Introduction: Methylphenidate is a central nervous system stimulant medicinally used in the treatment of attention-deficit disorder with or without hyperactivity (ADD/ADHD). Data on its use in human pregnancy are limited. The primary objective of the study was to evaluate the risk of major congenital anomalies after pregnancy exposure to methylphenidate for medical indications.
Methods: In a prospective, comparative, multicenter observational study performed in 4 participating Teratology Information Services (in Jerusalem, Berlin, Newcastle upon Tyne, and Toronto) between 1996 and 2013, methylphenidate-exposed pregnancies were compared with pregnancies counseled for nonteratogenic exposure (NTE) after matching by maternal age, gestational age, and year at initial contact.
Results: 382 methylphenidate-exposed pregnancies (89.5% in the first trimester) were followed up. The overall rate of major congenital anomalies was similar between the groups (10/309 = 3.2% [methylphenidate] vs 13/358 = 3.6% [NTE], P = .780). The rates of major congenital anomalies (6/247 = 2.4% [methylphenidate] vs 12/358 = 3.4% [NTE], P = .511) and cardiovascular anomalies (2/247 = 0.8% [methylphenidate] vs 3/358 = 0.8% [NTE], P = .970) were also similar after exclusion of genetic or cytogenetic anomalies and limiting methylphenidate exposure to the period of organogenesis (weeks 4-13 after the last menstrual period). There was a higher rate of miscarriages and elective terminations of pregnancy in the methylphenidate group. Significant predictors for the miscarriages using Cox proportional hazards model were methylphenidate exposure (adjusted hazard ratio [HR] = 1.98; 95% CI, 1.23-3.20; P = .005) and past miscarriage (adjusted HR = 1.35; 95% CI, 1.18-1.55; P < .001).
Conclusions: The present study suggests that methylphenidate does not seem to increase the risk for major malformations. Further studies are required to establish its pregnancy safety and its possible association with miscarriages.
Methylphenidate in Pregnancy:
A Multicenter, Prospective, Comparative, Observational Study

ABSTRACT
Introduction: Methylphenidate is a central nervous system stimulant medicinally used in the treatment of attention-deficit disorder with or without hyperactivity (ADD/ADHD). Data on its use in human pregnancy are limited. The primary objective of the study was to evaluate the risk of major congenital anomalies after pregnancy exposure to methylphenidate for medical indications.
Methods: In a prospective, comparative, multicenter observational study performed in 4 participating Teratology Information Services (in Jerusalem, Berlin, Newcastle upon Tyne, and Toronto) between 1996 and 2013, methylphenidate-exposed pregnancies were compared with pregnancies counseled for nonteratogenic exposure (NTE) after matching by maternal age, gestational age, and year at initial contact.
Results: 382 methylphenidate-exposed pregnancies (89.5% in the first trimester) were followed up. The overall rate of major congenital anomalies was similar between the groups (10/309 = 3.2% [methylphenidate] vs 13/358 = 3.6% [NTE], P = .780). The rates of major congenital anomalies (6/247 = 2.4% [methylphenidate] vs 12/358 = 3.4% [NTE], P = .511) and cardiovascular anomalies (2/247 = 0.8% [methylphenidate] vs 3/358 = 0.8% [NTE], P = .970) were also similar after exclusion of genetic or cytogenetic anomalies and limiting methylphenidate exposure to the period of organogenesis (weeks 4-13 after the last menstrual period). There was a higher rate of miscarriages and elective terminations of pregnancy in the methylphenidate group. Significant predictors for the miscarriages using Cox proportional hazards model were methylphenidate exposure (adjusted hazard ratio [HR] = 1.98; 95% CI, 1.23-3.20; P = .005) and past miscarriage (adjusted HR = 1.35; 95% CI, 1.18-1.55; P < .001).
Conclusions: The present study suggests that methylphenidate does not seem to increase the risk for major malformations. Further studies are required to establish its pregnancy safety and its possible association with miscarriages.
J Clin Psychiatry 2016;77(9):1176-1181
dx.doi.org/10.4088/JCP.15m10083
© Copyright 2016 Physicians Postgraduate Press, Inc.
aThe Israeli Teratology Information Service, Jerusalem, Israel Ministry of Health
bThe Hebrew University Hadassah Medical School, Jerusalem, Israel
cBerlin Institute for Clinical Teratology and Drug Risk Assessment in Pregnancy, Berlin, Germany
dThe United Kingdom Teratology Information Service, Newcastle upon Tyne, England
eThe Motherisk Program, The Hospital for Sick Children, Toronto, Canada
*Corresponding author: Orna Diav-Citrin, MD, The Israeli Teratology Information Service, Israel Ministry of Health, PO Box 1176, Jerusalem, 9446724, Israel ([email protected]).
Methylphenidate is a central nervous system stimulant medicinally used in the treatment of attention-deficit disorder with or without hyperactivity (ADD/ADHD) and narcolepsy. The prevalence rate of ADHD is estimated between 2%-18%.1 The worldwide pooled prevalence of ADHD in a recent systematic review was 5%.2 In recent years, methylphenidate has been increasingly prescribed in children and young adults with ADHD. It is also increasingly used for cognitive enhancement in the absence of ADHD.3 Methylphenidate carries a potential for abuse and can be used as a drug of abuse. A substantial increase in the incidence of pregnancies exposed to ADHD medication from 5 to 533 per 100,000 person-years between 2003 and the first quarter of 2010 was shown in Denmark.4
Methylphenidate is a piperidine derivative that is structurally related to amphetamine. Placental transport of amphetamines has been shown in mice and sheep.5,6 There are no human data regarding the placental transfer of methylphenidate. However, its molecular weight of 269.8 Da is low enough to suggest transfer. Methylphenidate has not been teratogenic in most animal reproductive studies.7,8 In 1 rabbit study,9 however, the racemic mixture produced a low incidence of spina bifida at a maternal dose of 200 mg/kg/d, which also caused some maternal lethality.
Until recently, published data on human pregnancy experience with methylphenidate have been limited. A total sample of 41 children exposed to methylphenidate in pregnancy were found in a systematic review of the literature dated until September 2012.10 In the Collaborative Perinatal Project,11 there were 11 pregnancies exposed to methylphenidate with no evidence of increased malformation risk. In the surveillance study12 of Michigan Medicaid recipients, 13 newborns had been exposed to methylphenidate during the first trimester, with 1 neonate with cardiovascular defect. Human pregnancy experience with methylphenidate is partly derived from women who abused it. Thus, in a study examining intravenous pentazocine and methylphenidate abuse in 38 pregnancies, there was a high rate of preterm deliveries, growth restriction, and withdrawal symptoms after birth.13 This study was confounded by concurrent abuse of alcohol and other drugs, as well as by the underlying disorders. In the Swedish Medical Birth Register, 104 children were reported following in utero exposure to methylphenidate, with 3 having a congenital malformation.14 Two children had ventricular septal defects, and 1 had a univentricular heart. In updated data from the Swedish Medical Birth Register, there were 5 infants with relatively severe malformations, all cardiovascular, among 208 pregnancies with early exposure to methylphenidate.15 The relative risk (RR) for cardiovascular malformations was 1.81 (95% CI, 0.59-4.21). In contrast, there was no increase in the risk of major or cardiac malformations among the offspring of 222 first trimester-exposed pregnancies from a Danish population-based cohort study.16 There was, however, an increased risk for miscarriage in another recent Danish register-based study, with 480 ADHD-medication-exposed pregnancies (of which 393 exposures were methylphenidate) compared with unexposed pregnancies.4 In addition, their data included 3 pregnancies exposed to ADHD medication that resulted in a child with a congenital malformation within the first year of life (prevalence of 3.1%; 95% CI, 0.6%-8.7%) and did not support an association between use of ADHD medication during pregnancy and congenital malformations.4
Another concern of methylphenidate treatment during pregnancy is its potential effect on the developing brain. A few studies have investigated the long-term neurobehavioral consequences of in utero exposure to methylphenidate in animal models, suggesting such effects.17-19
The primary objective of the present study was to evaluate the risk of major anomalies after early pregnancy exposure to methylphenidate for medical indications or for cognitive enhancement. Secondary objectives were to compare the rate of pregnancy outcome (live birth, miscarriage, elective termination of pregnancy, stillbirth), neonatal birth weight, rate of preterm delivery, and neonatal effects after pregnancy exposure to methylphenidate for these indications or enhancements.
METHODS
Setting
Pregnant women who contacted 1 of 4 selected Teratology Information Service (TIS) providers—the Israeli TIS (Jerusalem, Israel), the Institute for Clinical Teratology and Drug Risk Assessment in Pregnancy (Berlin, Germany), the United Kingdom TIS (Newcastle upon Tyne, England), or the Motherisk Program (Toronto, Canada)—between 1996 and 2013 regarding gestational exposure to methylphenidate were enrolled in the present multicenter, prospective, comparative, observational study. The 4 TIS providers are members of the European Network of Teratology Information Services (ENTIS) or the Organization of Teratology Information Specialists (OTIS); both are organizations for providers of counseling services with regard to environmental exposure during pregnancy, and they use a similar methodology. TIS providers offer risk assessment to pregnant women and health care professionals who spontaneously contact these services for consultation in pregnancy.

- Methylphenidate is increasingly prescribed in adults, while data on its use in pregnancy are limited.
- Methylphenidate in pregnancy did not show an increased risk of major congenital anomalies compared with nonteratogenic exposure.
- The higher rate of miscarriages observed in the methylphenidate group was associated with a history of miscarriages and might also be related to the mother’s underlying disorder or indication.
The participants consented to take part in the study. The study protocol was approved by the ethics committee of the Israeli Ministry of Health and by ethics committees of other TIS providers, where required. Retrospective cases were not included in the study. Pregnant women who contacted the TIS after an anomaly had been identified on prenatal testing were also excluded.
Exposure
Exposure during pregnancy was defined from conception till the end of pregnancy. Exposure to methylphenidate during the period of organogenesis was defined when the woman took the medication during pregnancy between 4 and 13 completed weeks from her last menstrual period. The methylphenidate-exposed group was compared with a group of women counseled by 1 of the 4 TIS providers for nonteratogenic exposure (NTE), at a 1:1 ratio, matched for maternal age, gestational age, and year at initial contact. Gestational age referred to the time from the last menstrual period. Common exposures in the comparison group were short-term courses of antibiotics (eg, penicillins, cephalosporins), oral contraceptives taken in the first 4-5 weeks of pregnancy, low-dose diagnostic irradiation, topical preparations (excluding retinoids) with negligible systemic absorption, occasional paracetamol (acetaminophen), and hair dye (non-occupational). Pregnancies of women who had chronic diseases were not included in the NTE group. Details of exposure were collected at the initial contact with the TIS and before pregnancy outcome was known using a structured questionnaire. In addition, the following information was recorded: maternal demographics, medical and obstetric history, and exposure details (dose, duration and timing in pregnancy, and additional exposures).
Outcome
Pregnancy outcome data were actively sought after the expected date of delivery in the exposed and comparison groups, and collection methods for both groups were similar. After at least 2 physical examinations were performed in the neonatal period, follow-up was conducted by a telephone interview with the woman or by a questionnaire mailed to the woman, her health care professional, and/or the child’s pediatrician to obtain details on the pregnancy outcome, gestational age at delivery, birth weight, congenital anomalies, and neonatal complications. In cases of anomalies, an attempt to obtain medical records was made. In addition, it was verified that the exposure(s) the woman was counseled for indeed occurred during pregnancy. Details on any dose modifications during pregnancy, the exact timing and duration of treatment, and additional exposures during pregnancy were also ascertained. .
Major anomalies were defined as structural anomalies in the offspring that have serious medical, surgical, or cosmetic consequences. Children with minor anomalies or functional problems without any morphological changes (eg, ankyloglossia, toe syndactyly, umbilical hernia, lacrimal duct stenosis, mild congenital ptosis, small dermoid cyst, hemangioma, pyelectasis not requiring an intervention, heart murmur with normal echocardiography) or infants with complications of preterm delivery were not considered as having major anomalies. Cardiac septal defects are structural anomalies of the heart and were considered major anomalies in the present study, even if spontaneously resolved, unless the closure occurred during the neonatal period. Patent foramen ovale was considered a minor anomaly. Abnormalities detected by prenatal ultrasonography (if verified postnatally or by autopsy) were included in our study, since antenatal screening for major anomalies is routinely performed in the participating countries. The classification of anomalies was done by a certified pediatrician blinded to the exposure group, using guidelines of 2 classification systems.20,21 The analysis of major congenital anomalies was performed among live-born infants and elective terminations of pregnancy due to prenatally diagnosed anomalies. In the case of multiple births, each live-born offspring was included in the analysis. Miscarriage was defined as spontaneous pregnancy loss before or at 20 completed weeks, whereas stillbirth was defined as spontaneous pregnancy loss beyond 20 completed weeks. Preterm delivery was defined as birth before 37 completed weeks.
Statistical Analysis
Categorical data were compared using χ2 or Fisher exact tests and are expressed as ratios or percentages. Continuous data are presented using mean ± SD or median with interquartile range (IQR), depending on whether they followed normal distribution, and were compared using Student t or Mann-Whitney tests. Hazard ratios (HR) and 95% CI for miscarriages were estimated using Cox proportional hazards models while accounting for left truncation due to varying time of gestation at enrollment. All statistical calculations were done using SPSS Version 19 (IBM Corp), Epi Info software (Centers for Disease Control and Prevention), or R version 2.15 (The R Foundation for Statistical Computing).
RESULTS
A total of 382 methylphenidate-exposed pregnancies and a similar number of pregnancies in women counseled for nonteratogenic exposure were prospectively followed up (Jerusalem n = 271 in the years 2000-2012, Berlin n = 80 in the years 1997-2012, Newcastle upon Tyne n = 19 in the years 1996-2012, Toronto n = 12 in the years 2009-2013). Of those who reported the medication formulation, regular methylphenidate (Ritalin or Medikinet) tablets were taken by 65.6%, while 34.4% took extended- or sustained-release formulations (Concerta or Methylphenidat HEXAL in 22.9%, Ritalin LA in 5.7%, and Ritalin SR or Medikinet Retard in 5.7%). The reported indications were as follows: ADHD in 55.3%, ADD in 34.6%, narcolepsy in 4.8%, and others in 5.3%. Exposure to methylphenidate occurred at least in the first trimester in 89.5% of pregnancies. In 15.2% of pregnancies, exposure occurred in all 3 trimesters. The median daily dose was 20 mg (IQR, 18-40). The majority of women (> 95%) started the treatment with methylphenidate before pregnancy. The median gestational age at treatment discontinuation was week 6 (IQR, 5-31). Concomitant psychiatric medications were taken by 25.4% of the women in the methylphenidate group.
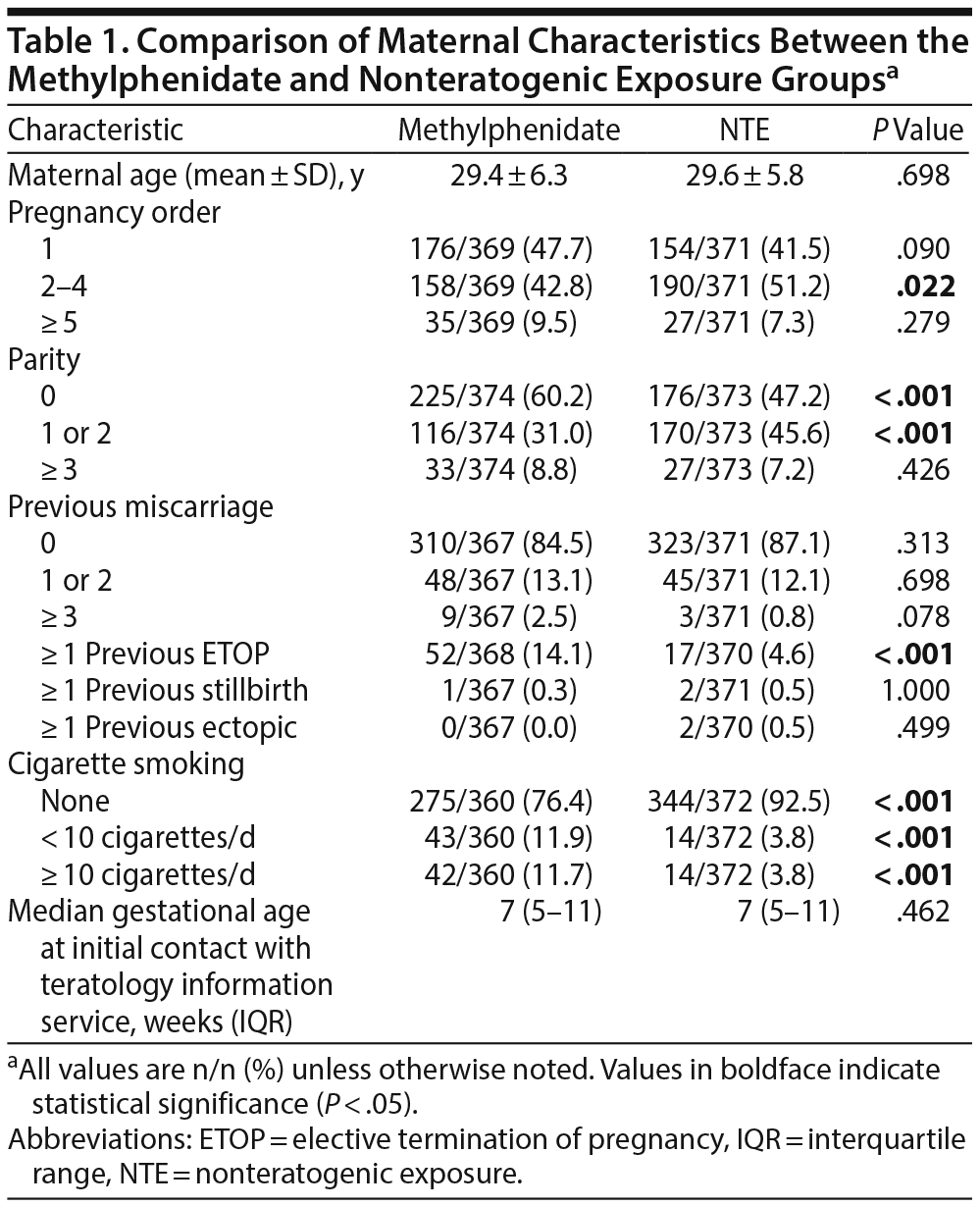
A comparison of maternal characteristics and obstetric history is presented in Table 1. Due to the matching procedure, the mean maternal age and the median gestational age at initial contact were similar between the groups. When compared with the NTE group, a lower percentage of women in the methylphenidate group contacted the TIS regarding their 2nd-4th pregnancy, a higher percentage were nulliparous when they called, and a lower percentage had 1 or 2 children. A higher percentage of women in the methylphenidate group reported 1 or more elective terminations of previous pregnancies, and there was a higher rate of cigarette smokers in the methylphenidate group.
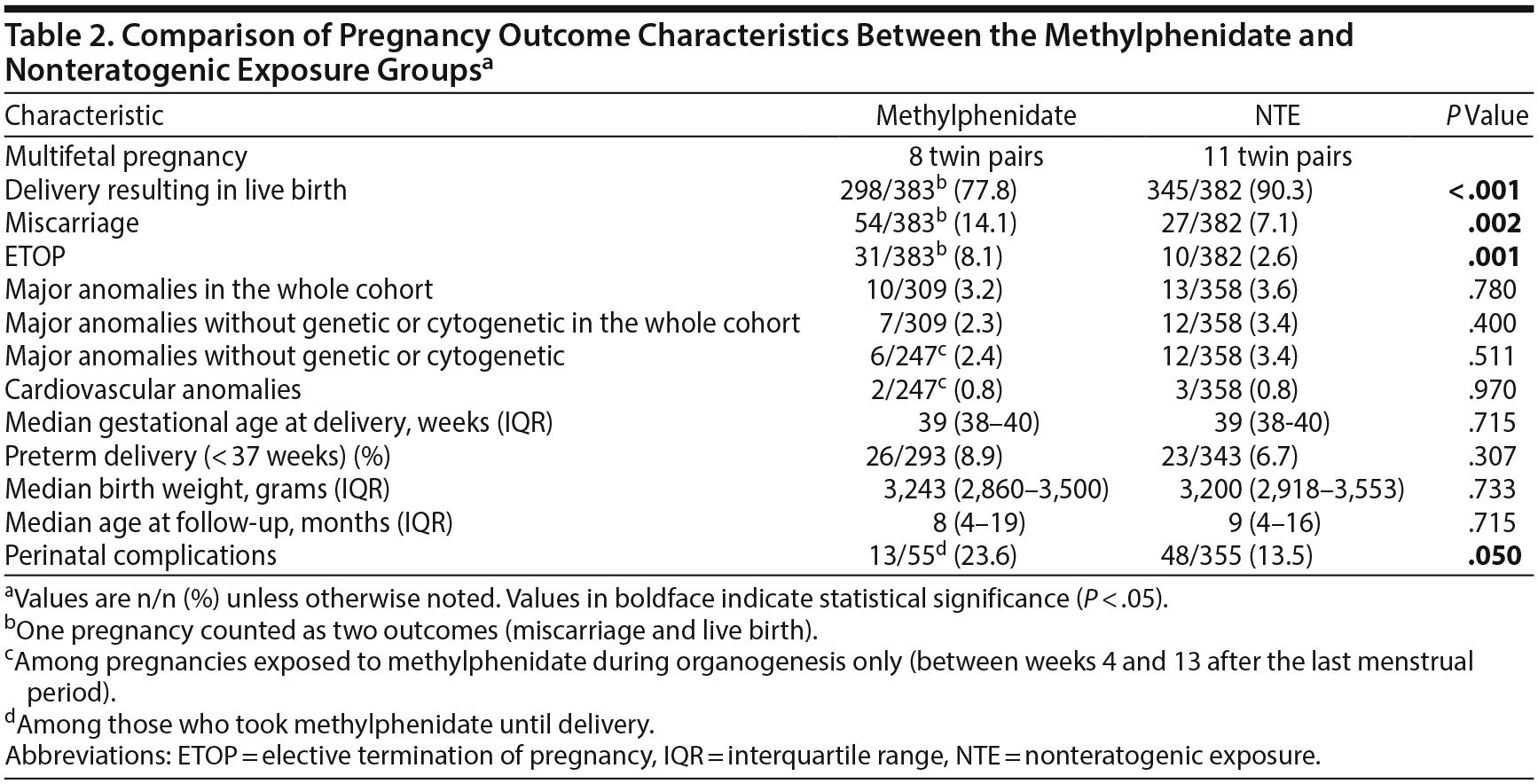
A comparison of pregnancy outcome is presented in Table 2. There was a higher crude rate of miscarriages and elective terminations of pregnancy in the methylphenidate group compared with the NTE group. Elective terminations of pregnancy were performed due to prenatally diagnosed anomalies in 3 of 31 and in 4 of 10 cases of the methylphenidate and comparison groups, respectively. In all other cases of elective termination of pregnancy, no gross malformations were observed. The rate of overall major anomalies was similar between the 2 groups (3.2% methylphenidate, 3.6% NTE, P = .780). In a reanalysis after excluding genetic or cytogenetic anomalies the difference remained nonsignificant (2.3% methylphenidate, 3.4% NTE, P = .400). Similarly, the rate did not significantly differ when the analysis was limited to pregnancies exposed to methylphenidate during the period of organogenesis (2.4% methylphenidate, 3.4% NTE, P = .511). The rate of cardiovascular anomalies was also similar between the groups. There were no significant differences in the median gestational age at delivery, rate of preterm delivery, median birth weight, and age at follow-up between the groups. There was, however, a higher rate of perinatal complications among neonates exposed to methylphenidate close to term compared with the NTE group. The most common perinatal complication in both groups was jaundice. It is noteworthy that the mothers of 3 neonates who had neurologic complications in the methylphenidate late-exposure group took concurrent psychiatric medications (benzodiazepines, selective serotonin reuptake inhibitors, or both). An additional comparison of perinatal complications between 2 methylphenidate subgroups (those who stopped the medication vs those who continued until term) showed an nonsignificant difference [18.3% (44/240) vs 23.6% (13/55), respectively, P = .369].
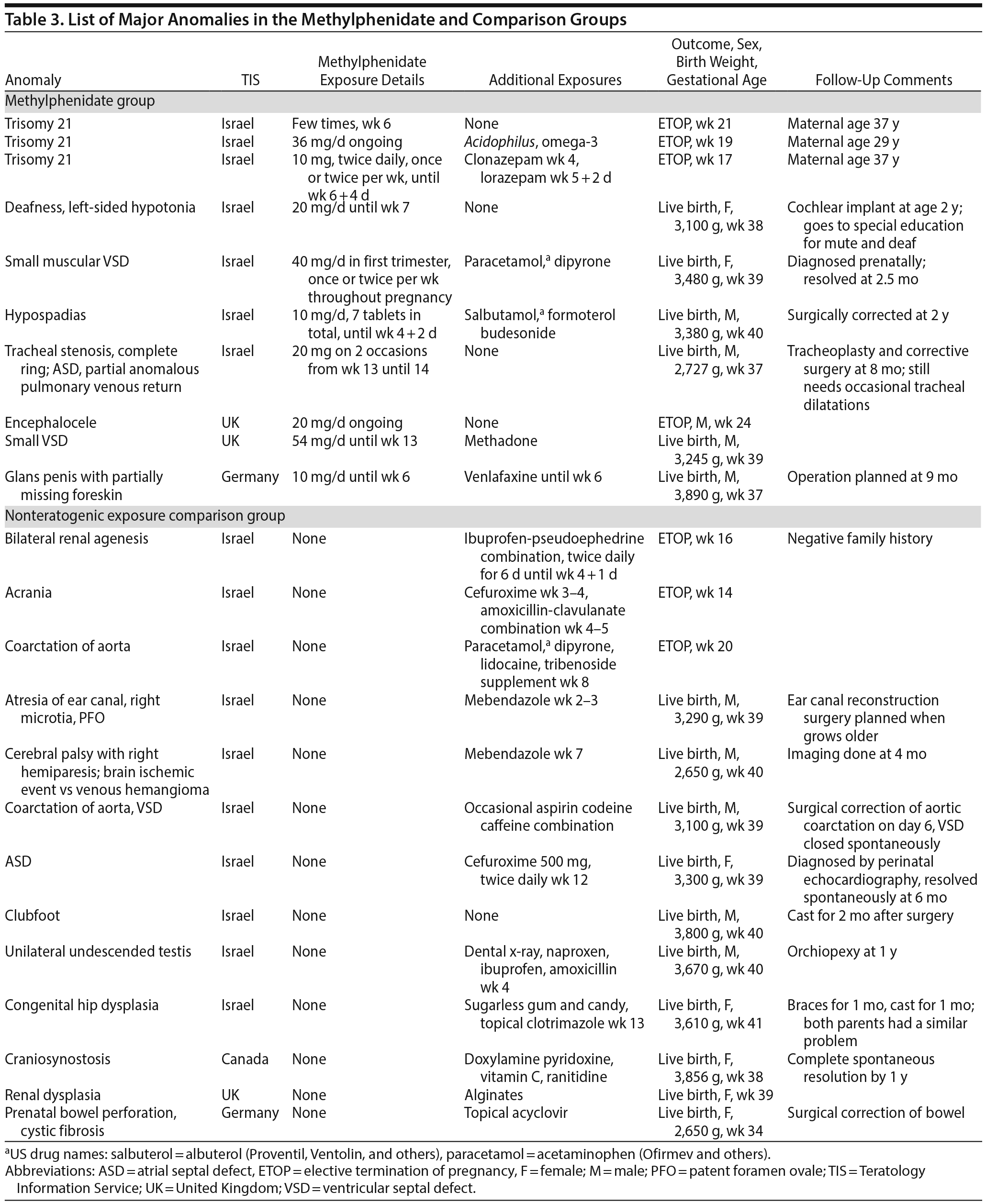
The crude HR for miscarriages was 2.08; 95% CI, 1.31-3.31; P = .002. To evaluate the relative contribution of various predictors to the differences in the miscarriage rate, regression analysis using a Cox proportional hazard model was performed. The following predictors were entered into the model: gestational age at initial contact, maternal age, exposure group, and history of miscarriage. Significant predictors included methylphenidate exposure (P = .005) with an adjusted HR = 1.98 (95% CI, 1.23-3.20) and history of miscarriage (P < .001) with an adjusted HR = 1.35 (95% CI, 1.18-1.55). Adding smoking and concomitant medications (psychiatric or nonsteroidal anti-inflammatory drugs) as covariates in an additional analysis did not further attenuate the associations. The reported major anomalies and the details of exposures in the methylphenidate and NTE groups are listed in Table 3. There was no specific pattern of anomalies in the methylphenidate group.
DISCUSSION
The results of the present study suggest that methylphenidate treatment, in the context of medical indications or for cognitive enhancement, is not associated with an increased risk of major anomalies or cardiovascular anomalies. These results are consistent with the study published by Potteg×¥rd et al.16 The rate of major anomalies in the present study did not significantly differ between the methylphenidate and the NTE groups. There was no specific pattern of anomalies in the offspring of women in the methylphenidate group, supporting lack of teratogenic effect.
A sample size of 247 live births or elective terminations of pregnancy due to prenatally diagnosed anomalies in the methylphenidate group exposed during the period of organogenesis, with 358 in the NTE comparison group, 80% power, and a baseline risk of 2.4% for major anomalies, enables detection of a 3.2-fold increase in the rate of overall major anomalies, ie, it was not powered to detect a smaller increase. The study is also not powered to find an increase in the rate of specific rare anomalies. The current study, taken together with previous studies,10-12,15,16 includes data on over 800 human pregnancies with early exposure to methylphenidate and no apparent increase in the rate of major anomalies.
The higher rate of miscarriages seen in the present study is consistent with the finding reported by Haervig et al.4 The higher rate of miscarriages in the methylphenidate group found in the current study was associated with the exposure and with a history of miscarriage, as verified by the Cox proportional hazards model. In a recent Danish population-based cohort study,22 exposure to methylphenidate or atomoxetine was also associated with an increased risk of spontaneous abortion (adjusted RR = 1.55; 95% CI, 1.03-2.36). However, a similar increased risk among pregnant women with ADHD who did not use these medications during pregnancy was demonstrated (adjusted RR = 1.56; 95% CI, 1.11-2.20). These results suggest that the observed risk of miscarriage may, at least partly, be explained by indication.
The subgroup comparison of perinatal complications, although based on small numbers, and the fact that these neonates were frequently exposed to concurrent psychiatric medications, suggests that these neonatal adverse effects may have been associated with comorbid conditions that could accompany the mother’s underlying disorder that may require psychiatric medications.
The present study has certain limitations and advantages. It is based on TIS user population, which may not represent the general population, but has an appropriate comparison group from the same TIS providers. A substantial part of the methylphenidate cohort stopped the medication early in pregnancy. Such exposure, from an embryologic point of view, may only be relevant mainly for early developmental effects. However, for future women with inadvertent early pregnancy exposure to methylphenidate, the reassuring lack of increased rate of major anomalies is important. Other limitations of the study include reliance on maternal interviews as a source for outcome data in most cases, a nonrandomized design, and limited power for specific rare defects. However, applying the same method to both arms of the study, and the study’s prospective nature, minimize the potential biases. Due to ethical considerations randomized controlled trials are often not feasible in pregnancy. In addition, medical records—including discharge letters, echocardiography results, or information from the child’s pediatrician—were available for most cases with anomalies. Another advantage of the present study is that data were available on elective terminations of pregnancies and were included in the analysis.
In conclusion, the present prospective, observational, comparative, multicenter cohort study suggests that the use of methylphenidate during early pregnancy is not associated with an increased risk of major anomalies. Larger studies are needed to establish the safety of methylphenidate during pregnancy and its possible association with miscarriages. Further long-term studies are needed to address its potential neurodevelopmental effect on human offspring.
Submitted: April 27, 2015; accepted July 30, 2015.
Online first: May 24, 2016.
Drug names: acetaminophen (Ofirmev and others), acyclovir (Zovirax and others), albuterol (Proventil, Ventolin, and others)amoxicillin (Amoxil and others), amoxicillin-clavulanate (Augmentin and others), amphetamine (Adzenyx XR-ODT, Adderall XR, and others), atomoxetine (Strattera and others), cefuroxime (Ceftin and others), clonazepam (Klonopin and others), doxylamine pyridoxine (Diclegis), formoterol budesonide (Symbicort), ibuprofen (Ibu-tab and others), lorazepam (Ativan and others), methadone (Methadose and others), methylphenidate (Ritalin and others), naproxen (Naprosyn and others), pentazocine (Talwin), ranitidine (Zantac and others).
Potential conflicts of interest: The authors have no conflicts of interest, financial or other, to disclose.
Funding/support: None.
Previous presentation: Previous presentation of preliminary data at the First International ENTIS (European Network of Teratology Information Services) and OTIS (Organization of Teratology Information Specialists) joint meeting, Jerusalem, Israel, March 26-30, 2011.
Additional information: The Israeli Teratology Information Service (TIS) database belongs to the Israeli government. All data for this study now reside at the Israeli TIS.
REFERENCES
1. Rowland AS, Lesesne CA, Abramowitz AJ. The epidemiology of attention-deficit/hyperactivity disorder (ADHD): a public health view. Ment Retard Dev Disabil Res Rev. 2002;8(3):162-170. PubMed doi:10.1002/mrdd.10036
2. Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942-948. PubMed doi:10.1176/ajp.2007.164.6.942
3. Bagot KS, Kaminer Y. Efficacy of stimulants for cognitive enhancement in non-attention deficit hyperactivity disorder youth: a systematic review. Addiction. 2014;109(4):547-557. PubMed doi:10.1111/add.12460
4. Haervig KB, Mortensen LH, Hansen AV, et al. Use of ADHD medication during pregnancy from 1999 to 2010: a Danish register-based study. Pharmacoepidemiol Drug Saf. 2014;23(5):526-533. PubMed doi:10.1002/pds.3600
5. Shah NS, Yates JD. Placental transfer and tissue distribution of dextro-amphetamine in the mouse. Arch Int Pharmacodyn Ther. 1978;233(2):200-208. PubMed
6. Burchfield DJ, Lucas VW, Abrams RM, et al. Disposition and pharmacodynamics of methamphetamine in pregnant sheep. JAMA. 1991;265(15):1968-1973. PubMed doi:10.1001/jama.1991.03460150072026
7. Teo SK, Stirling DI, Thomas SD, et al. The perinatal and postnatal toxicity of d-methylphenidate and d,l-methylphenidate in rats. Reprod Toxicol. 2002;16(4):353-366. PubMed doi:10.1016/S0890-6238(02)00044-8
8. Teo SK, Stirling DI, Hoberman AM, et al. d-Methylphenidate and d,l-methylphenidate are not developmental toxicants in rats and rabbits. Birth Defects Res B Dev Reprod Toxicol. 2003;68(2):162-171. PubMed doi:10.1002/bdrb.10018
9. Beckman DA, Schneider M, Youreneff M, et al. Developmental toxicity assessment of d,l-methylphenidate and d-methylphenidate in rats and rabbits. Birth Defects Res B Dev Reprod Toxicol. 2008;83(5):489-501. PubMed
10. Bolea-Alamanac BM, Green A, Verma G, et al. Methylphenidate use in pregnancy and lactation: a systematic review of evidence. Br J Clin Pharmacol. 2014;77(1):96-101. PubMed doi:10.1111/bcp.12138
11. Heinonen OP, Slone D, Shapiro S. Birth Defects and Drugs in Pregnancy. Littleton, MA: Publishing Sciences Group; 1977:346-347.
12. Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A reference guide to Fetal and Neonatal Risk. 9th ed. Philadelphia, PA: Lippincott, Williams and Wilkins; 2011:942-943.
13. Debooy VD, Seshia MM, Tenenbein M, et al. Intravenous pentazocine and methylphenidate abuse during pregnancy: maternal lifestyle and infant outcome. Am J Dis Child. 1993;147(10):1062-1065. PubMed doi:10.1001/archpedi.1993.02160340048012
14. Dideriksen D, Potteg×¥rd A, Hallas J, et al. First trimester in utero exposure to methylphenidate. Basic Clin Pharmacol Toxicol. 2013;112(2):73-76. PubMed doi:10.1111/bcpt.12034
15. Kפllén B, Borg N, Reis M. The use of central nervous system active drugs during pregnancy. Pharmaceuticals (Basel). 2013;6(10):1221-1286. PubMed doi:10.3390/ph6101221
16. Potteg×¥rd A, Hallas J, Andersen JT, et al. First-trimester exposure to methylphenidate: a population-based cohort study. J Clin Psychiatry. 2014;75(1):e88-e93. PubMed doi:10.4088/JCP.13m08708
17. McFadyen-Leussis MP, Lewis SP, Bond TL, et al. Prenatal exposure to methylphenidate hydrochloride decreases anxiety and increases exploration in mice. Pharmacol Biochem Behav. 2004;77(3):491-500. PubMed doi:10.1016/j.pbb.2003.12.011
18. Lloyd SA, Oltean C, Pass H, et al. Prenatal exposure to psychostimulants increases impulsivity, compulsivity, and motivation for rewards in adult mice. Physiol Behav. 2013;119:43-51. PubMed doi:10.1016/j.physbeh.2013.05.038
19. Lepelletier FX, Tauber C, Nicolas C, et al. Prenatal exposure to methylphenidate affects the dopamine system and the reactivity to natural reward in adulthood in rats. Int J Neuropsychopharmacol. 2015;18(4):pyu044. PubMed doi:10.1093/ijnp/pyu044
20. Rasmussen SA, Olney RS, Holmes LB, et al; National Birth Defects Prevention Study. Guidelines for case classification for the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol. 2003;67(3):193-201. PubMed doi:10.1002/bdra.10012
21. Merks JH, van Karnebeek CD, Caron HN, et al. Phenotypic abnormalities: terminology and classification. Am J Med Genet A. 2003;123A(3):211-230. PubMed doi:10.1002/ajmg.a.20249
22. Bro SP, Kjaersgaard MI, Parner ET, et al. Adverse pregnancy outcomes after exposure to methylphenidate or atomoxetine during pregnancy. Clin Epidemiol. 2015;7:139-147. PubMed doi:10.2147/CLEP.S72906
Please sign in or purchase this PDF for $40.00.
Save
Cite