cholesterol (mmol/L), triglycerides (mmol/L), glucose (mmol/L), and hemoglobin A1c (HbA1c, % of total hemoglobin) (participants were asked to refrain from caloric intake for 8 hours before the blood sample was collected, but fasting status was not always reported; therefore we evaluated both total glucose [the entire sample] and fasting glucose concentrations [patients with confirmed fasting status]); (e) severity of psychotic symptoms, assessed with the Positive and Negative Syndrome Scale (PANSS)17 remission criteria18; (f) self-reported smoking (yes/no), alcohol use (units per week), and cannabis use (yes/no); and (g) prescribed pharmacotherapy for metabolic disorders (ie, antihypertensive, lipid-lowering, and antihyperglycemic drugs) and antipsychotic drugs used (ie, clozapine, olanzapine, risperidone, quetiapine, aripiprazole, haloperidol, or other) as reported by the patient or as recorded in the patient’s files.

- High premature cardiovascular mortality rates and the generally increased metabolic risk in people with psychotic disorders emphasize the importance of treating metabolic risk factors in these patients.
- Despite the increased metabolic risk, low rates of treatment for metabolic risk factors are seen even among patients most compliant with follow-up screenings when adequate health monitoring is installed.
Defining Metabolic Syndrome
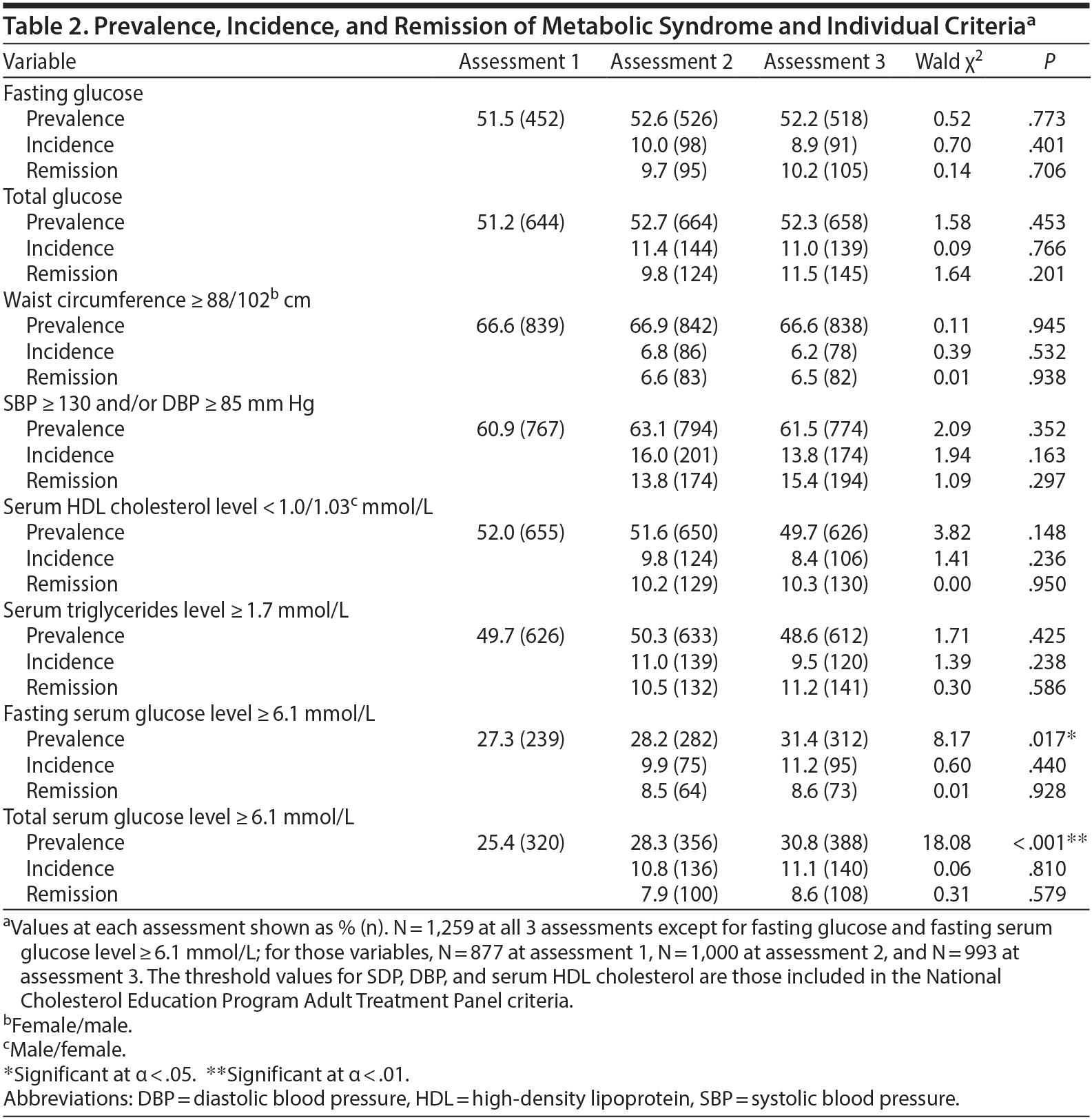
MetS was diagnosed when 3 or more of the following National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP-III) criteria4 were fulfilled: waist circumference ≥ 88 or ≥ 102 cm (female or male, respectively); systolic blood pressure ≥ 130 mm Hg and/or diastolic blood pressure ≥ 85 mm Hg or receiving antihypertensive drug treatment; serum HDL cholesterol concentration < 1.3 or < 1.03 mmol/L (female or male, respectively) or receiving lipid-lowering drugs; serum triglycerides concentration ≥ 1.7 mmol/L or receiving lipid-lowering drugs; and fasting serum glucose concentration ≥ 6.1 mmol/L or receiving antihyperglycemic drug treatment, in accordance with World Health Organization guidelines.19,20
Treatment Guidelines
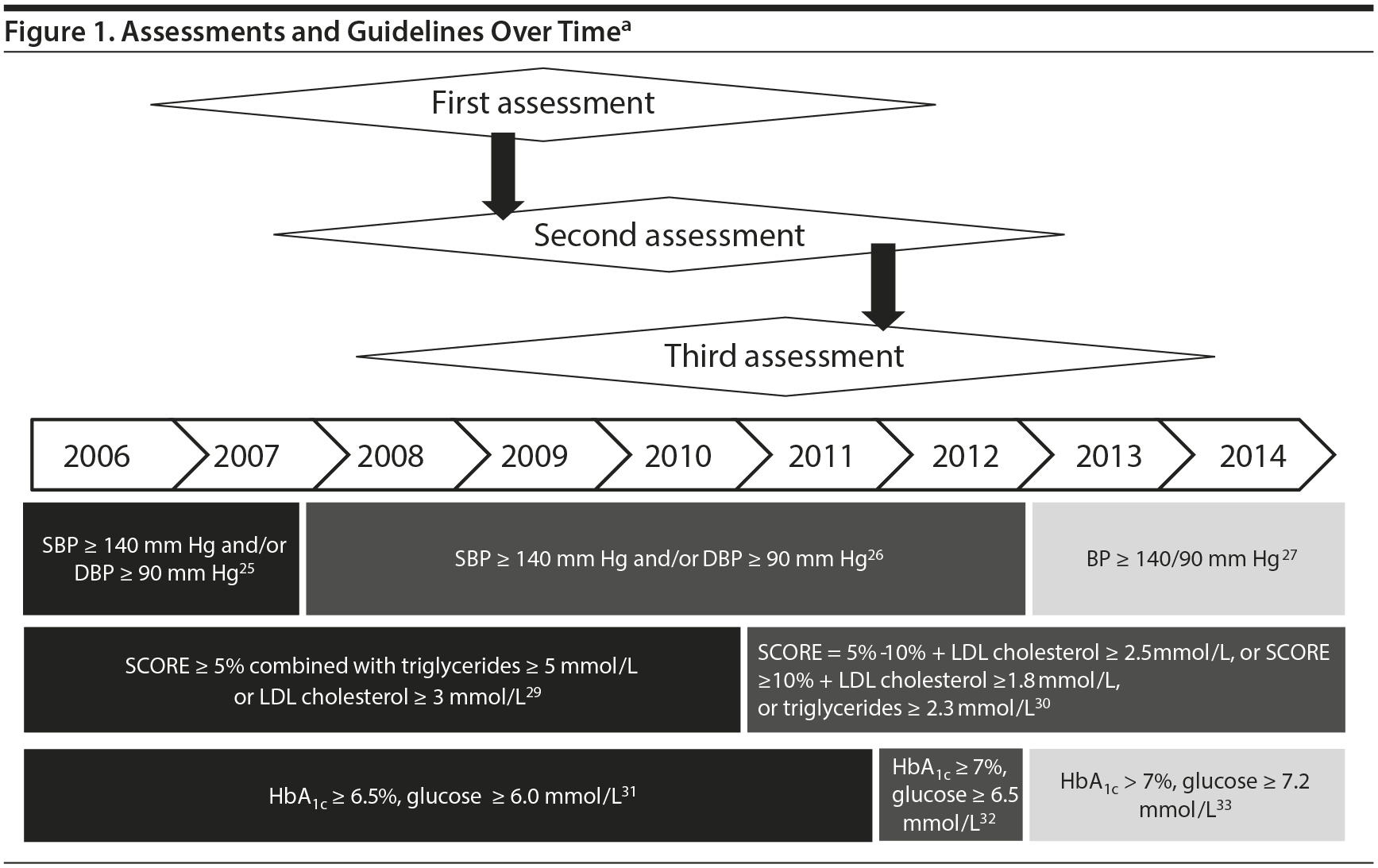
International guidelines of the European Society of Cardiology (ESC), the International Diabetes Federation (IDF), and the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus were used to determine whether drug treatment for metabolic risk factors was recommended. The European guidelines concur with the American guidelines for hypertension, dyslipidemia, and diabetes.21-24 Treatment recommendation rates are based on guidelines valid at the time of assessment, as described in Figure 1. Patients with a prescription for antihypertensive, lipid-lowering, or antihyperglycemic pharmacotherapy were considered both to have needed and to have received treatment.
Hypertension
The European Society of Hypertension (ESH) and ESC guidelines25-27 recommend antihypertensive drug treatment for systolic blood pressure ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg.
Dyslipidemia
The ESC and European Atherosclerosis Society (EAS) treatment guidelines, based on risk estimation by the SCORE model,28 recommend lipid-lowering drugs for patients with SCORE ≥ 5 combined with triglyceride level ≥ 5 mmol/L or LDL cholesterol level ≥ 3 mmol/L (until 2010)29 and for patients with SCORE ≥ 5 and < 10 and LDL cholesterol level ≥ 2.5 mmol/L, SCORE ≥ 10 and LDL cholesterol level ≥ 1.8 mmol/L, or triglyceride level ≥ 2.3 mmol/L (2011 and onward).30
Diabetes Mellitus
According to guidelines, a diagnosis of diabetes mellitus is required for treatment with antihyperglycemic drugs.31-33 Patients reported whether they had been diagnosed with diabetes mellitus by their treating physician during the interview as part of the assessments. In addition, patients were considered to have diabetes mellitus at the third study assessment when they had fasting glucose concentrations ≥ 7.0 mmol/L at the first 2 study assessments,34 regardless of whether or not a diagnosis of diabetes mellitus was self-reported in the third interview.
The IDF, ESC, and European Association for the Study of Diabetes (EASD) guidelines recommend antihyperglycemic drugs for patients diagnosed with diabetes mellitus when HbA1c is ≥ 6.5% and/or fasting glucose concentrations ≥ 6.0 mmol/L or postprandial glucose concentrations ≥ 8.0 mmol/L (2005 to 2011),31 HbA1c is ≥ 7% and/or fasting glucose concentrations ≥ 6.5 mmol/L or postprandial glucose concentrations ≥ 9.0 mmol/L (2012),32 or HbA1c is > 7% and/or fasting glucose concentrations ≥ 7.2 mmol/L or postprandial glucose concentrations ≥ 10.0 mmol/L (2013 and onwards)33 are measured.
Data Analysis
To establish the prevalence of MetS in each assessment, the number of patients with MetS was divided by the total number of patients. Incidence in each assessment was calculated by dividing the new MetS cases by the number of patients who did not meet the MetS criteria in the previous assessment. New cases were patients who met the MetS criteria in 1 assessment, but not in previous assessments. Remission was not meeting the MetS criteria in 1 assessment but meeting the criteria in the previous assessment. Remission rates were calculated by dividing the number of remitted cases by the number of MetS patients in the previous assessment.
Second, with generalized estimating equations (GEE), we examined whether the rates of patients needing drug treatment according to guidelines differed over the 3 assessments. GEE tests were also used to examine whether rates of patients receiving the recommended antihypertensive, lipid-lowering, and antihyperglycemic drugs differed over the 3 assessments to see whether treatment rates increased over time.
Third, to investigate what factors predict treatment, we used a multinomial logistic regression model with a backward elimination approach and Pearson correction. The following potential predictors were included: age and illness duration,14 sex,35 total score for the PANSS remission criteria,36 smoking,37 alcohol intake,38 cannabis use,39 and type and dosage (in chlorpromazine equivalents) of antipsychotic medication.40,41 For the purpose of this model, all patients for whom treatment was recommended during at least 1 assessment were divided into 3 categories as the dependent variable: (1) never received treatment while treatment was recommended; (2) received treatment at some, but not all assessments while treatment was recommended; and (3) receiving appropriate treatment at all assessments when treatment was indicated. Wald test statistics and odds ratios (ORs) with 95% confidence intervals (CIs) between categories were calculated to determine the significance of the predictors.
Last, the course of different metabolic risk factors over the 3 assessments was examined with mixed modeling. At each assessment, all patients included in the study were divided into 3 treatment categories, separately for antihypertensive medication (systolic blood pressure and diastolic blood pressure), lipid-lowering drugs (total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides), and antihyperglycemic medication (glucose and HbA1c): (1) patients for whom drug treatment was not recommended, (2) patients who did not receive pharmacotherapy when treatment was recommended, and (3) patients who received the recommended drug treatment. The longitudinal structure of the data imposed a correlation between the repeated observations that was addressed by assuming an unstructured covariance matrix: no constraints were imposed on the values and each (co)variance was estimated uniquely from the data. Covariates age, gender, illness duration, type of antipsychotic medication, smoking, alcohol intake, and cannabis use were included in the model as fixed factors. Differences between treatment categories were tested using the Wald test statistic. We included time (assessments 1, 2, and 3) and treatment groups as an interaction term to examine if differences were consistent throughout the 3 assessments.
SPSS 22.0 was used for all statistical analyses.42
RESULTS
In total, 1,259 patients fulfilled the inclusion criteria for this study (see Supplementary eFigure 1 at Psychiatrist.com). This subsample was similar to the overall cohort, in which the mean age was 43.3 years, the mean PANSS remission score was 16.0, the male prevalence was 65.2%, and the prevalence of MetS was 55.5%. The total mean (SD) follow-up time was 27.7 (5.2) months (range, 19-46 months), with a mean (SD) interval of 13.7 (3.6) months between the first 2 assessments and 13.5 (3.4) months between the second and third assessment.
Diagnosis of Diabetes Mellitus
At the first assessment, 10.9% of the patients had a diabetes mellitus diagnosis; the percentage significantly increased to 12.7% at the second and 15.3% at the third assessment (Wald χ2 = 58.17, P < .001). Of the diabetes mellitus patients at the third assessment, 14.7% reported being diagnosed with diabetes mellitus. An additional 8 patients (0.6%) did not report having diabetes mellitus, but had fasting glucose levels ≥ 7.0 mmol/L at the first 2 assessments and were therefore considered to have diabetes mellitus at the third assessment. Fasting blood samples were collected in 69.7% (first assessment), 79.4% (second assessment), and 78.9% (third assessment) of the patients.
MetS and Treatment Rates
Over half of the patients were diagnosed with MetS; prevalence, incidence, and reversal rates did not differ throughout the assessments. An overview of the prevalence rates of MetS and its individual components is presented in Table 2.
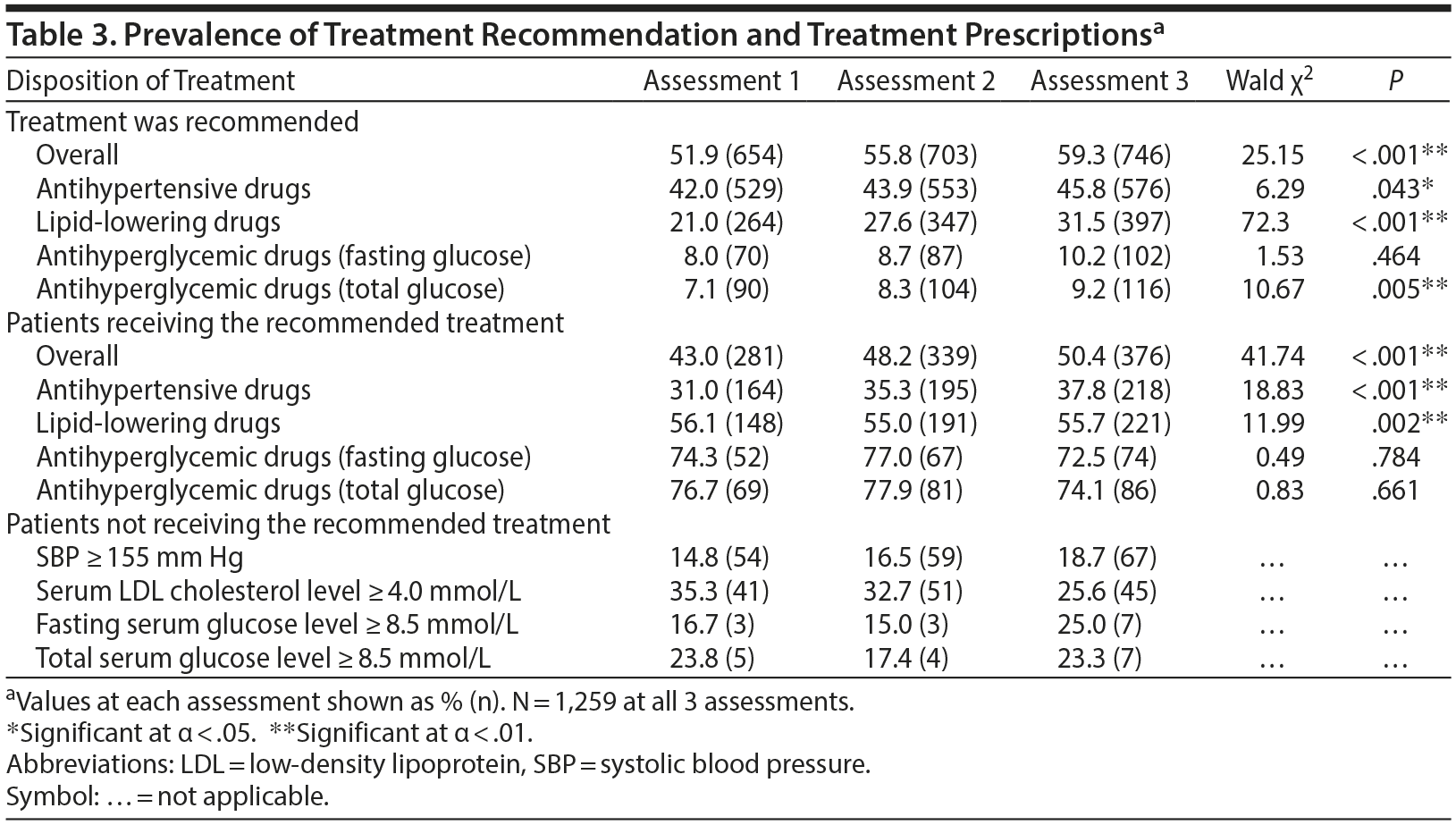
The rates of patients for whom antihypertensive and lipid-lowering treatment was recommended according to the guidelines significantly increased over the 3 assessments. The number of patients for whom treatment with antihyperglycemic drugs was recommended did not change significantly based on fasting glucose, but slightly increased when total glucose was examined. An increasing number of patients received treatment with antihypertensive and lipid-lowering drugs during the consecutive assessments, but treatment rates with antihyperglycemic drugs did not change. Of the patients not receiving the recommended treatment, up to one-third had severe risk factors, ie, systolic blood pressure ≥ 155 mm Hg, LDL cholesterol level ≥ 4.0 mmol/L, or fasting glucose level ≥ 8.5 mmol/L. An overview of treatment rates is presented in Table 3.
Receiving Treatment for Other Metabolic Risk Factors
Of the patients remaining untreated for hypertension, 11.8% received drug treatment for at least 1 other metabolic risk factor at the first assessment; the percentage significantly increased to 15.4% at the second and 16.8% at the third assessment (Wald χ2 = 14.48, P = .001). Of the patients who remained untreated for dyslipidemia, 20.7% received drug treatment for at least 1 other metabolic risk factor at the first assessment, and the percentage significantly increased to 20.5% at the second and 26.1% at the third assessment (Wald χ2 = 7.04, P = .030). Of the patients who remained untreated for diabetes mellitus, 38.9% (fasting glucose) or 38.1% (total glucose) received pharmacotherapy for at least 1 other metabolic risk factor at the first assessment. This percentage did not significantly increase during the follow-up assessments (Wald χ2 = 1.84, P = .398 and Wald χ2 = 4.11, P = .128 for fasting glucose and total glucose, respectively).
Predicting Treatment
Only age significantly predicted receipt of treatment. When age increased, patients were more likely to be treated as recommended by guidelines than to be treated at some but not all assessments (Wald χ21 = 12.96, P < .001, OR = 1.081, 95% CI, 1.04-1.13).
Treatment and Severity of Metabolic Risk Factors
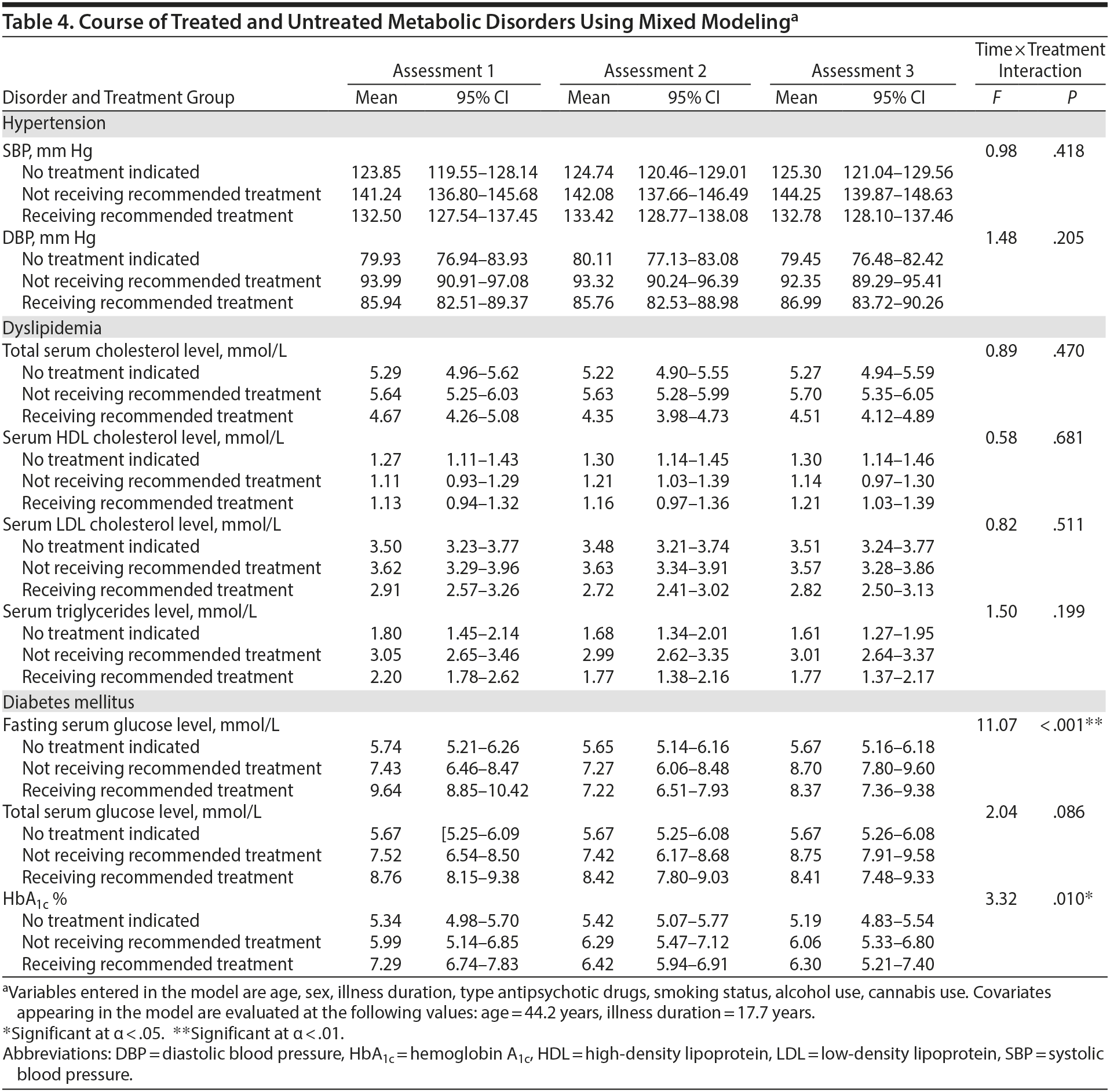
The mean values of metabolic risk factors, separately for patients receiving the recommended pharmacotherapy, patients not receiving the recommended pharmacotherapy, and patients without a treatment indication for metabolic risk factors to demonstrate the severity of metabolic risk in each group, are presented in Table 4. At all 3 assessments, systolic and diastolic blood pressure and total cholesterol, LDL, cholesterol and triglyceride concentrations were lower in patients receiving treatment compared to patients not receiving the recommended pharmacotherapy. HDL cholesterol concentrations did not differ between patients who did and patients who did not receive the recommended pharmacotherapy. Fasting glucose concentrations were lower in patients receiving treatment compared to patients not receiving the recommended treatment at the second and third assessments, but not at the first assessment (F = 11.07, P < .001). HbA1c level was higher in treated patients compared to patients not receiving the recommended treatment, but the differences became smaller throughout the assessments (F = 3.32, P = .010).
DISCUSSION
In this study, over half of the patients with psychotic disorders suffered from MetS, and in almost two-thirds, pharmacologic treatment for metabolic risk factors was recommended according to internationally accepted guidelines. Prevalence of MetS in our study (> 50%) was higher than in the general Dutch population aged 40-49 years (22%)43 and higher than reported in a large meta-analysis of schizophrenia patients (33%),14 a finding that is probably related to the older age and longer illness duration of the patients in our sample.14,44 There was a higher prevalence of patients in our sample for whom treatment for hypertension was recommended (42%-46%) compared to the general European population (30%), but twice as many people in the general population received the recommended antihypertensive drug treatment.45 The prevalence of diabetes mellitus in our study was considerably higher (11%-15%) than in the general European population (6%).46 Rates of patients receiving antihyperglycemic drugs were lower than treatment rates in the general US adult population with diabetes mellitus (86%).47 The rates of receiving lipid-lowering drugs (54%) found in the general US population with dyslipidemia were comparable with our results.48
Pharmacotherapy for metabolic disorders was recommended for up to 60% of the patients according to the guidelines. Over half of these patients did not receive any treatment at all. Pharmacologic treatment for metabolic disorders seemed effective in lowering systolic and diastolic blood pressure and total cholesterol, LDL cholesterol, and triglycerides concentrations. For glucose concentrations, this effect was reversed, although the differences for HbA1c became smaller throughout the assessments. Managing glucose control has been proven difficult in both the general and the psychiatric population, which may explain these inconsistent findings.49
Our findings tap a serious health problem that in reality may be even worse. In spite of American Diabetes Association/American Psychiatric Association guidelines that recommend frequent screening of metabolic risk factors in people taking antipsychotic drugs,50 a recent review demonstrated that metabolic risk factors still remain unscreened in 70% of these patients.51 However, the current study shows that screening rates have increased throughout the last decade. Furthermore, patients’ actual metabolic risk may be underestimated because the current guidelines and risk models are based on the general population. Guidelines for metabolic disturbances might need updating to include metabolic risk models specifically designed for people with psychotic disorders, such as the recently proposed PRIMROSE models.52 These models include psychiatric diagnosis and the use of antidepressant and antipsychotic drugs as additional predictors on top of the standard risk factors used in most other prediction models (ie, age, sex, systolic blood pressure, cholesterol, smoking).
The data do not reveal why patients are not treated with recommended pharmacotherapy. Maybe patients are referred to general practitioners but do not actually visit them, or physicians might be reluctant to prescribe pharmacotherapy due to the relatively young age of patients and might prefer to suggest lifestyle interventions. Also, when multiple metabolic dysregulations are present, often only one antimetabolic drug is prescribed. Possibly, treating physicians anticipate that treating one risk factor might improve overall metabolic health. Finally, patients themselves can refuse treatment. To what extent these factors contribute to the low treatment rates cannot be extrapolated from the present data.
Limitations
The observational nature of our data does not allow the examination of medication effects, which may be overestimated or underestimated due to confounding by indication. This subsample may be biased in containing only patients with 3 consecutive completed assessments, with having participated in less than 3 assessments being the main reason for exclusion (60% of the original sample). However, patients in this subsample did not differ from the total PHAMOUS cohort on demographical variables, MetS indicators, and psychopathology. No information is available on the number of patients opting out of participation in one or more PHAMOUS screenings.
It is not known whether all patients had refrained from caloric intake for 8 hours before glucose was measured. Although in our sample fasting glucose and total glucose were not considerably different, we cannot rule out that fasting glucose levels have been overestimated. Also, no information was available on patients’ compliance with the prescribed pharmacotherapy, which may have led to an underestimation of treatment effects.
Information was available only on pharmacologic treatment and not on whether patients participated in lifestyle interventions. Participating in lifestyle interventions could explain why some patients were not receiving treatment, but not all considering that 15%-35% of the untreated patients had severe metabolic risk factors.
Although it is expected that treatment for metabolic risk factors will ultimately reduce cardiovascular mortality in people with psychotic disorders, data to support this hypothesis were not available.
Clinical Implications
Despite increasing knowledge about metabolic risk in psychotic disorders; well-established effectiveness of antihypertensive, lipid-lowering, and antihyperglycemic drugs; as well as patients’ readiness to accept physical health monitoring,53 treatment rates for metabolic disorders in this cohort were similar to the results of 2 large US trials published during the previous decade.7,8 Low rates of treatment for metabolic risk factors are seen even among patients most compliant with follow-up screenings when adequate health monitoring is installed. Our findings strongly support the "Don’ t just screen, intervene!" claim that is receiving growing international attention.54 Routine outcome monitoring of metabolic health is not a goal in and of itself, and if monitoring does not result in better care, we should urgently examine current monitoring, referral, and treatment practices.
Although psychiatrists seem to be well aware of increased metabolic risk, and most international guidelines acknowledge the importance of monitoring,55,56 psychiatrists and general practitioners may hold different views as to who is responsible for monitoring and treating metabolic disorders.57,58 Thus, clear communication between psychiatric services and medical care physicians is highly needed, a fact that might become self-evident when services are more integrated.59 Ideally, general practitioners, psychiatrists, and patients will together acknowledge the increased risk and set up an adequate (pharmacotherapeutic) treatment plan based on the monitoring results.54,57,60 To our view, doing so would greatly improve the metabolic care of psychiatric patients.
Submitted: March 24, 2016; accepted August 12, 2016.
Online first: April 11, 2017.
Potential conflicts of interest: The authors declare no conflict of interest. The authors report no financial or other relationship relevant to the subject of this article.
Funding/support: This work was supported by the Rob Giel Research Center at the University Center for Psychiatry of the University Medical Center Groningen, Friesland Mental Health Services, GGZ Drenthe and Lentis Mental Health Organization. The study was funded by the mental health institutions participating in PHAMOUS screenings: University Medical Center Groningen, Lentis Mental Health Organization, GGZ Drenthe, Friesland Mental Health Services. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Role of the sponsor: The funding sources had no role in the data collection, management, analyses, and publication of this study.
Acknowledgments: The authors are grateful to all the staff members of the different institutions for collecting the data.
Supplementary material: Available at Psychiatrist.com.
REFERENCES
1. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders, I: prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52-77. PubMed doi:10.1002/j.2051-5545.2011.tb00014.x
2. Hennekens CH, Hennekens AR, Hollar D, et al. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115-1121. PubMed doi:10.1016/j.ahj.2005.02.007
3. Suvisaari JM, Saarni SI, Perפlפ J, et al. Metabolic syndrome among persons with schizophrenia and other psychotic disorders in a general population survey. J Clin Psychiatry. 2007;68(7):1045-1055. PubMed doi:10.4088/JCP.v68n0711
4. Grundy SM, Cleeman JI, Daniels SR, et al; American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112(17):2735-2752. PubMed doi:10.1161/CIRCULATIONAHA.105.169404
5. Bruins J, Jörg F, Bruggeman R, et al. The effects of lifestyle interventions on (long-term) weight management, cardiometabolic risk and depressive symptoms in people with psychotic disorders: a meta-analysis. PLoS One. 2014;9(12):e112276. PubMed doi:10.1371/journal.pone.0112276
6. Maayan L, Correll CU. Management of antipsychotic-related weight gain. Expert Rev Neurother. 2010;10(7):1175-1200. PubMed doi:10.1586/ern.10.85
7. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22. PubMed doi:10.1016/j.schres.2006.06.026
8. Correll CU, Druss BG, Lombardo I, et al. Findings of a US national cardiometabolic screening program among 10,084 psychiatric outpatients. Psychiatr Serv. 2010;61(9):892-898. PubMed doi:10.1176/ps.2010.61.9.892
9. Correll CU. Balancing efficacy and safety in treatment with antipsychotics. CNS Spectr. 2007;12(10 suppl 17):12-20, 35. PubMed doi:10.1017/S1092852900026298
10. Correll CU, Robinson DG, Schooler NR, et al. Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: baseline results from the RAISE-ETP study. JAMA Psychiatry. 2014;71(12):1350-1363. PubMed doi:10.1001/jamapsychiatry.2014.1314
11. Fleischhacker WW, Cetkovich-Bakmas M, De Hert M, et al. Comorbid somatic illnesses in patients with severe mental disorders: clinical, policy, and research challenges. J Clin Psychiatry. 2008;69(4):514-519. PubMed doi:10.4088/JCP.v69n0401
12. Falissard B, Mauri M, Shaw K, et al. The METEOR study: frequency of metabolic disorders in patients with schizophrenia: focus on first and second generation and level of risk of antipsychotic drugs. Int Clin Psychopharmacol. 2011;26(6):291-302. PubMed doi:10.1097/YIC.0b013e32834a5bf6
13. Meyer JM, Stahl SM. The metabolic syndrome and schizophrenia. Acta Psychiatr Scand. 2009;119(1):4-14. PubMed doi:10.1111/j.1600-0447.2008.01317.x
14. Mitchell AJ, Vancampfort D, Sweers K, et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders—a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306-318. PubMed doi:10.1093/schbul/sbr148
15. Schorr SG, Slooff CJ, Bruggeman R, et al. Prevalence of metabolic syndrome in patients with psychotic disorders in the Netherlands. J Clin Psychopharmacol. 2009;29(4):399-402. PubMed doi:10.1097/JCP.0b013e3181acceeb
16. Risselada AJ, Vehof J, Bruggeman R, et al. Association between HTR2C gene polymorphisms and the metabolic syndrome in patients using antipsychotics: a replication study. Pharmacogenomics J. 2012;12(1):62-67. PubMed doi:10.1038/tpj.2010.66
17. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276. PubMed doi:10.1093/schbul/13.2.261
18. Andreasen NC, Carpenter WT Jr, Kane JM, et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162(3):441-449. PubMed doi:10.1176/appi.ajp.162.3.441
19. Forouhi NG, Balkau B, Borch-Johnsen K, et al; EDEG. The threshold for diagnosing impaired fasting glucose: a position statement by the European Diabetes Epidemiology Group. Diabetologia. 2006;49(5):822-827. PubMed doi:10.1007/s00125-006-0189-4
20. WHO. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia. Report of a WHO/IDF Consultation. Geneva, Switzerland: WHO Document Production Services; 2006.
21. Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich). 2014;16(1):14-26. PubMed doi:10.1111/jch.12237
22. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1-S45. PubMed doi:10.1161/01.cir.0000437738.63853.7a
23. Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364-1379. PubMed doi:10.2337/dc12-0413
24. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S49-S73. PubMed doi:10.1161/01.cir.0000437741.48606.98
25. Mancia G, Agabiti Rosei E, Cifkova R, et al; European Society of Hypertension-European Society of Cardiology Guidelines Committee. 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21(6):1011-1053. PubMed doi:10.1097/00004872-200306000-00001
26. Mancia G, De Backer G, Dominiczak A, et al; ESH-ESC Task Force on the Management of Arterial Hypertension. 2007 ESH-ESC practice guidelines for the management of arterial hypertension: ESH-ESC Task Force on the Management of Arterial Hypertension. J Hypertens. 2007;25(9):1751-1762. PubMed doi:10.1097/HJH.0b013e3282f0580f
27. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159-2219. PubMed doi:10.1093/eurheartj/eht151
28. Conroy RM, Pyörפlפ K, Fitzgerald AP, et al; SCORE project group. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24(11):987-1003. PubMed doi:10.1016/S0195-668X(03)00114-3
29. De Backer G, Ambrosioni E, Borch-Johnsen K, et al; Third Joint Task Force of European and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. European guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2003;24(17):1601-1610. PubMed doi:10.1016/S0195-668X(03)00347-6
30. Reiner Z, Catapano AL, De Backer G, et al; European Association for Cardiovascular Prevention & Rehabilitation; ESC Committee for Practice Guidelines (CPG) 2008-2010 and 2010-2012 Committees. ESC/EAS guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European Society of cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32(14):1769-1818. PubMed doi:10.1093/eurheartj/ehr158
31. Global guideline for type 2 diabetes. International Diabetes Federation website. https://www.idf.org/webdata/docs/IDF%20GGT2D.pdf. 2005.
32. IDF Clinical Guidelines Task Force. Global Guideline for Type 2 Diabetes. Brussels, Belgium: International Diabetes Federation; 2012.
33. Rydén L, Grant PJ, Anker SD, et al; Authors/Task Force Members; ESC Committee for Practice Guidelines (CPG); Document Reviewers. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34(39):3035-3087. PubMed doi:10.1093/eurheartj/eht108
34. Genuth S, Alberti KG, Bennett P, et al; Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160-3167. PubMed doi:10.2337/diacare.26.11.3160
35. Galletly CA, Foley DL, Waterreus A, et al. Cardiometabolic risk factors in people with psychotic disorders: the second Australian national survey of psychosis. Aust N Z J Psychiatry. 2012;46(8):753-761. PubMed doi:10.1177/0004867412453089
36. Bobes J, Arango C, Aranda P, et al; CLAMORS Study Collaborative Group. Cardiovascular and metabolic risk in outpatients with schizoaffective disorder treated with antipsychotics: results from the CLAMORS study. Eur Psychiatry. 2012;27(4):267-274. PubMed doi:10.1016/j.eurpsy.2010.09.001
37. Bobes J, Arango C, Garcia-Garcia M, et al. Healthy lifestyle habits and 10-year cardiovascular risk in schizophrenia spectrum disorders: an analysis of the impact of smoking tobacco in the CLAMORS schizophrenia cohort. Schizophr Res. 2010;119(1-3):101-109. PubMed doi:10.1016/j.schres.2010.02.1030
38. Sun K, Ren M, Liu D, et al. Alcohol consumption and risk of metabolic syndrome: a meta-analysis of prospective studies. Clin Nutr. 2014;33(4):596-602. PubMed doi:10.1016/j.clnu.2013.10.003
39. Isaac M, Isaac M, Holloway F. Is cannabis an anti-antipsychotic? the experience in psychiatric intensive care. Hum Psychopharmacol. 2005;20(3):207-210. PubMed doi:10.1002/hup.674
40. Bushe CJ, Slooff CJ, Haddad PM, et al. Weight change by baseline BMI from three-year observational data: findings from the Worldwide Schizophrenia Outpatient Health Outcomes Database. J Psychopharmacol. 2013;27(4):358-365. PubMed doi:10.1177/0269881112473789
41. Chadda RK, Ramshankar P, Deb KS, et al. Metabolic syndrome in schizophrenia: differences between antipsychotic-naׯve and treated patients. J Pharmacol Pharmacother. 2013;4(3):176-186. PubMed doi:10.4103/0976-500X.114596
42. SPSS statistics for Windows, version 22.0 [computer program]. Armonk, NY: IBM Corp; 2013.
43. Prevalence of the metabolic syndrome divided by age and gender. National Institute for Public Health and the Environment website. www.rivm.nl. Updated 2012.
44. Termorshuizen F, Wierdsma AI, Smeets HM, et al. Cause-specific mortality among patients with psychosis: disentangling the effects of age and illness duration. Psychosomatics. 2013;54(6):536-545. PubMed doi:10.1016/j.psym.2013.05.011
45. Joffres M, Falaschetti E, Gillespie C, et al. Hypertension prevalence, awareness, treatment and control in national surveys from England, the USA and Canada, and correlation with stroke and ischaemic heart disease mortality: a cross-sectional study. BMJ Open. 2013;3(8):e003423. PubMed doi:10.1136/bmjopen-2013-003423
46. Whiting DR, Guariguata L, Weil C, et al. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94(3):311-321. PubMed doi:10.1016/j.diabres.2011.10.029
47. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and its Burden in the United States, 2014. Atlanta, Georgia: US Department of Health and Human Services; 2014.
48. Goff DC Jr, Bertoni AG, Kramer H, et al. Dyslipidemia prevalence, treatment, and control in the Multi-Ethnic Study of Atherosclerosis (MESA): gender, ethnicity, and coronary artery calcium. Circulation. 2006;113(5):647-656. PubMed doi:10.1161/CIRCULATIONAHA.105.552737
49. Rathmann W, Pscherer S, Konrad M, et al. Diabetes treatment in people with type 2 diabetes and schizophrenia: retrospective primary care database analyses. Prim Care Diabetes. 2016;10(1):36-40. PubMed doi:10.1016/j.pcd.2015.04.001
50. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601. PubMed doi:10.2337/diacare.27.2.596
51. Mangurian C, Newcomer JW, Modlin C, et al. Diabetes and cardiovascular care among people with severe mental illness: a literature review. J Gen Intern Med. 2016;31(9):1083-1091. PubMed doi:10.1007/s11606-016-3712-4
52. Osborn DPJ, Hardoon S, Omar RZ, et al. Cardiovascular risk prediction models for people with severe mental illness: results from the prediction and management of cardiovascular risk in people with severe mental illnesses (PRIMROSE) research program. JAMA Psychiatry. 2015;72(2):143-151. PubMed doi:10.1001/jamapsychiatry.2014.2133
53. De Hert M, van Winkel R, Silic A, et al. Physical health management in psychiatric settings. Eur Psychiatry. 2010;25(suppl 2):S22-S28. PubMed doi:10.1016/S0924-9338(10)71702-8
54. The Lancet Psychiatry. Physical and mental health: activate and integrate. Lancet Psychiatry. 2014;1(3):163. PubMed doi:10.1016/S2215-0366(14)70322-0
55. Montejo AL. The need for routine physical health care in schizophrenia. Eur Psychiatry. 2010;25(suppl 2):S3-S5. PubMed doi:10.1016/S0924-9338(10)71699-0
56. Larsen JT, Fagerquist M, Holdrup M, et al. Metabolic syndrome and psychiatrists’ choice of follow-up interventions in patients treated with atypical antipsychotics in Denmark and Sweden. Nord J Psychiatry. 2011;65(1):40-46. PubMed doi:10.3109/08039488.2010.486443
57. Cohn TA, Sernyak MJ. Metabolic monitoring for patients treated with antipsychotic medications. Can J Psychiatry. 2006;51(8):492-501. PubMed doi:10.1177/070674370605100804
58. Mangurian C, Giwa F, Shumway M, et al. Primary care providers’ views on metabolic monitoring of outpatients taking antipsychotic medication. Psychiatr Serv. 2013;64(6):597-599. PubMed doi:10.1176/appi.ps.002542012
59. Druss BG, Rohrbaugh RM, Levinson CM, et al. Integrated medical care for patients with serious psychiatric illness: a randomized trial. Arch Gen Psychiatry. 2001;58(9):861-868. PubMed doi:10.1001/archpsyc.58.9.861
60. Millar H. Management of physical health in schizophrenia: a stepping stone to treatment success. Eur Neuropsychopharmacol. 2008;18(suppl 2):S121-S128. PubMed doi:10.1016/j.euroneuro.2008.02.002
Please sign in or purchase this PDF for $40.00.
Save
Cite