Objective: Current dopamine-blocking antipsychotic drugs have little impact on the cognitive deficits associated with schizophrenia. We evaluated whether MIN-101, a molecule that combines sigma-2 antagonism and 5-HT2A antagonism, might improve cognitive deficits in individuals with moderate to severe negative symptoms in schizophrenia.
Methods: Individuals (N = 244) aged 18 to 60 years with stable symptoms of DSM-5-defined schizophrenia and moderate to severe negative symptoms were randomized to placebo (n = 83), MIN-101 32 mg (n = 78), or MIN-101 64 mg (n = 83) in a 12-week, phase 2b, prospective, double-blind, placebo-controlled, parallel-group trial between May 2015 and December 2015. In a post hoc analysis, mean z and T score changes from baseline at 12 weeks of treatment in the cognitive composite score and individual tests on the Brief Assessment of Cognition in Schizophrenia (BACS) Battery were compared between MIN-101 and placebo.
Results: A total of 79 patients (95.2%) from the placebo group, 76 (97.4%) from the MIN-101 32 mg group, and 79 (95.2%) from the MIN-101 64 mg group completed the BACS at baseline. The BACS token motor (P = .04), verbal fluency (P = .01), and composite z scores (P = .05) showed significant improvements in the MIN-101 32 mg group compared to the placebo group. At week 4, the clinical improvements from baseline in the Positive and Negative Syndrome Scale (PANSS) negative factor showed a significant correlation with improvements from baseline on the BACS composite in the 64 mg group (r = −0.292, P = .020). At week 12, improvement in the PANSS negative factor showed significant correlations with improvements in the BACS composite (r = −0.408, P = .002), Trail Making Test (r = −0.394, P = .003), and verbal memory (r = −0.322, P = .017) for the 64 mg group.
Conclusions: Results suggest a possible benefit of MIN-101 on cognitive performance in individuals with schizophrenia with stable positive symptoms and concurrent clinically significant negative symptoms.
Trial Registration: EU Clinical Trials Register identifier: 2014-004878-42‘ ‹
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Cognitive Effects of MIN-101 in Patients With Schizophrenia and Negative Symptoms:
Results From a Randomized Controlled Trial

ABSTRACT
Objective: Current dopamine-blocking antipsychotic drugs have little impact on the cognitive deficits associated with schizophrenia. We evaluated whether MIN-101, a molecule that combines sigma-2 antagonism and 5-HT2A antagonism, might improve cognitive deficits in individuals with moderate to severe negative symptoms in schizophrenia.
Methods: Individuals (N = 244) aged 18 to 60 years with stable symptoms of DSM-5-defined schizophrenia and moderate to severe negative symptoms were randomized to placebo (n = 83), MIN-101 32 mg (n = 78), or MIN-101 64 mg (n = 83) in a 12-week, phase 2b, prospective, double-blind, placebo-controlled, parallel-group trial between May 2015 and December 2015. In a post hoc analysis, mean z and T score changes from baseline at 12 weeks of treatment in the cognitive composite score and individual tests on the Brief Assessment of Cognition in Schizophrenia (BACS) Battery were compared between MIN-101 and placebo.
Results: A total of 79 patients (95.2%) from the placebo group, 76 (97.4%) from the MIN-101 32 mg group, and 79 (95.2%) from the MIN-101 64 mg group completed the BACS at baseline. The BACS token motor (P = .04), verbal fluency (P = .01), and composite z scores (P = .05) showed significant improvements in the MIN-101 32 mg group compared to the placebo group. At week 4, the clinical improvements from baseline in the Positive and Negative Syndrome Scale (PANSS) negative factor showed a significant correlation with improvements from baseline on the BACS composite in the 64 mg group (r = −0.292, P = .020). At week 12, improvement in the PANSS negative factor showed significant correlations with improvements in the BACS composite (r = −0.408, P = .002), Trail Making Test (r = −0.394, P = .003), and verbal memory (r = −0.322, P = .017) for the 64 mg group.
Conclusions: Results suggest a possible benefit of MIN-101 on cognitive performance in individuals with schizophrenia with stable positive symptoms and concurrent clinically significant negative symptoms.
Trial Registration: EU Clinical Trials Register identifier: 2014-004878-42
J Clin Psychiatry 2018;79(3):17m11753
To cite: Keefe RSE, Harvey PD, Khan A, et al. Cognitive effects of MIN-101 in patients with schizophrenia and negative symptoms: results from a randomized controlled trial. J Clin Psychiatry. 2018;79(3):17m11753.
To share: https://doi.org/10.4088/JCP.17m11753
© Copyright 2018 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry and Behavioral Sciences, Duke University Medical Center, Durham, North Carolina
bNeuroCog Trials, Durham, North Carolina
cDepartment of Psychiatry and Behavioral Sciences, University of Miami Leonard Miller School of Medicine, Miami, Florida
dNathan S. Kline Institute for Psychiatric Research, Orangeburg, New York
ePPRS RESEARCH, Inc, Colmar, France
fMinerva Neurosciences, Inc., Waltham, Massachusetts
gDepartment of Psychiatry, Sackler Medical School Tel Aviv University, Tel Aviv, Israel
*Corresponding author: Richard S. E. Keefe, PhD, Duke University Medical Center, Box 3270, Durham, NC 27710 ([email protected]).
The majority of individuals with schizophrenia demonstrate cognitive impairment, which is severe across multiple domains, including learning and memory, attention/vigilance, executive functioning, verbal fluency, and speed of processing.1,2 Cognitive impairment is usually evident at the time of a first psychotic episode3,4 and persists throughout the longitudinal course of illness. The magnitude of cognitive impairment is on average 2 standard deviations below the healthy control mean,5,6 and the severity of impairment is more predictive of functional outcomes than are positive and negative symptoms.2 Cognitive impairment in this population has been shown to be consistently associated with various aspects of functioning, including reduced employment, limited social functioning, and poor quality of life. Such impairment is the chief predictor of long-term disability.7,8
The severity of cognitive impairment and its role as one of the primary drivers of functional disability in schizophrenia define it as one of society’s largest unmet medical needs. Yet, no medications are approved to treat cognitive impairment in schizophrenia. Any treatment that can improve cognition in these participants would have a substantial impact on the treatment of schizophrenia.
MIN-101 is a novel cyclic amido derivative with specific affinities for sigma-2, 5-hydroxytryptamine-2A (5-HT2A), and α1-adrenergic receptors. MIN-101 exhibits low affinity for other receptors including dopaminergic, muscarinic, cholinergic, and histaminergic receptors. In vivo functional studies have established that MIN-101 is an antagonist at both 5-HT2A and sigma-2 receptors.9 By contrast, MIN-101 increased dopamine (DA) turnover and the production of DA metabolites such as dihydroxyphenyl acetic acid and homovanillic acid, as well as slightly elevated prolactin, only at high dose levels, suggesting it has a weak antagonistic effect at the DA D2 receptor (data on file, Minerva Neurosciences, 2015).9
This study examines the effects of MIN-101 versus placebo on cognitive function as measured by the Brief Assessment of Cognition in Schizophrenia (BACS). As this compound lacks the detrimental anticholinergic and antihistaminergic effects associated with other medications that might worsen cognitive performance in people with severe mental illness, it would be expected that the potential beneficial effects on cognition induced by 5-HT2A antagonism and sigma antagonism would not be disrupted by other aspects of the compound’s mechanism of action. Further, given that some medications that have failed in schizophrenia (eg, guanfacine)10 have been reported to enhance cognition in individuals with schizotypal personality disorder who have never received D2 blocking antipsychotic medications,11 it has been suggested that concurrent D2 blockade may be partially responsible for the high rates of failure of previous attempts at pharmacologic cognitive enhancement in schizophrenia.12 Hence, a drug like MIN-101 given as monotherapy might have significant advantages for cognitive functioning.
METHODS
Study Design and Setting
The BACS was utilized as a secondary outcome measure in a 12-week, phase 2b, prospective, randomized, double-blind, placebo-controlled, parallel-group trial that compared the effect of MIN-101 versus placebo in 244 subjects selected for the presence of negative symptoms of schizophrenia (EU Clinical Trials Register identifier: 2014-004878-42). Between May 2015 and December 2015, participants were randomized in equal groups to receive daily doses of MIN-101 32 mg, MIN-101 64 mg, or placebo, administered as monotherapy, at 32 sites in Russia and 5 European countries. All 3 cohorts were balanced with respect to demographic and baseline disease characteristics. The study achieved its primary endpoint, demonstrating a statistically significant benefit of MIN-101 over placebo in improving negative symptoms, as measured by the Positive and Negative Syndrome Scale (PANSS).13 The effect was observed for both the 32-mg and 64-mg doses of MIN-101 (P ≤ .022 and P ≤ .003, respectively).9 All participants provided written informed consent, and the study was approved by the relevant ethics committees and regulatory authorities at each site and was conducted per Good Clinical Practice guidelines.

- Cognitive dysfunction is a core feature of schizophrenia and is stable throughout the course of the illness. There are no FDA-approved medications for the treatment of cognitive impairment in schizophrenia.
- For patients with negative symptoms, a compound with affinities for sigma-2 and 5-HT2A receptors and no direct dopamine affinities is a viable consideration to treat cognitive deficits.
Participants
All participants were 18-60 years of age and had been diagnosed with schizophrenia based on the DSM-5 criteria as established with the Mini-International Neuropsychiatric Interview.14 Participants were included if they met all of the following criteria: (1) stability of positive and negative symptoms of schizophrenia over the last 3 months according to treating psychiatrists; (2) manifestation of negative symptoms of schizophrenia over the last 3 months according to treating psychiatrists; (3) a PANSS score ≥ 20 on the negative symptoms subscale; (4) scores of < 4 on each of the following PANSS items: excitement, hyperactivity, hostility, suspiciousness, uncooperativeness, and poor impulse control (a score of < 4 on these items was selected in order to maintain study power and reduce the dropout rate, which is generally around 40% in schizophrenia trials; although suspiciousness and hostility were included, active social avoidance was not included as it can also be a manifestation of negative symptoms and hence a target of treatment); and (5) extensive metabolism for P450 CYP2D6, as determined by genotyping test before the first drug dose was administered. The exclusion criteria were (1) comorbid serious physical disorder, (2) active suicidal ideation, (3) history of attempted suicide, (4) history of significant drug or alcohol abuse, and (5) pregnancy.
All psychotropic drugs were discontinued 5 days before baseline. For the duration of the study, subjects could take anticholinergic agents for new extrapyramidal symptoms, continue drugs for concomitant medical conditions if they started taking them before the enrollment in the study, and receive rescue medication for insomnia and agitation. Administration of antipsychotics was not allowed during the study.
Outcome Measures
Cognitive performance was assessed with the BACS,15,16 which was administered to all subjects at baseline, week 4, and week 12. The BACS is a tool for measuring cognitive function in participants with schizophrenia that consists of 6 domains: verbal memory (list learning), working memory (digit sequencing task), motor speed (token motor task), verbal fluency (category instances, letter fluency), attention and processing speed (symbol coding), and executive function (Tower of London test).15 Using the extensive normative data collected on the BACS,16 the primary outcome measures were standardized into age- and gender-adjusted z scores. To calculate cognitive subscale scores, all raw scores were first converted to z scores. The PANSS was also administered at each visit. We also converted the z scores to T scores as per standard conventional guidelines.17 A T score of 50 on each scale indicates average functioning for the normal population of the same age range and gender, and every 10 points represents 1 standard deviation (SD). Like z scores, T scores are also a conversion of individual scores into a standard form.
Statistical Analysis
The analysis was performed based on an intent-to-treat (ITT) population. To ensure group comparability, baseline clinical characteristics were tested by t tests or Pearson χ2 tests as appropriate. Two models were tested. For Model 1: least squares mean, standard error, and P value from a mixed-model repeated measures (MMRM) with treatment (placebo, MIN-101 32 mg, MIN-101 64 mg), visit, pooled study center, and treatment-by-visit interaction terms as fixed effects, subject nested in treatment as a random effect with baseline value as covariate, was performed. For Model 2: least squares mean, standard error, and P value from an MMRM with treatment (placebo, MIN-101 Total), visit, pooled study center, and treatment-by-visit interaction terms as fixed effects, subject nested in treatment as a random effect with baseline value as covariate, was performed. An unstructured covariance matrix was used for both models. The Hochberg procedure was applied to maintain the type I error rate due to multiple comparisons of the results at or below 0.050%. Pearson correlation coefficients were calculated to study the correlation between the BACS change from baseline and the PANSS negative factor score change from baseline.
RESULTS
Demographic and Clinical Characteristics
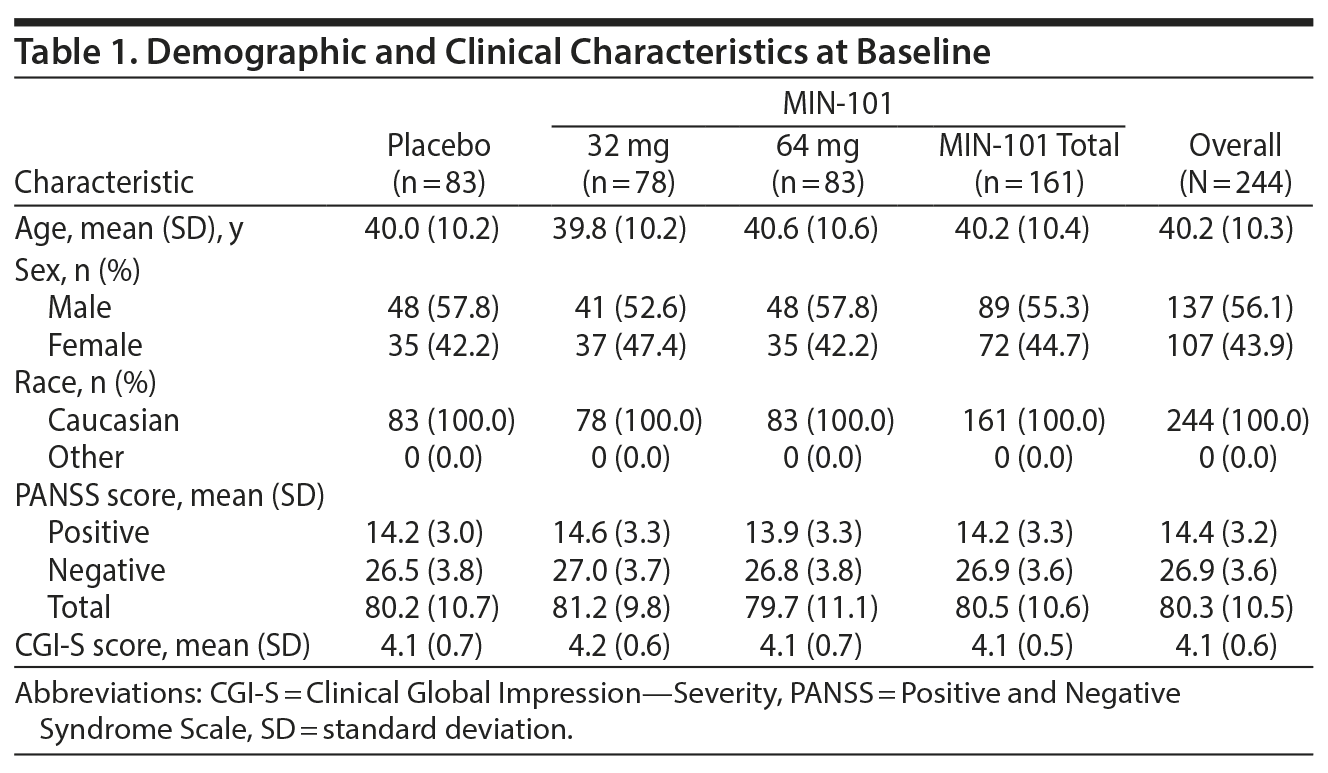
Participants were allocated into the 3 groups (placebo group, n = 83; MIN-101 32 mg group, n = 78; MIN-101 64 mg group, n = 83) at the start of the study. The clinical and demographic characteristics of the sample are shown in Table 1. At baseline, the 3 groups did not differ significantly in age, sex, PANSS positive score, PANSS negative score, PANSS total score, or Clinical Global Impression-Severity18 score (P > .05).
Cognitive Function
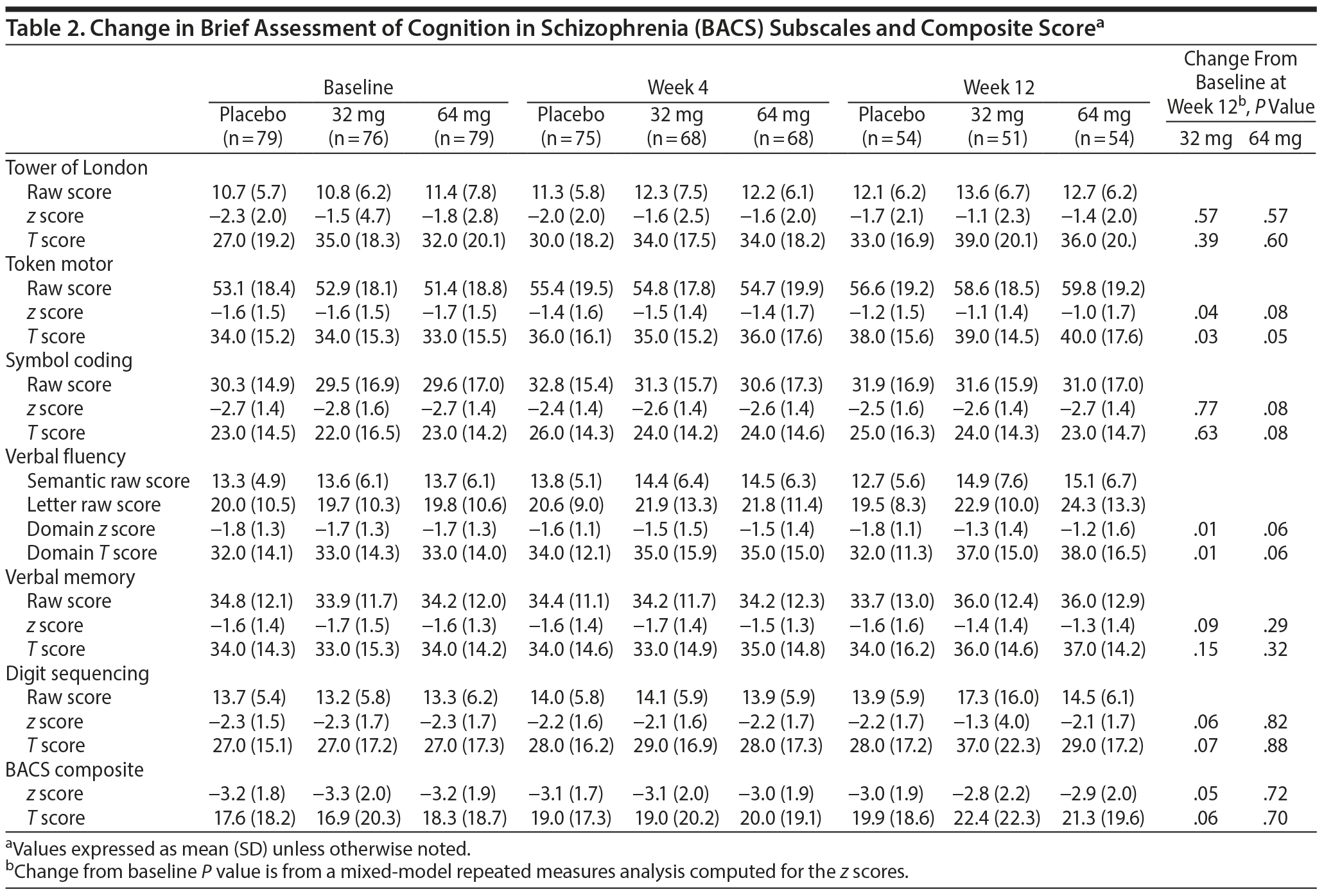
Of the enrolled participants, 79 (95.2%) from the placebo group, 76 (97.4%) from the MIN-101 32 mg group, and 79 (95.2%) from the MIN-101 64 mg group completed the BACS at baseline. The groups did not demonstrate baseline differences in the BACS composite score or individual test scores. In the analysis of the changes in the BACS z scores and T scores from baseline to the study endpoint (week 12), the token motor test (z score distribution, P = .04 and T score distribution, P = .03; 95% CI for z score, 0.02 to 0.75; Table 2), verbal fluency (z score distribution, P = .01 and T score distribution, P = .01; 95% CI for z score, 0.13 to 0.76; Table 2), and BACS composite z scores (z score distribution P = .05; 95% CI, −0.00 to 0.71; Table 2) showed statistically significant improvement in the MIN-101 32 mg group compared to the placebo group. The T score for the BACS composite did not show statistically significant improvements (P = .06). There were no significant intergroup differences in the changes in the other BACS domains.
Correlations With Negative Symptoms
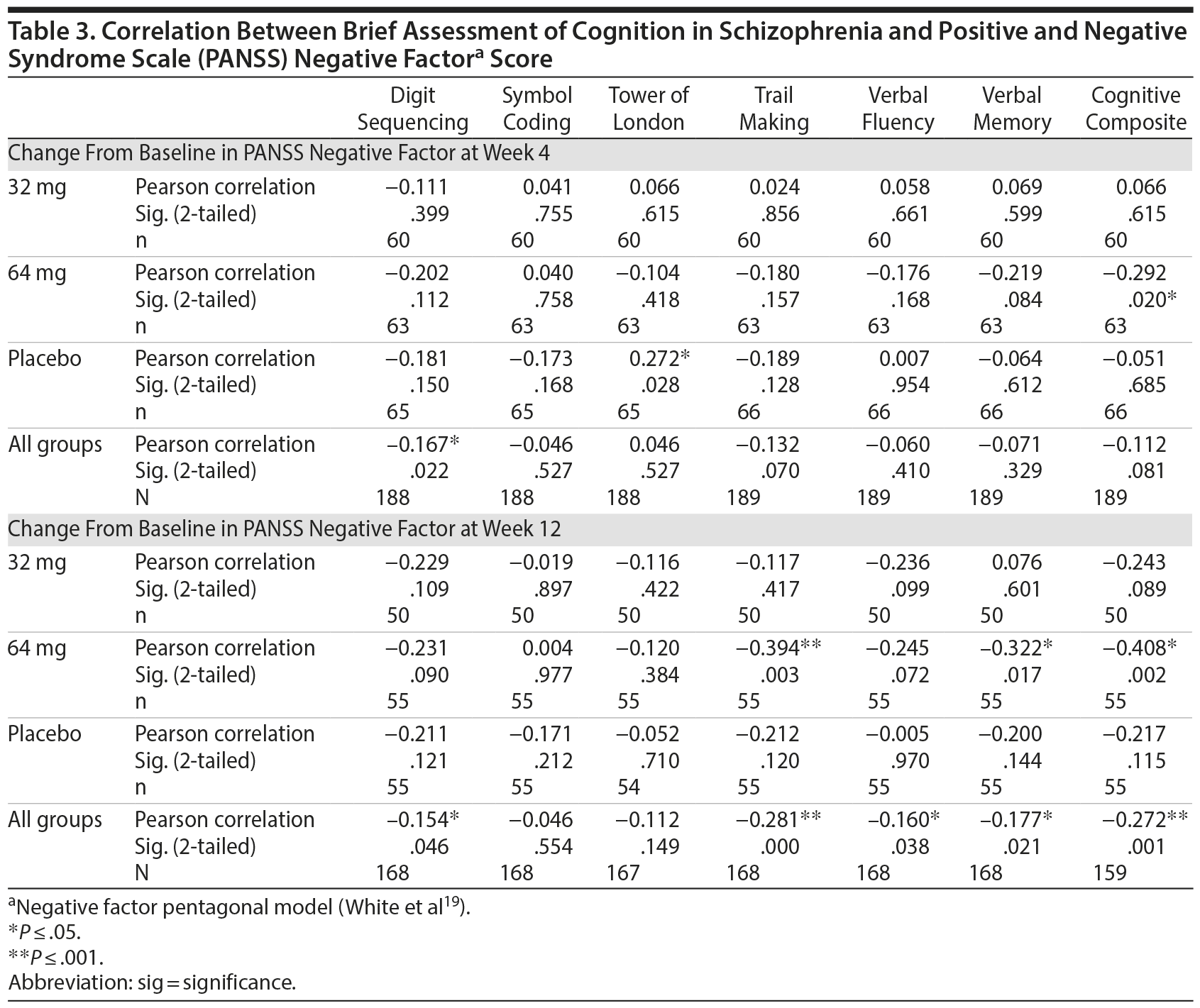
Our analyses of the relationship between treatment-related changes in cognition and negative symptoms are presented in Table 3, which illustrates the correlations between change from baseline in the BACS measures and change from baseline in negative symptoms (PANSS negative factor score).
At week 4, the clinical improvements from baseline in the PANSS negative factor were significantly correlated with improvements from baseline on the BACS cognitive composite in the 64 mg group (Pearson r = −0.292, P = .020). The only other significant change observed at week 4 was in the placebo group, where the change from baseline in PANSS negative factor was significantly correlated with change from baseline in the Tower of London task (measuring executive functioning; Pearson r = 0.272, P = .028).
At week 12, improvement in the PANSS negative factor again was significantly correlated with improvements in the BACS cognitive composite (Pearson r = −0.408, P = .002), as well as the Trail Making task (measuring attention and processing speed, Pearson r = −0.394, P = .003) and verbal memory (Pearson r = −0.322, P = .017), for the 64 mg group.
No significant correlations were observed for the 32 mg group at week 4 or week 12.
DISCUSSION
The results of this study suggest a possible benefit of MIN-101 on cognitive performance in individuals with stable schizophrenia symptoms and the concurrent presence of moderate to severe negative symptoms. The novel pharmacologic profile of MIN-101 suggests that it acts as an antagonist at both 5-HT2A and sigma-2 receptors, which might explain the effect. Composite z scores on the BACS were significantly improved compared to placebo on the 32 mg dose. This improvement does not appear be pseudospecific, as improvements in negative symptoms did not covary with these changes. Two tests of processing speed showed significant improvement, with trend-level improvements in verbal memory and verbal working memory for both z score change and T score change. Treatment with the 64-mg dose did not separate from placebo on the composite score or any subscales and in general was associated with lower levels of improvement across all of the individual BACS domains other than symbol coding, a measure of processing speed and executive functioning. Interestingly, in this group, changes in cognitive test performance were correlated with improvements in negative symptoms, although the overall cognitive changes were not significant.
The effect size for improvement on the composite score was 0.5 SD for the 32-mg dose and 0.2 SD for placebo. Thus, the effect size for improvement with the 32-mg dose was about 0.3 SD over placebo. This amount of improvement is about halfway between what Cohen considered a "small" and "medium" effect size. While no single magnitude of effect can be considered "clinically meaningful,"20 Cohen described a 0.5 SD effect size as "visible to the naked eye." Given the distributional properties of change in this study, about 40% of participants demonstrate an improvement of 0.5 or greater, suggesting that a substantial minority of participants experienced benefit that was clinically meaningful.
To understand which aspects of cognition may have driven the overall improvement in participants receiving the 32 mg dose, we investigated the impact of treatment on individual test scores. Two additional cognitive domains manifested an improvement in performance, the token motor test and a test of verbal fluency. Both tests require the ability to perform speeded processing. The improvements on these specific tests (along with a trend level improvement on symbol coding in the 64-mg dose group) suggest that MIN-101 may improve the ability to process information, complete simple tasks, and express knowledge. These skills are essential for important functional activities such as employment. Processing speed has been suggested to be one of the most important domains for functional outcomes in participants with schizophrenia.21
Positive psychotic symptoms may fluctuate during the illness, but negative symptoms and cognitive dysfunction are relatively constant. Conventional antipsychotics treat the positive symptoms of schizophrenia but have little effect on primary negative symptoms and cognition. Our results showed that improvement in the PANSS negative factor was significantly correlated with improvements in the BACS cognitive composite, and the main study9 showed no change in positive symptoms as assessed by the PANSS; however, it should be noted that the baseline PANSS positive symptom score was low, with a mean score of 14.3 (SD = 3.3) for the combined groups. Therefore, little improvement in positive symptoms was expected, as patients can be classified as stable with moderately high negative symptoms (mean = 26.9, SD = 3.7).9 Additionally, the mean total score for all participants of 80.4 (SD = 10.5) was primarily driven by the high negative symptoms scores (mean = 26.9, SD = 3.7) and general psychopathology (mean = 39.3, SD = 6.9) rather than acute psychosis; hence, deterioration of the participants in the placebo arm was not observed after 12 weeks.
The study has several limitations. No information is available yet as to why a lower dose of MIN-101 would be associated with reduced changes in cognitive performance. When compared to the dose-related effects of MIN-101 on negative symptoms, the cognitive performance results are divergent and will require additional research. Also, this is a post hoc analysis of a phase 2 trial.9 It is not clear if the same results would be found in participants with a different symptom profile or in participants receiving this medication as add-on therapy.
CONCLUSIONS
This study is important for several reasons. First, there is a desperate need for the development of novel schizophrenia treatments with innovative mechanisms of action that target clinically significant negative symptoms and cognitive impairment. All currently available drugs for monotherapy of schizophrenia bind to the dopamine D2 receptor, and an agent that is meant to block both 5-HT2A and sigma-2 receptors represents a potentially important new strategy. Second, very few studies have demonstrated meaningful correlated improvements in negative symptoms and cognitive deficits resulting from pharmacotherapy. Finally, since there are no available treatments for cognitive impairment or negative symptoms in schizophrenia, the current results that MIN-101 provides benefit for both of these aspects of schizophrenia suggest the potential to alleviate 2 significant unmet needs.
Submitted: June 15, 2017; accepted November 1, 2017.
Published online: May 15, 2018.
Potential conflicts of interest: Dr Keefe currently or in the past 36 months has received investigator-initiated research funding support from the Department of Veterans Affairs, National Institute of Mental Health, and the Singapore National Medical Research Council; currently or in the past 36 months has received honoraria, served as a consultant, speaker, or advisory board member for Abbvie, Acadia, Aeglea, Akebia, Akili, Alkermes, ArmaGen, Astellas, Avanir, AviNeuro/ChemRar, Axovant, Biogen, Boehringer-Ingelheim, Cerecor, CoMentis, Critical Path Institute, FORUM, Global Medical Education (GME), GW Pharmaceuticals, Intracellular Therapeutics, Janssen, Lundbeck, Lysogene, MedScape, Merck, Minerva Neurosciences, Mitsubishi, Monteris, Moscow Research Institute of Psychiatry, Neuralstem, Neuronix, Novartis, NY State Office of Mental Health, Otsuka, Pfizer, Regenix Bio, Reviva, Roche, Sangamo, Sanofi, Sunovion, Takeda, Targacept, University of Moscow, University of Texas Southwest Medical Center, and WebMD; receives royalties from the BACS testing battery, the MATRICS Battery (BACS Symbol Coding), and the Virtual Reality Functional Capacity Assessment Tool (VRFCAT); and is a shareholder in NeuroCog Trials and Sengenix. Dr Harvey, in the past 36 months, has served as an advisor to Abbvie, Acadia, Allergan, Boehringer-Ingelheim, Forum, Genentech, Lundbeck, Otsuka Digital Health, Sanofi, Sunovion, and Takeda and has received grant support from Takeda. Dr Khan has received funding from National Institute of Mental Health and is an employee at Nathan S. Kline Institute for Psychiatric Research, Manhattan Psychiatric Center, and NeuroCog Trials. Drs Saoud and Staner are employees of PPRS Research. Drs Davidson and Luthringer are employees of Minerva Neurosciences.
Funding/support: This study was funded by Minerva Neurosciences Inc.
Role of sponsor: Minerva Neurosciences provided funding for the clinical trial. The authors were not paid to generate or review this manuscript.
Previous presentation: Poster presented at the American College of Neuropsychopharmacology annual meeting; December 4-8, 2016; Hollywood, Florida.
REFERENCES
1. Heinrichs RW, Zakzanis K. Neurocognitive deficits in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12(3):426-445. PubMed CrossRef
2. Harvey PD, Keefe RS. Cognitive impairment schizophrenia and implications of atypical neuroleptic treatment. CNS Spectr. 1997;2(8):1-11.
3. Saykin AJ, Shtasel DL, Gur RE, et al. Neuropsychological deficits in neuroleptic naive participants with first-episode schizophrenia. Arch Gen Psychiatry. 1994;51(2):124-131. PubMed CrossRef
4. Bilder RM, Goldman RS, Robinson D, et al. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am J Psychiatry. 2000;157(4):549-559. PubMed CrossRef
5. Saykin AJ, Gur RC, Gur RE, et al. Neuropsychological function in schizophrenia: selective impairment in memory and learning. Arch Gen Psychiatry. 1991;48(7):618-624. PubMed CrossRef
6. Keefe RSE, Fox KH, Harvey PD, et al. Characteristics of the MATRICS Consensus Cognitive Battery in a 29-site antipsychotic schizophrenia clinical trial. Schizophr Res. 2011;125(2-3):161-168. PubMed CrossRef
7. Mohamed S, Rosenheck R, Swartz M, et al. Relationship of cognition and psychopathology to functional impairment in schizophrenia. Am J Psychiatry. 2008;165(8):978-987. PubMed CrossRef
8. Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res. 2004;72(1):41-51. PubMed CrossRef
9. Davidson M, Saoud J, Staner C, et al. Efficacy and safety of MIN-101: a 12-week randomized, double-blind, placebo-controlled trial of a new drug in development for the treatment of negative symptoms in schizophrenia. Am J Psychiatry. 2017;174(12):1195-1202. PubMed CrossRef
10. Friedman JI, Adler DN, Temporini HD, et al. Guanfacine treatment of cognitive impairment in schizophrenia. Neuropsychopharmacology. 2001;25(3):402-409. PubMed CrossRef
11. McClure MM, Barch DM, Romero MJ, et al. The effects of guanfacine on context processing abnormalities in schizotypal personality disorder. Biol Psychiatry. 2007;61(10):1157-1160. PubMed CrossRef
12. Harvey PD, Bowie CR. Cognitive enhancement in schizophrenia: pharmacological and cognitive remediation approaches. Psychiatr Clin North Am. 2012;35(3):683-698. PubMed CrossRef
13. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276. PubMed CrossRef
14. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22-33. PubMed
15. Keefe RS, Goldberg TE, Harvey PD, et al. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68(2-3):283-297. PubMed CrossRef
16. Keefe RS, Harvey PD, Goldberg TE, et al. Norms and standardization of the Brief Assessment of Cognition in Schizophrenia (BACS). Schizophr Res. 2008;102(1-3):108-115. PubMed CrossRef
17. Lyman HB. Test Scores and What They Mean. 2nd ed. Englewood Cliffs, NJ: Prentice-Hall, Inc; 1971.
18. Guy W. ECDEU Assessment Manual for Psychopharmacology, Revised. DHEW publication no. (ADM) 76-338. Rockville, MD: US Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976:534-537.
19. White L, Harvey PD, Opler L, et al, and the PANSS Study Group. Empirical assessment of the factorial structure of clinical symptoms in schizophrenia: a multisite, multimodel evaluation of the factorial structure of the Positive and Negative Syndrome Scale. Psychopathology. 1997;30(5):263-274. PubMed CrossRef
20. Keefe RS, Kraemer HC, Epstein RS, et al. Defining a clinically meaningful effect for the design and interpretation of randomized controlled trials. Innov Clin Neurosci. 2013;10(5-6 suppl A):4S-19S. PubMed
21. Dickinson D, Ragland JD, Gold JM, et al. General and specific cognitive deficits in schizophrenia: Goliath defeats David? Biol Psychiatry. 2008;64(9):823-827. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite