ABSTRACT
Apathy is a common and important yet often ignored neuropsychiatric symptom of Alzheimer’s disease (AD). Cholinesterase inhibitors and memantine, used to treat AD, appear ineffective against apathy. A meta-analysis of 4 randomized, placebo-controlled trials (RCTs) found that psychostimulants significantly attenuated apathy ratings in AD. However, the pooled sample size in this meta-analysis was just 156, and one of the trials was a 2-week crossover study with a large effect. A large RCT (n = 200) has now been published. This study found that methylphenidate (MPH; 20 mg/d) was superior to placebo in the attenuation of apathy scores in patients with possible or probable, mild to moderate AD; the advantage was evident by the end of the second month of treatment and remained evident to the end of 6 months. The effect size at 6 months was small (Cohen d = 0.37). In this RCT, disappointingly, MPH was not superior to placebo on secondary outcomes, including informant-rated apathy, dependence, activities of daily living, quality of life, and neurocognitive performance; caregiver burden was not formally studied. Speculatively, the psychosocial intervention provided to all participants in this RCT may have boosted response in the placebo group, thereby attenuating differences in outcomes between the MPH and placebo groups. A reasonable conclusion is that whereas MPH may attenuate the severity of apathy in patients with AD across as long as 6 months, the absence of improvements in measures of dependence, activities of daily living, and quality of life suggest that this effect of MPH on apathy may not be clinically significant. An unanswered question is whether the benefits of MPH may be clinically significant in real world practice settings in which the delivery of behavioral interventions is not feasible.
J Clin Psychiatry 2022;83(1):22f14398
To cite: Andrade C. Methylphenidate and other pharmacologic treatments for apathy in Alzheimer’s disease. J Clin Psychiatry. 2022;83(1):22f14398.
To share: https://doi.org/10.4088/JCP.22f14398
© Copyright 2022 Physicians Postgraduate Press, Inc.
Alzheimer’s disease (AD) is the commonest form of dementia. In addition to suffering from cognitive deterioration, patients with AD, especially later in the course of the illness, commonly exhibit neuropsychiatric symptoms. These are also referred to as behavioral and psychological symptoms of dementia, and they include anxiety, agitation, irritability, aggression, delusions, hallucinations, and other symptoms. Neuropsychiatric symptoms are best treated with nonpharmacologic interventions but, if severe, may necessitate the use of pharmacologic interventions such as antipsychotic drugs.3,4Dementia is a neurocognitive disorder characterized by a clinically significant, sustained decline in a wide range of cognitive functions; there is an associated decrease in independence resulting from impairments in instrumental activities of daily living. The estimated global prevalence of dementia was 43.8 million in 20161 and 57.4 million in 2019.2
Apathy in Dementia
Apathy is an example of a common neuropsychiatric symptom in patients with dementia. In a systematic review and meta-analysis of mood symptoms reported in 20 studies that included 5,897 persons with dementia, Leung et al5 found that the pooled prevalence of apathy was 43%–59%, depending on the severity of the dementia; these prevalence rates for apathy were higher than those for depression (37%–41%) and anxiety (also 37%–41%).
Apathy is characterized by indifference, loss of spontaneity, lack of motivation, decreased interest in and emotional reactivity to the surroundings, and reduced participation in domestic and social activities; diagnostic criteria for apathy have recently been suggested.6 Apathy receives less clinical attention because it is not as disturbing a symptom as other neuropsychiatric symptoms (such as aggression and agitation) can be. Apathy is nevertheless problematic. In a population-based, longitudinal cohort study of aging (n = 3,626), apathy was found to be one of the most stable among behavioral and psychological symptoms in subjects with cognitive impairment. Apathy increased disability, increased caregiver burden, and compromised the management of other diseases. Apathy was also strongly associated with mortality.7
Apathy in dementia is a difficult symptom to treat. In a systematic review and meta-analysis of 4 randomized, placebo-controlled trials (RCTs) of cholinesterase inhibitors (pooled N for drug vs placebo = 1,669 vs 967), 4 RCTs of memantine (pooled N = 902 vs 833), and 3 RCTs of psychostimulants (pooled N = 53 vs 55), Sepehry et al8 found that no category of drugs attenuated ratings of apathy severity in patients with AD.
Treatment of Apathy in Alzheimer’s Disease: Meta-Analysis
Many authors have speculated about the neurobiology of apathy in AD.8,9 One view is that apathy results from degeneration of the prefrontal cortex and that manifestations of apathy can be attenuated by increasing the levels of the catecholamines norepinephrine and dopamine in prefrontal-striatal-thalamocortical circuits.9 If this is true, then psychostimulant drugs such as modafinil and methylphenidate (MPH) may reduce apathy in AD. In this context, Kishi et al10 described a systematic review and meta-analysis of RCTs of psychostimulants in AD.
These authors searched online research publication databases, clinical trial registries, and reference lists and identified 3 parallel-group and 1 crossover RCT of psychostimulants for apathy in AD11–14; only 1 of these, examining modafinil (200 mg/d), was industry-sponsored. The remaining 3 RCTs, examining MPH (20 mg/d), were industry-independent. These RCTs were conducted in the US and Canada. The duration of the crossover trial was 2 weeks; the remaining trials were 6–12 (median, 8) weeks long. Sample sizes were n = 13 in the crossover trial and n = 23 to 60 in the parallel group trials.
In random effects meta-analysis, the meta-analysis authors10 found psychostimulants superior to placebo for the attenuation of apathy ratings (standardized mean difference [SMD], −0.63; 95% CI, −1.22 to −0.04; 4 RCTs; N = 166) as well as for improvement in Mini-Mental State Examination (MMSE) scores (SMD, −0.58; 95% CI, −1.14 to −0.02; 3 RCTs; N = 144). Both analyses were characterized by moderate to high heterogeneity.
In sensitivity analyses, when the crossover RCT was excluded, psychostimulants were no longer superior to placebo against apathy (SMD, −0.63; 95% CI, −1.41 to 0.17); however, when the modafinil RCT was excluded, the advantage for MPH over placebo was statistically significant (SMD, −0.82; 95% CI, −1.43 to −0.20). Heterogeneity was moderate to high in both of these analyses, as well. In meta-analysis of 1 modafinil and 1 MPH RCT, psychostimulants were not superior to placebo for instrumental activities of daily living and for caregiver burden outcomes. Finally, all-cause discontinuation, discontinuation due to adverse events, and report of at least 1 adverse event did not differ between psychostimulant and placebo groups.
Comments on the Meta-Analysis
Although the results of this meta-analysis10 suggest that psychostimulants are safe and effective against apathy in AD, they must be viewed with much caution for many reasons. There were only 4 RCTs. Of these 4, 1 examined modafinil, and so there were only 3 MPH RCTs. Of the 3 MPH RCTs, 1 was a crossover trial, leaving only 2 parallel-group RCTs. Study sample sizes were small. Study durations were short; as short as 2 weeks in 1 RCT. Heterogeneity for all efficacy outcomes was moderate to high. Adverse effects specific to psychostimulants were not examined. These are not data based on which confident guidance can be offered.
Methylphenidate for Apathy in Alzheimer’s Disease: Randomized Controlled Trial
A large, well-designed, well-conducted, and well-analyzed RCT is the gold standard in research, and one such study investigating MPH for apathy in AD has now been published by Mintzer et al.15 This study was named Apathy in Dementia Methylphenidate Trial 2 (ADMET 2). In this RCT, 200 patients with clinically significant apathy (of at least 4 weeks duration) and possible or probable AD were recruited from 9 US and 1 Canadian dementia clinic. Patients with a current or past major depressive episode and those with clinically significant other neuropsychiatric symptoms were excluded. All patients were clinically stable for at least the past month. No patient had recent clinically significant weight loss or contraindications for MPH.
The median age of the sample was 76 years. The sample was 66% male and 90% white. Patients had been diagnosed with dementia for a mean of 3 years. The mean MMSE score was about 19, indicating mild to moderate dementia. The mean apathy score was nearly 8 on the apathy subscale of the Neuropsychiatric Inventory. Very surprisingly, the mean apathy score was only 1.9 on a 16-item informant-rated interview.
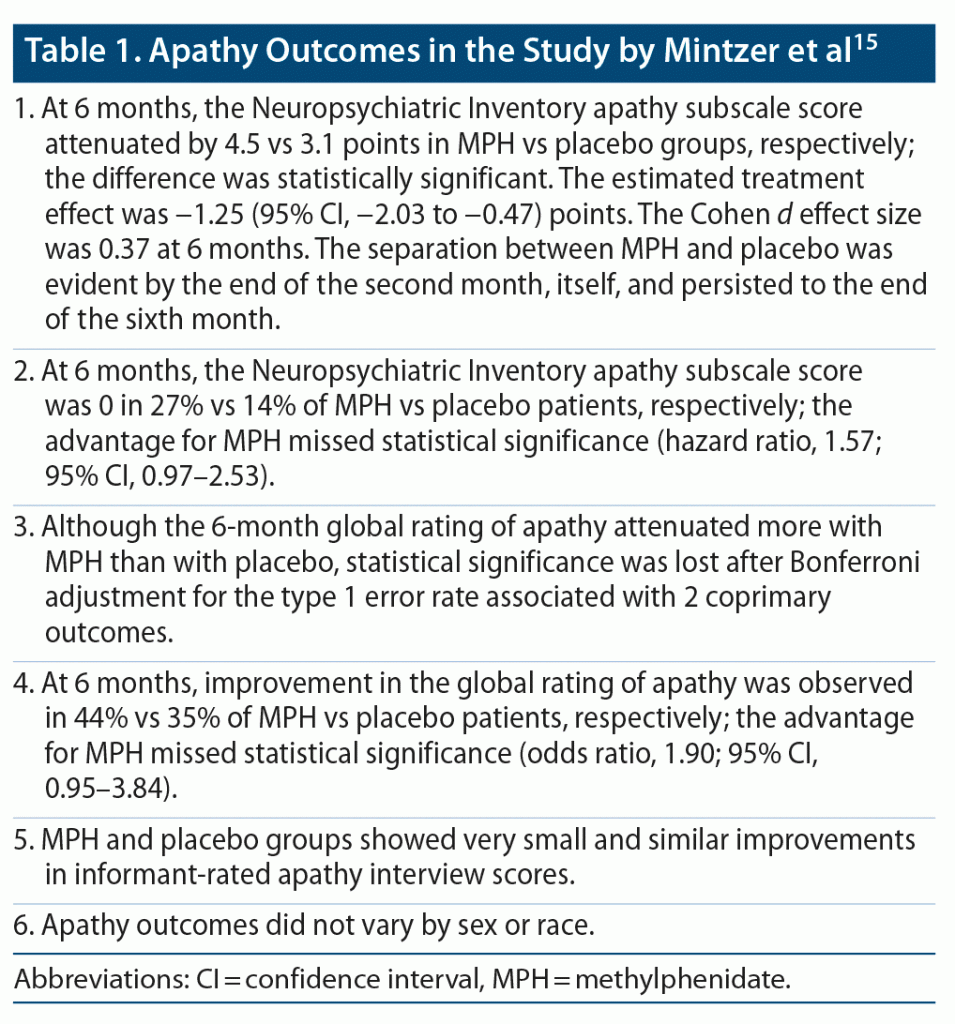
These patients were randomized to receive MPH (n = 99) or placebo (n = 101). The dose of MPH was uptitrated across 3 days to 10 mg twice daily; all concurrent medications, including psychotropic and antidementia medications, were continued unchanged. All patients and their caregivers also received a standardized psychosocial intervention at each study visit. Visits were scheduled monthly for 6 months. At the 6-month study endpoint, data for analysis were available for 89 MPH and 92 placebo patients. Important findings from the RCT are presented in Table 1 and Table 2.
In summary, 6 months of treatment with MPH (20 mg/d) was associated with a small but statistically significant advantage over placebo in the attenuation of apathy scores in patients with mild to moderate AD who were on treatment with antidementia drugs; the advantage became apparent by the end of the second month of treatment and persisted to the end of 6 months. However, importantly, MPH was not superior to placebo on a wide range of other outcomes, including informant-rated apathy, dependence, activities of daily living, quality of life, and neurocognitive performance. MPH appeared safe and well tolerated across 6 months of treatment.
Comments on the RCT by Mintzer et al15
Neuropsychiatric Inventory apathy subscale scores improved substantially: by about 56% vs 41% in the MPH vs placebo groups, respectively. However, in absolute terms, the estimated MPH vs placebo treatment effect was just 1.25 points, and the effect size (Cohen d = 0.37) was correspondingly small. There was little to no advantage associated with MPH treatment across a range of neuropsychological and real-life outcomes. These findings do not encourage the use of MPH to treat apathy in AD. But there is an elephant in the room. All patients and caregivers in the RCT received a psychosocial intervention that comprised a 20–30 minute counseling session at the monthly study visits. Caregivers were also provided with educational materials and 24-hour access to study staff for crisis management. Whereas this is excellent clinical practice, it is unlikely to be standard clinical care or treatment as usual in most parts of the world. What if the psychosocial intervention boosted the response in the placebo group; in other words, might the advantage for MPH have been greater had the placebo group received what passes as treatment as usual elsewhere? This is an important clinical question because MPH, as dosed in the study, was safe and well tolerated, and effective against apathy for as long as 6 months. Given that MPH is available as a generic drug in many parts of the world, it would have been useful to learn how effective it is against apathy in AD patients who are seen in routine, real world practice.
It is surprising that Mintzer et al15 did not study caregiver burden. However, if ratings of dependence, activities of daily living, and quality of life did not differ between MPH and placebo groups, it is unlikely that differences would have been observed for assessments of caregiver burden.
There were 2 other curiosities. One is that MPH was dosed “twice a day.” The first dose was presumably administered in the morning. It is not clear whether the second dose was administered later in the day or at night; if the latter, one wonders whether the second dose, with its actions spent during the hours of sleep, contributed to benefit. The other curiosity is that the mean 16-item, informant-rated interview apathy score was just 1.9 at baseline. The informants, presumably, were the caregivers. Why did the caregivers rate apathy so low, and could this explain why MPH did not separate from placebo on other outcomes for which caregivers would have contributed information?
Take-Home Message
Although both meta-analysis and a large, well-designed, well-conducted, and well-analyzed RCT suggest that psychostimulants in general and MPH in particular attenuate the severity of apathy in patients with AD, and that the benefit with MPH (20 mg/d) may extend for as long as 6 months, the absence of improvements in measures of dependence, activities of daily living, and quality of life suggest that this effect of MPH may not be clinically significant. An unanswered question is whether the benefits of MPH may be clinically significant in real world practice settings in which the delivery of behavioral interventions is not feasible.
Parting Note
Bupropion is a catecholaminergic antidepressant; it inhibits the reuptake of dopamine and norepinephrine.16 Maier et al17 described a 12-week multicenter German RCT that examined the efficacy of bupropion (300 mg/d) vs placebo in treating apathy in 108 patients (mean age, 75 years; 62% male) with mild to moderate AD. Bupropion was no better than placebo in improving apathy, and, unexpectedly, improvements in neuropsychiatric symptoms, depression, and quality of life were actually greater with placebo than with bupropion.
Published online: February 1, 2022.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
References (17)

- GBD 2016 Dementia Collaborators. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):88–106. PubMed CrossRef
- GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019 [online ahead of print January 6, 2022]. Lancet Public Health. 2022. CrossRef
- Gerlach LB, Kales HC. Managing behavioral and psychological symptoms of dementia. Psychiatr Clin North Am. 2018;41(1):127–139. PubMed CrossRef
- Dyer SM, Harrison SL, Laver K, et al. An overview of systematic reviews of pharmacological and non-pharmacological interventions for the treatment of behavioral and psychological symptoms of dementia. Int Psychogeriatr. 2018;30(3):295–309. PubMed CrossRef
- Leung DKY, Chan WC, Spector A, et al. Prevalence of depression, anxiety, and apathy symptoms across dementia stages: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 2021;36(9):1330–1344. PubMed CrossRef
- Miller DS, Robert P, Ereshefsky L, et al. Diagnostic criteria for apathy in neurocognitive disorders. Alzheimers Dement. 2021;17(12):1892–1904. PubMed CrossRef
- van der Linde RM, Matthews FE, Dening T, et al. Patterns and persistence of behavioural and psychological symptoms in those with cognitive impairment: the importance of apathy. Int J Geriatr Psychiatry. 2017;32(3):306–315. PubMed CrossRef
- Sepehry AA, Sarai M, Hsiung GR. Pharmacological therapy for apathy in Alzheimer’s disease: a systematic review and meta-analysis. Can J Neurol Sci. 2017;44(3):267–275. PubMed CrossRef
- van Dyck CH, Arnsten AFT, Padala PR, et al. Neurobiologic rationale for treatment of apathy in Alzheimer’s disease with methylphenidate. Am J Geriatr Psychiatry. 2021;29(1):51–62. PubMed CrossRef
- Kishi T, Sakuma K, Iwata N. Efficacy and safety of psychostimulants for Alzheimer’s disease: a systematic review and meta-analysis. Pharmacopsychiatry. 2020;53(3):109–114. PubMed CrossRef
- Herrmann N, Rothenburg LS, Black SE, et al. Methylphenidate for the treatment of apathy in Alzheimer disease: prediction of response using dextroamphetamine challenge. J Clin Psychopharmacol. 2008;28(3):296–301. PubMed CrossRef
- Frakey LL, Salloway S, Buelow M, et al. A randomized, double-blind, placebo-controlled trial of modafinil for the treatment of apathy in individuals with mild-to-moderate Alzheimer’s disease. J Clin Psychiatry. 2012;73(6):796–801. PubMed CrossRef
- Rosenberg PB, Lanctôt KL, Drye LT, et al; ADMET Investigators. Safety and efficacy of methylphenidate for apathy in Alzheimer’s disease: a randomized, placebo-controlled trial. J Clin Psychiatry. 2013;74(8):810–816. PubMed CrossRef
- Padala PR, Padala KP, Lensing SY, et al. Methylphenidate for apathy in community-dwelling older veterans with mild Alzheimer’s disease: a double-blind, randomized, placebo-controlled trial. Am J Psychiatry. 2018;175(2):159–168. PubMed CrossRef
- Mintzer J, Lanctôt KL, Scherer RW, et al; ADMET 2 Research Group. Effect of methylphenidate on apathy in patients with Alzheimer disease: the ADMET 2 randomized clinical trial. JAMA Neurol. 2021;78(11):1324–1332. PubMed CrossRef
- Stahl SM, Pradko JF, Haight BR, et al. A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Prim Care Companion J Clin Psychiatry. 2004;6(4):159–166. PubMed CrossRef
- Maier F, Spottke A, Bach J-P, et al. Bupropion for the treatment of apathy in Alzheimer disease: a randomized clinical trial. JAMA Netw Open. 2020;3(5):e206027. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite