Objective: To determine whether neurocognitive performance and clinical outcomes can be enhanced by a mindfulness intervention in older adults with stress disorders and cognitive complaints. To explore decreased hypothalamic-pituitary-adrenal (HPA) axis activity as a possible mechanism.
Methods: 103 adults aged 65 years or older with an anxiety or depressive disorder (diagnosed according to DSM-IV criteria) and subjective neurocognitive difficulties were recruited in St. Louis, Missouri, or San Diego, California, from September 2012 through August 2013 and randomly assigned in groups of 5-8 to mindfulness-based stress reduction (MBSR) or a health education control condition matched for time, attention, and credibility. The primary outcomes were memory (assessed by immediate and delayed paragraph and list recall) and cognitive control (Delis-Kaplan Executive Function System Verbal Fluency Test and Color Word Interference Test). Other outcomes included clinical symptoms (worry, depression, anxiety, and global improvement). HPA axis activity was assessed using peak salivary cortisol. Outcomes were measured immediately post-intervention and (for clinical outcomes only) at 3- and 6-month follow up.
Results: On the basis of intent-to-treat principles using data from all 103 participants, the mindfulness group experienced greater improvement on a memory composite score (P = .046). Groups did not differ on change in cognitive control. Participants receiving MBSR also improved more on measures of worry (P = .042) and depression (P = .049) at posttreatment and on worry (P = .02), depression (P = .002), and anxiety (P = .002) at follow-up and were more likely to be rated as much or very much improved as rated by the Clinical Global Impressions-Improvement scale (47% vs 27%, χ2 = 4.5, P = .03). Cortisol level decreased to a greater extent in the mindfulness group, but only among those participants with high baseline cortisol.
Conclusions: In this population of older adults with stress disorders and neurocognitive difficulties, a mindfulness intervention improves clinical outcomes such as excessive worry and depression and may include some forms of immediate memory performance.
Trial Registration: ClinicalTrials.gov identifier: NCT01693874
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.

Mindfulness-Based Stress Reduction for Older Adults With Stress Disorders and Neurocognitive Difficulties:
A Randomized Controlled Trial

ABSTRACT
Objective: To determine whether neurocognitive performance and clinical outcomes can be enhanced by a mindfulness intervention in older adults with stress disorders and cognitive complaints. To explore decreased hypothalamic-pituitary-adrenal (HPA) axis activity as a possible mechanism.
Methods: 103 adults aged 65 years or older with an anxiety or depressive disorder (diagnosed according to DSM-IV criteria) and subjective neurocognitive difficulties were recruited in St. Louis, Missouri, or San Diego, California, from September 2012 through August 2013 and randomly assigned in groups of 5-8 to mindfulness-based stress reduction (MBSR) or a health education control condition matched for time, attention, and credibility. The primary outcomes were memory (assessed by immediate and delayed paragraph and list recall) and cognitive control (Delis-Kaplan Executive Function System Verbal Fluency Test and Color Word Interference Test). Other outcomes included clinical symptoms (worry, depression, anxiety, and global improvement). HPA axis activity was assessed using peak salivary cortisol. Outcomes were measured immediately post-intervention and (for clinical outcomes only) at 3- and 6-month follow up.
Results: On the basis of intent-to-treat principles using data from all 103 participants, the mindfulness group experienced greater improvement on a memory composite score (P = .046). Groups did not differ on change in cognitive control. Participants receiving MBSR also improved more on measures of worry (P = .042) and depression (P = .049) at posttreatment and on worry (P = .02), depression (P = .002), and anxiety (P = .002) at follow-up and were more likely to be rated as much or very much improved as rated by the Clinical Global Impressions-Improvement scale (47% vs 27%, χ2 = 4.5, P = .03). Cortisol level decreased to a greater extent in the mindfulness group, but only among those participants with high baseline cortisol.
Conclusions: In this population of older adults with stress disorders and neurocognitive difficulties, a mindfulness intervention improves clinical outcomes such as excessive worry and depression and may include some forms of immediate memory performance.
Trial Registration: ClinicalTrials.gov identifier: NCT01693874
J Clin Psychiatry 2017;78(7):e734-e743
https://doi.org/10.4088/JCP.16m10947
© Copyright 2017 Physicians Postgraduate Press, Inc.
aVA San Diego Healthcare System, San Diego, California
bDepartment of Psychiatry, University of California, San Diego
cHealthy Mind Laboratory, Department of Psychiatry, Washington University School of Medicine, St Louis, Missouri
dJoint Doctoral Program in Clinical Psychology, San Diego State University/University of California San Diego
*Corresponding author: Julie Loebach Wetherell, PhD, UCSD Department of Psychiatry, 9500 Gilman Dr, Dept. 9111N-1, San Diego, CA 92093-9111 ([email protected]).
Neurocognitive aging may be accelerated by stress and stress-related disorders such as clinical depression and anxiety disorders.1,2 These are common disorders that contribute significantly to both suffering and neurocognitive decline in the growing demographic of elderly persons.3-6 This decline is most evident in the domains of memory and cognitive control (also called executive function).7,8 Even among nondemented older adults, declines in memory and cognitive control can impair the ability to perform meaningful daily activities.9,10 There is much current interest in early, timely intervention for neurocognitive difficulties, before they advance to the stage of major neurocognitive disorder.11
One hypothesized mechanism by which anxiety and depression may lead to neurocognitive decline in older adults involves the hypothalamic-pituitary-adrenal (HPA) axis. According to this theory, stress (manifested as worry or depression) in older people causes an exaggerated physiological response, resulting in excess cortisol production and, consequently, impaired neurocognition via cellular and synaptic changes particularly affecting the hippocampus and prefrontal cortex.12-15
This stress-induced neurobiological pathway to neurocognitive decline may be reversible.16 For example, memory and hippocampal volumes in depression and anxiety disorders appear to be dynamic, increasing with successful treatment even in older individuals.17,18 Specifically relevant to our model, HPA axis hyperactivity can be reduced by stress reduction interventions in elderly people; the resulting reduced levels of cortisol should result in neurocognitive improvements in addition to the clinical benefits.19-21
Mindfulness is a state of nonjudgmental awareness of present moment experience.22,23 Often associated with meditation, increased mindfulness appears to improve control of the neuroendocrine stress response, resulting in reduced cortisol output.24-27 Mindfulness-based stress reduction (MBSR) is a popular complementary and alternative medicine intervention that combines meditation and yoga and has been shown to reduce anxiety and depression, and possibly modify substrates of attention, in younger adults.28-34 Mindfulness-based stress reduction is taught in many community settings and is appealing to older adults; it is more widely available and potentially less stigmatizing than conventional treatments for anxiety, depression, and stress, such as cognitive-behavioral therapy. Possible benefits of MBSR include reductions in emotional distress, loneliness, and proinflammatory gene expression.35-39 Because of its focus on reducing stress and worry, MBSR could potentially correct HPA axis hyperactivity in anxious and depressed older adults, with consequent benefits for neurocognition as well as its clinical stress reduction benefits.20,40
Only a handful of preliminary investigations have explored the impact of mindfulness interventions on neurocognitive function.41,42 Among older adults with and without neurocognitive impairment, preliminary findings suggest positive effects on attention, processing speed, memory, and cognitive control.43,44 Despite the need for stress-reducing interventions acceptable to older adults and their potential benefits with respect to neurocognitive aging, only 2 studies to date to our knowledge have tested the impact of mindfulness training and practice as a cognitive enhancer for older adults. In a study45 of 14 older individuals with mild neurocognitive impairment randomized to MBSR or usual care, investigators found a trend toward improvement on cognition for the MBSR group.
In an initial study46 of MBSR in 34 older adults, we found preliminary evidence for increases in mindfulness, reductions in worry, and improvement in memory and cognitive control relative to baseline. In the present study, we conducted a single-blind, randomized controlled trial to compare this MBSR protocol to an active control condition (health education) to test the hypotheses that (1) MBSR will lead to superior neurocognitive performance in the domains of cognitive control and memory than will health education; (2) MBSR will lead to superior clinical outcomes in terms of worry, depression, anxiety, and global mental health than health education; and (3) MBSR will reduce salivary cortisol levels more than health education.

- Mood disorders and mild cognitive impairment are common and disabling among older adults.
- Mindfulness-based stress reduction is an acceptable intervention that can improve depression, anxiety, and worry, and possibly improve memory, among older adults with anxiety or depressive disorders and subjective cognitive complaints.
METHODS
Participants
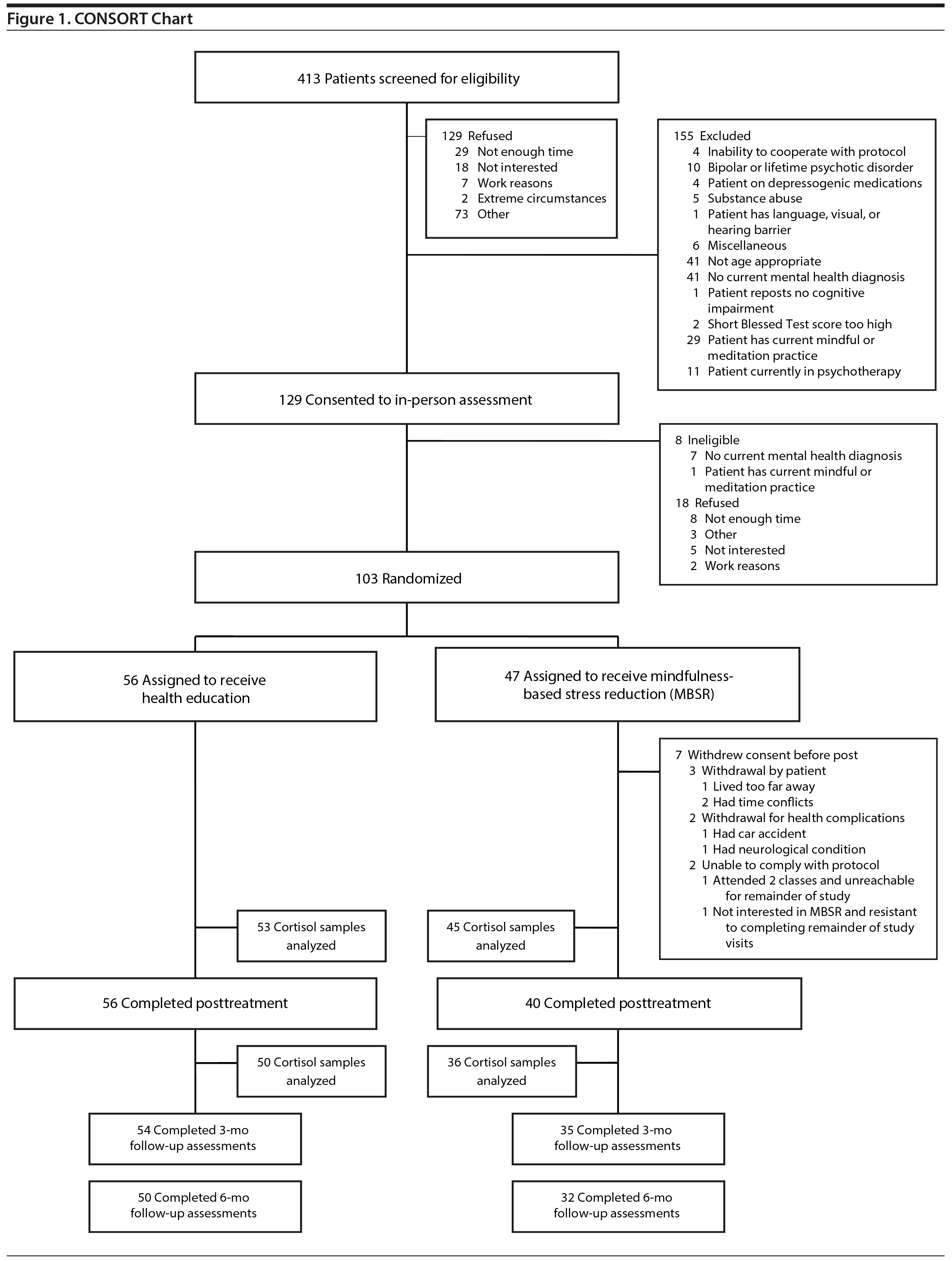
Participants were 103 adults over the age of 65 years enrolled and treated at 2 sites: St. Louis, Missouri (Washington University, n = 52), and San Diego (University of California San Diego [UCSD], n = 51). They were recruited via newspaper advertising, university registries, and word of mouth. Recruitment material described the study as a comparison of 2 interventions intended to improve neurocognitive function, and potential participants were made aware that the health education condition was based on a well-researched, empirically based program developed by researchers at Stanford University (Stanford, California) in order to reduce expectation bias. All participants gave informed consent to participate, and the study was reviewed and approved by the institutional review boards at both sites. The study was registered on ClinicalTrials.gov (identifier: NCT01693874).
Inclusion criteria included clinically significant anxiety or depressive symptoms, as defined by a Patient-Reported Outcomes Measurement Information System (PROMIS)47 Anxiety score ≥ 14 or a PROMIS Depression score ≥ 16 at screening, plus a current diagnosis of a depressive or anxiety disorder (ie, major depressive disorder, dysthymia, depressive disorder not otherwise specified [NOS], generalized anxiety disorder, panic disorder with or without agoraphobia, or anxiety disorder NOS), ascertained using the Structured Diagnostic Interview for DSM-IV Axis I Disorders.48 All participants also endorsed current subjective aging-related neurocognitive problems. Exclusion criteria included dementia, as defined by a score of ≥ 10 errors on the Short Blessed Test49; a chart diagnosis of dementia; or prescription of cognitive-enhancing medication such as donepezil. Other exclusion criteria included alcohol or substance use disorders within the past 6 months, current or lifetime psychotic or bipolar disorder, current participation in psychotherapy or regular engagement in mindfulness practice or yoga, corticoid steroid use, and serious medical illness that would prevent study participation or accurate data collection (eg, congestive heart failure, oxygen dependent). Individuals currently taking antidepressants or anxiolytics were eligible if they had been on a stable daily dose for at least a month prior to enrollment and agreed to remain stable throughout the intervention. A CONSORT chart illustrating the patient flow is shown in Figure 1.
Assessments
The primary outcomes for this study were memory and cognitive control (executive function). Memory was assessed by immediate and delayed paragraph and list recall. Tests of list and paragraph learning and recall are commonly used to assess verbal memory function.50,51 The specific paragraph recall test used in the present study52 was based on the Wechsler Memory Scale-Revised Logical Memory Test53; in it, subjects hear short narratives containing 44 bits of information and are tested on immediate and delayed (30 minutes) verbatim recall of paragraph bits. Participants were also tested on immediate and 30-minute delayed recall of a 16-word list similar to that in the Rey Auditory Verbal Learning Test, which has been used extensively by the Washington University Alzheimer’s Disease Research Center.54
Cognitive control was assessed by the Delis-Kaplan Executive Function System (DKEFS) Verbal Fluency Test and the DKEFS Color Word Interference Test.55,56 In these tests, respondents are required to retrieve words, select them according to specific rules, and inhibit incorrect responses, all of which are assumed to draw on executive function. In the DKEFS Verbal Fluency Test, the subject is required to generate as many words as possible in 1 minute that (1) begin with a given letter (eg, F; letter or phonemic fluency), (2) fall into a particular category (eg, "animals"; category or semantic fluency), and (3) fall into 1 of 2 alternating categories (category switching). The score is the number of unique correct words in each condition.
The DKEFS Color Word Interference Test, similar to other Stroop tests, requires respondents to identify colors (color naming), read a series of color words (word reading), and then identify the ink color of color words that do not match the ink (eg, the word "blue" printed in red ink; interference). The DKEFS Color Word Interference Test differs from other Stroop tests in that it also includes an inhibition and switching trial in which half the words appear in boxes. Words without boxes are treated as in the interference condition (ie, identifying the ink color), whereas words in boxes are treated as in the word reading condition (ie, the actual color word is read). The test is scored on the basis of the amount of time required to complete each task.
In order to reduce the number of measures analyzed, memory and cognitive control composite scores were created by averaging Z scores for each measure. We also assessed concerns about memory using the PROMIS47 Cognitive Concerns scale. In addition to the memory and cognitive control tests, participants were administered the Wechsler Test of Adult Reading (WTAR)57 to assess for premorbid neurocognitive function. To control for Hawthorne effects, we also assessed attention using the Digit Span subtest from the Repeatable Battery for the Assessment of Neuropsychological Status58 and motor function using the Grooved Pegboard Test.59 We did not expect these variables to change in response to either intervention.
Other outcomes included the various components of our theoretical model; specifically, chronic worry as assessed by the Penn State Worry Questionnaire-Abbreviated (PSWQ),60 depression and anxiety as measured by the PROMIS scales,47 and mindfulness as assessed by the Cognitive and Affective Mindfulness Scale-Revised.61 All assessments were performed by blind raters who also made an overall assessment of each participant’s progress using the Clinical Global Impressions-Improvement scale (CGI-I).62
Saliva was collected for cortisol from participants at pre- and post-intervention according to published techniques.63 In brief, we used Salivette assays to gather saliva for cortisol on 3 consecutive days at waking, 30 minutes after waking, and bedtime. These procedures have been shown to be reliable in other research, correlate with serum cortisol (salimetrics.com), and are consistent with our laboratory’s methods, described elsewhere.60 Saliva was assayed for cortisol level using standard procedures (Salimetrics, LLC, State College, Pennsylvania). Peak daily cortisol was computed from the higher of the 2 (waking or wake + 30 minutes) values on each day, and the median value of the 3 days was used. Peak cortisol was used because previous research suggests that peak cortisol levels are most strongly associated both with cognitive function20,64 and with worry severity.65
Interventions
Participants were randomized in groups of 5-8 people to either MBSR or health education. Randomization, based on block sequence, was generated and held by the study statistician, who had no contact with participants or raters. After each group of participants completed their baseline assessments, the statistician provided information on randomization to the site study coordinator, who notified the appropriate group instructor. Mindfulness-based stress reduction was conducted according to the protocol developed by Jon Kabat-Zinn, PhD,66 and colleagues at the University of Massachusetts, Boston, and included 8 sessions of meditation and light yoga, as modified in our pilot study to reduce risk of injury to older patients, along with a half-day meditation retreat. The San Diego site instructor was an associate professor of psychiatry and director of the UCSD Center for Mindfulness. He supervised the 2 St Louis MBSR instructors by watching videotapes and holding weekly supervision conference calls. All instructors had training and credentials in relevant health professions (OT, MSW, PsyD), had long-standing personal meditation practices, and had served as MBSR instructors for at least 4 years (range, 4-10 years).
Health education was based on the Stanford health care self-management book by Kate Lorig and colleagues,67 with references to relaxation and meditation strategies removed. The program was 8 sessions and covered topics including understanding and managing common conditions and symptoms, healthy eating, managing medications, and communicating with health care providers. Delivery of health education was similar to the MBSR condition (once weekly, group-delivered, approximately 90 minutes). The instructor in San Diego was an associate professor of psychiatry who served as Health Behavior Coordinator at the VA San Diego Healthcare System. She trained and supervised the health education instructor in St Louis, an MSW with experience in working with older adults with chronic medical conditions.
Participants in both groups received manuals and had between-session assignments. The conditions did not differ on credibility as measured by a version of Borkovec’s Credibility scale68 administered after the first group session (t = −1.3, P = .19).
Data Analysis
SAS 9.4 (SAS Institute Inc) was used for all statistical analyses. To create the memory domain score, Z scores were calculated to replace all the raw data (number of items/bits of information recalled) for each of the 4 memory measures (immediate paragraph recall, immediate list recall, delayed paragraph recall, and delayed list recall). The Z scores adjust for the scale differences among the 4 individual measures so that they could be combined. These Z scores were averaged to produce a single total memory composite variable for each individual at each time point. A similar procedure was used to create the cognitive control variable using the raw verbal fluency score and 2 variables from the DKEFS Color-Word Interference Test (the difference between the Color-Word condition and the Word condition, and the difference between the Color-Word condition and the average of the Word and Color conditions).
Our primary analyses used analysis of covariance models to test the effects of the categorical variable treatment group on each continuous dependent variable, controlling for the effects of the covariates: baseline score on the variable, WTAR score, and site (St Louis or San Diego). Analysis of covariance was used because it had greater statistical power than alternatives, by virtue of reducing within-group variance. Initially the models also included as covariates age, ethnicity, Cumulative Illness Rating Scale for Geriatrics69 (medical burden), and use of selective serotonin reuptake inhibitor antidepressant medications, all of which varied across treatment condition or across site. To avoid overfitting the model, a stepwise variable selection method was employed. Only baseline score, condition, WTAR, and site were retained in the models. Including other variables in the analyses did not appreciably change the results.
Missingness was tested via Little MCAR (Missing Completely At Random) test in IBM SPSS Statistics 23 using the Missing Value Analysis add-on module. Expectation maximization was used as the estimation method. For the Little test, χ2 = 15.7, P = .61; the nonsignificant result suggested that the data may be assumed to be MCAR.
Multiple imputation with 20 imputations using the SAS procedure PROC MI was used as the missing value treatment. Missing data are a common issue and, rather than deal with the matter in an ad hoc fashion, the well-documented method of multiple imputation was selected. Multiple imputation has statistical advantages over more traditional methods such as hot-deck imputation and maximum likelihood-based imputation. Listwise deletion involves discarding observations, which may introduce bias or affect the representativeness of results. Multiple imputation preserves all observations by replacing missing data with an estimated value based on other available information. In order to deal with the problem of increased noise due to single imputation, multiple imputation averages the outcomes across multiple imputed data sets.
The SAS procedure PROC MIANALYZE was used to roll up the regression models from the 20 imputations. A P value for treatment group less than .05 indicated that there was a statistically significant difference in change over time between the treatment groups, adjusting for the covariates.
Mixed effects models were employed to analyze the data for the PSWQ, PROMIS depression, PROMIS anxiety, and cognitive concerns over an extended time course to include the 3-month and 6-month assessments.
RESULTS
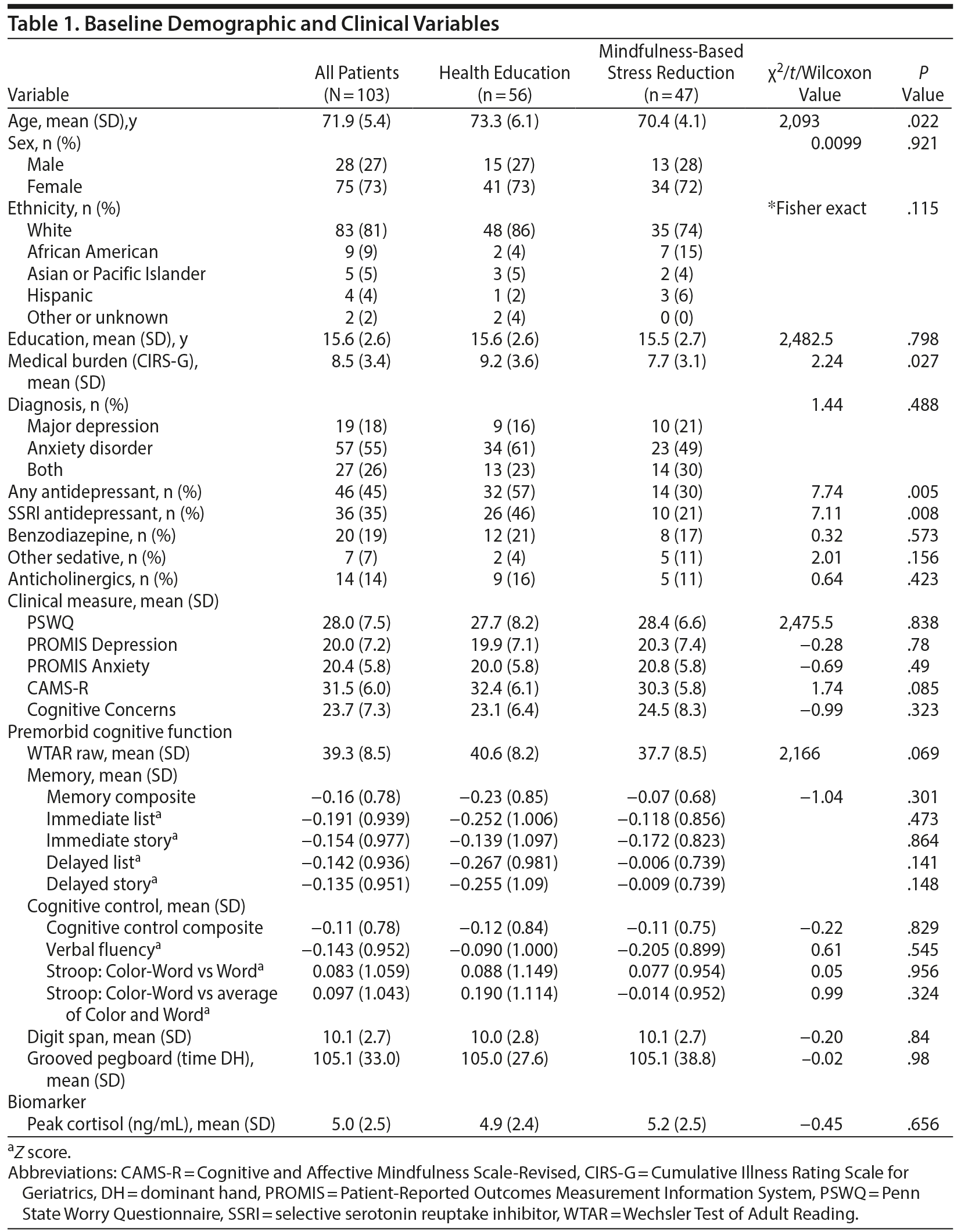
Descriptive information for the sample appears in Table 1. Despite randomization, the treatment groups differed on age (health education > MBSR), medical burden (health education > MBSR), and current treatment with antidepressants (health education > MBSR). Baseline values for outcome variables are also displayed in Table 1. There were no significant differences between the treatment conditions at baseline on any clinical or neurocognitive variable, including the individual neurocognitive tests that made up the composites (eg, paragraph recall, DKEFS Stroop test; results not shown).
The 2 groups also did not differ in their attendance at classes, with MBSR participants attending a mean of 7.3 sessions (SD = 2.8) and health education participants attending a mean of 7.6 sessions (SD = 2.5, t = 0.6, P = .56).
Neurocognitive Outcomes
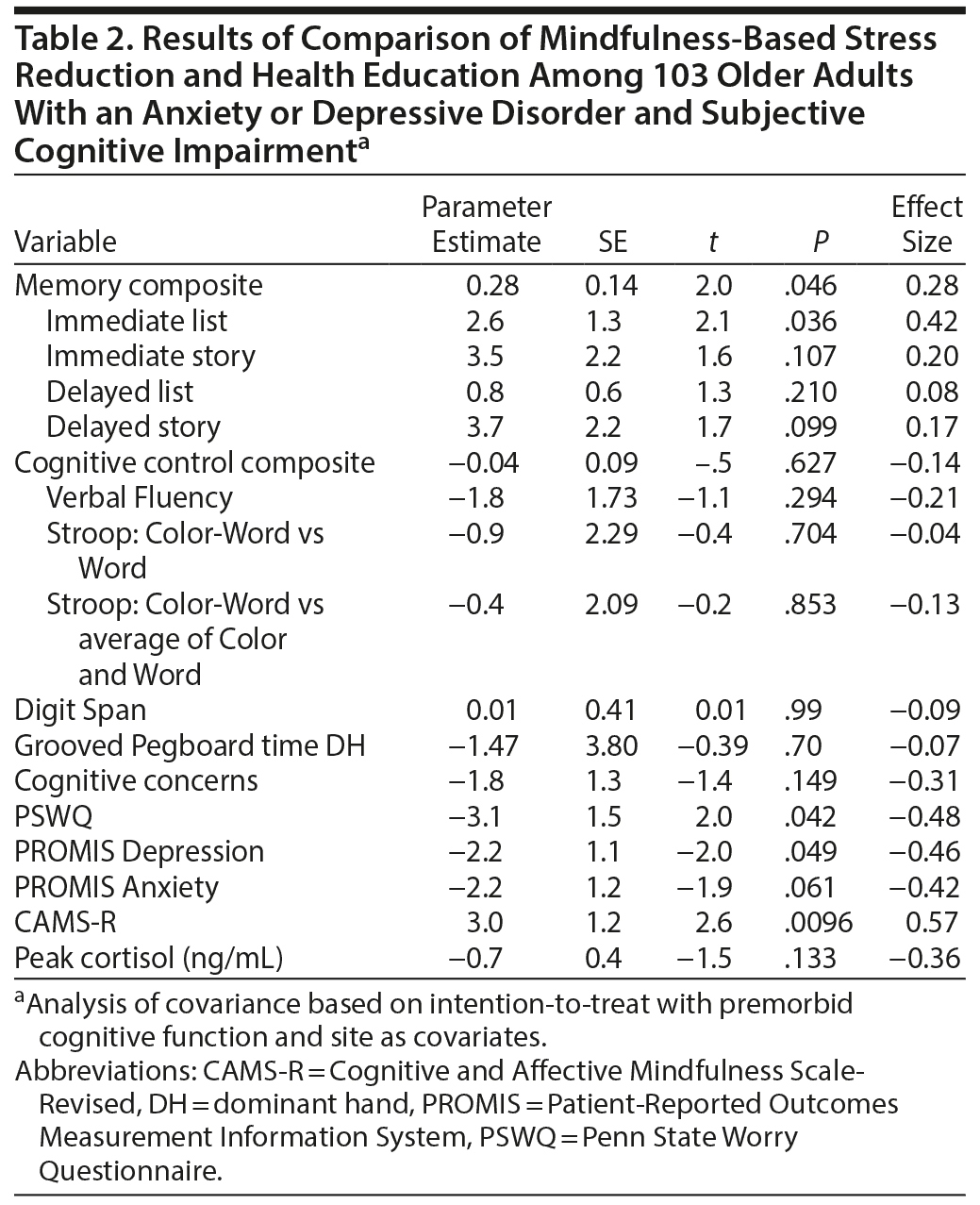
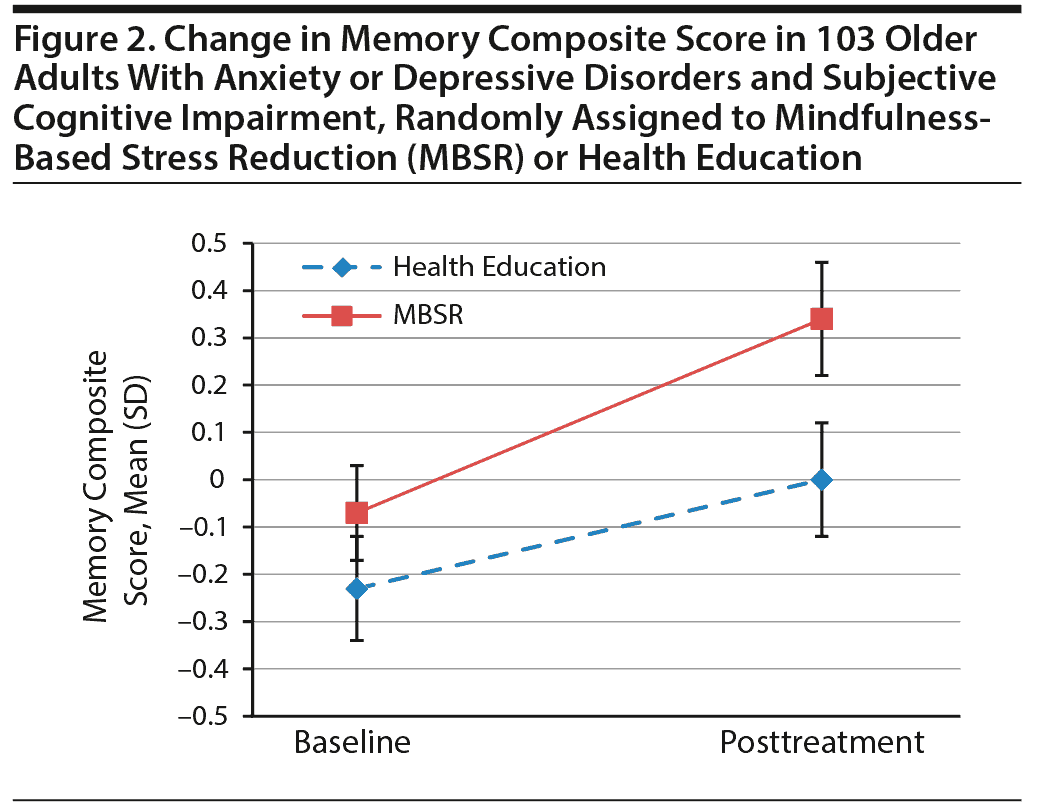
Results for change over time are presented in Table 2. Consistent with our hypotheses, MBSR participants improved significantly more in their memory composite score (Figure 2) than did health education participants. This finding was driven by the scores on Immediate List Recall (Table 2). We did not find significant differences in changes in the cognitive control composite score or any of the other individual cognitive tests (Table 2) between the intervention groups. We also did not find significant differences in Digit Span or Grooved Pegboard, suggesting that memory results were not driven by nonspecific performance improvements. Participants in both groups improved equivalently in their subjective perceptions of neurocognitive performance, suggesting that the memory results were not driven by participants’ self-perceptions of improvement.
Clinical Outcomes
Lending support to our theoretical model, MBSR participants reported more reduction in pathological worry than did health education participants. They also reported larger improvements in mindfulness and depression at posttreatment. Overall, 47% of the MBSR participants were rated by blind raters as much improved or very much improved on the CGI-I, compared with 27% of the health education participants, following treatment (χ21 = 4.5, P = .03).
We collected data on PSWQ and PROMIS Depression, Anxiety, and Cognitive Concerns at 3- and 6-month follow-up time points. The results for time by condition were significant for PSWQ (F = 2.86, P = .02), Depression (F = 3.87, P = .002), and Anxiety (F = 3.96, P = .002), but not for Cognitive Concerns (F = 1.42, P = .22).
Cortisol Results
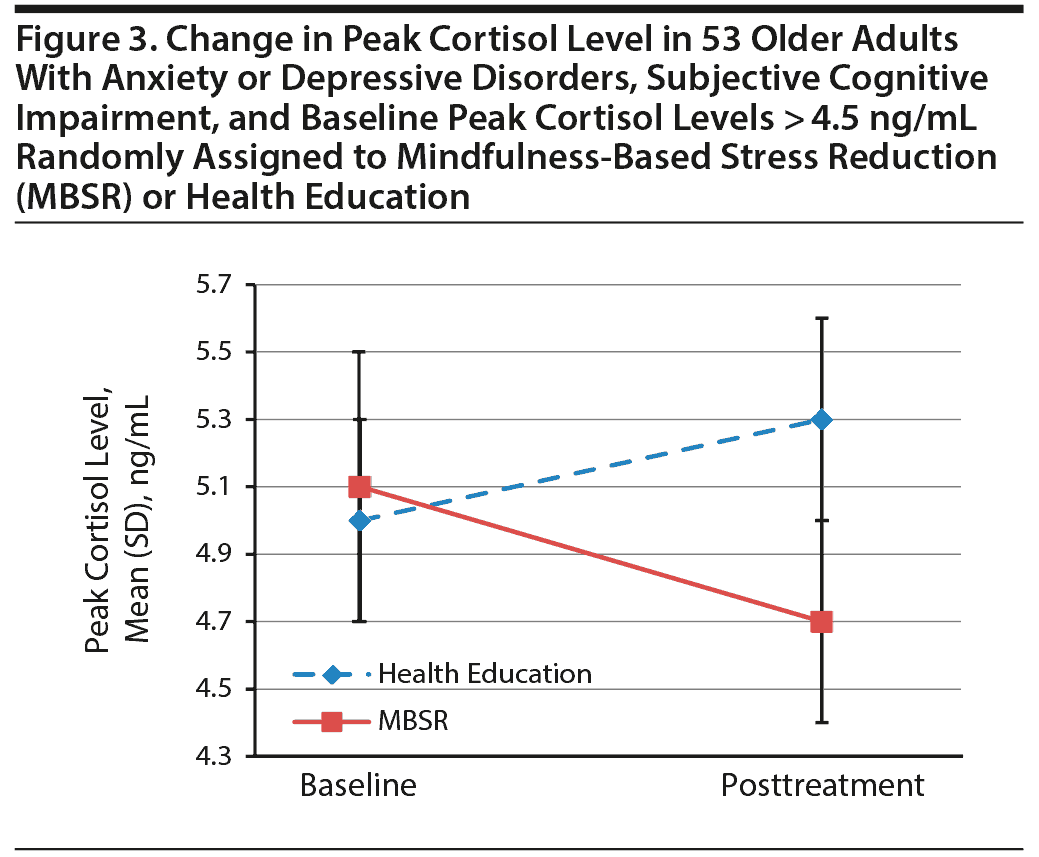
There were no overall between-group differences in reduction in peak cortisol (Table 2). We then used a median split to examine change in participants with high and low baseline cortisol levels separately. Fifty participants were classified as having low baseline cortisol, defined as levels at or below 4.5 ng/mL; of those, 28 were randomized to health education and 22 to MBSR. Forty-eight participants were classified as having high baseline cortisol (over 4.5 ng/mL). Of these, 25 were randomized to health education and 23 to MBSR. Five participants had missing values for baseline cortisol levels. We found no differences in demographics, mood, or cognitive measures between those with high and low baseline cortisol levels. Among those with higher baseline levels of cortisol, however, cortisol decreased significantly in the MBSR group (paired t = 3.8, P = .0015) but not in the health education group (paired t = 1.9, P = .07; Figure 3).
Among the participants who received MBSR, most responded to questionnaires at 3 (n = 35) and 6 (n = 36) months regarding their continued use of mindfulness practice. Every participant reported continuing to engage in at least some mindfulness practice on a weekly basis. The most commonly maintained strategies were mindful breathing (86%), informal mindfulness (86%), and formal meditation (78%). The least commonly maintained strategies were body scan (53%) and yoga (58%). Yoga was also identified as the least favorite technique by 50% of respondents. Mindful breathing was identified as the most favorite technique by 33% of responders.
DISCUSSION
In this randomized comparison of MBSR to an active health education control condition among older adults with anxiety or depressive disorders and subjective neurocognitive difficulties, we found greater improvements in memory, worry, depression, and global clinical improvement associated with MBSR. We also found a decrease in cortisol with MBSR relative to health education among those with high baseline levels. This finding is consistent with other observations about MBSR’s cortisol-reducing properties.
Furthermore, older adults were willing and able to participate in mindfulness activities. Not only was adherence to between-session assignments high, but all participants reported continuing to engage in mindfulness practice 6 months after the termination of their intervention. As well, those randomized to MBSR continued to have a superior clinical outcome in terms of worry severity, depression, and anxiety at 3 and 6 months post-intervention compared to those who received health education. This self-maintained mindfulness practice and sustained clinical improvement, as well as the wide availability of MBSR, bode well for its use to improve the stress-related health of the large and growing population of older adults.
Contrary to expectations, we did not find differences in cognitive control. This may be due to the fact that paper-and-pencil measures of cognitive control are not sufficiently sensitive among older people without identified neurocognitive impairment to detect change over a relatively short period.
It should be noted that some degree of improvement was also experienced by the health education group. This may have been due to a number of factors, including possibly greater self-efficacy resulting from participating in a credible alternative intervention, or practice effects associated with the neurocognitive tests. However, even in this context, we found more improvement in immediate memory associated with MBSR relative to health education.
Limitations of the study include the fact that although eligible participants reported subjective neurocognitive problems, we did not restrict enrollment to those with documented neurocognitive impairment. Attrition was higher among the MBSR participants, although all but 1 who dropped out cited reasons that had nothing to do with the intervention (eg, car accident, significant health event). Because of the group randomization and fluctuations in recruitment, fewer individuals were randomized to MBSR than to health education, which may have had implications for power. The cognitive tests were administered via paper and pencil rather than on a computer, which may have limited their sensitivity.
The study’s strengths included a sample of older adults with diagnosed anxiety or depressive disorders, as opposed to nondistressed volunteers often recruited for studies of MBSR, and a broad range of outcome measures, including neurocognitive function, worry, anxiety, depression, and global clinical status, and a biological marker of HPA axis activity. Results suggest that MBSR shows promise as an intervention for anxious or depressed older adults reporting subjective neurocognitive complaints.
Submitted: May 16, 2016; accepted October 31, 2016.
Published online: July 5, 2017.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, no investigational information about pharmaceutical agents that is outside US Food and Drug Administration-approved labeling has been presented in this article.
Financial disclosure: Dr Lenze receives funding from Lundbeck and Roche. Drs Wetherell, Hershey, Hickman, Tate, and Dixon and Ms Bower have no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
Funding/support: National Center for Complementary and Integrative Health R34 AT007064 (Dr Lenze) and R34 AT007070 (Dr Wetherell); Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448.
Role of the sponsor: The sponsor, the National Institutes of Health, had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCES
1. Peavy GM, Salmon DP, Jacobson MW, et al. Effects of chronic stress on memory decline in cognitively normal and mildly impaired older adults. Am J Psychiatry. 2009;166(12):1384-1391. PubMed doi:10.1176/appi.ajp.2009.09040461
2. Wilson RS, Begeny CT, Boyle PA, et al. Vulnerability to stress, anxiety, and development of dementia in old age. Am J Geriatr Psychiatry. 2011;19(4):327-334. PubMed doi:10.1097/JGP.0b013e31820119da
3. Wilson RS, Capuano AW, Boyle PA, et al. Clinical-pathologic study of depressive symptoms and cognitive decline in old age. Neurology. 2014;83(8):702-709. PubMed doi:10.1212/WNL.0000000000000715
4. Zeki Al Hazzouri A, Vittinghoff E, Byers A, et al. Long-term cumulative depressive symptom burden and risk of cognitive decline and dementia among very old women. J Gerontol A Biol Sci Med Sci. 2014;69(5):595-601. PubMed doi:10.1093/gerona/glt139
5. Beaudreau SA, O’ Hara R. The association of anxiety and depressive symptoms with cognitive performance in community-dwelling older adults. Psychol Aging. 2009;24(2):507-512. PubMed doi:10.1037/a0016035
6. Beaudreau SA, O’ Hara R. Late-life anxiety and cognitive impairment: a review. Am J Geriatr Psychiatry. 2008;16(10):790-803. PubMed doi:10.1097/JGP.0b013e31817945c3
7. Beats BC, Sahakian BJ, Levy R. Cognitive performance in tests sensitive to frontal lobe dysfunction in the elderly depressed. Psychol Med. 1996;26(3):591-603. PubMed doi:10.1017/S0033291700035662
8. Schuitevoerder S, Rosen JW, Twamley EW, et al. A meta-analysis of cognitive functioning in older adults with PTSD. J Anxiety Disord. 2013;27(6):550-558. PubMed doi:10.1016/j.janxdis.2013.01.001
9. Rog LA, Park LQ, Harvey DJ, et al. The independent contributions of cognitive impairment and neuropsychiatric symptoms to everyday function in older adults. Clin Neuropsychol. 2014;28(2):215-236. PubMed doi:10.1080/13854046.2013.876101
10. Rodakowski J, Skidmore ER, Reynolds CF 3rd, et al. Can performance on daily activities discriminate between older adults with normal cognitive function and those with mild cognitive impairment? J Am Geriatr Soc. 2014;62(7):1347-1352. PubMed doi:10.1111/jgs.12878
11. Kozauer N, Katz R. Regulatory innovation and drug development for early-stage Alzheimer’s disease. N Engl J Med. 2013;368(13):1169-1171. PubMed doi:10.1056/NEJMp1302513
12. Lupien SJ, Maheu F, Tu M, et al. The effects of stress and stress hormones on human cognition: implications for the field of brain and cognition. Brain Cogn. 2007;65(3):209-237. PubMed doi:10.1016/j.bandc.2007.02.007
13. Venero C, D×az-Mardomingo C, Pereda-Pérez I, et al. Increased morning salivary cortisol levels in older adults with nonamnestic and multidomain mild cognitive impairment. Psychoneuroendocrinology. 2013;38(4):488-498. PubMed doi:10.1016/j.psyneuen.2012.07.007
14. Franz CE, O’ Brien RC, Hauger RL, et al. Cross-sectional and 35-year longitudinal assessment of salivary cortisol and cognitive functioning: the Vietnam Era twin study of aging. Psychoneuroendocrinology. 2011;36(7):1040-1052. PubMed doi:10.1016/j.psyneuen.2011.01.002
15. Sotiropoulos I, Catania C, Pinto LG, et al. Stress acts cumulatively to precipitate Alzheimer’s disease-like tau pathology and cognitive deficits. J Neurosci. 2011;31(21):7840-7847. PubMed doi:10.1523/JNEUROSCI.0730-11.2011
16. Barch DM, DʼAngelo G, Pieper C, et al. Cognitive improvement following treatment in late-life depression: relationship to vascular risk and age of onset. Am J Geriatr Psychiatry. 2012;20(8):682-690. PubMed doi:10.1097/JGP.0b013e318246b6cb
17. Hou Z, Yuan Y, Zhang Z, et al. Longitudinal changes in hippocampal volumes and cognition in remitted geriatric depressive disorder. Behav Brain Res. 2012;227(1):30-35. PubMed doi:10.1016/j.bbr.2011.10.025
18. Frodl T, Jפger M, Smajstrlova I, et al. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. J Psychiatry Neurosci. 2008;33(5):423-430. PubMed
19. Lenze EJ, Mantella RC, Shi P, et al. Elevated cortisol in older adults with generalized anxiety disorder is reduced by treatment: a placebo-controlled evaluation of escitalopram. Am J Geriatr Psychiatry. 2011;19(5):482-490. PubMed doi:10.1097/JGP.0b013e3181ec806c
20. Lenze EJ, Dixon D, Mantella RC, et al. Treatment-related alteration of cortisol predicts change in neuropsychological function during acute treatment of late-life anxiety disorder. Int J Geriatr Psychiatry. 2012;27(5):454-462. PubMed doi:10.1002/gps.2732
21. Rosnick CB, Wetherell JL, White KS, et al. Cognitive-behavioral therapy augmentation of SSRI reduces cortisol levels in older adults with generalized anxiety disorder: a randomized clinical trial. J Consult Clin Psychol. 2016;84(4):345-352. PubMed doi:10.1037/a0040113
22. Bishop SR. What do we really know about mindfulness-based stress reduction? Psychosom Med. 2002;64(1):71-83. PubMed doi:10.1097/00006842-200201000-00010
23. Shapiro SL, Carlson LE, Astin JA, et al. Mechanisms of mindfulness. J Clin Psychol. 2006;62(3):373-386. PubMed doi:10.1002/jclp.20237
24. Bergen-Cico D, Possemato K, Pigeon W. Reductions in cortisol associated with primary care brief mindfulness program for veterans with PTSD. Med Care. 2014;52(suppl 5):S25-S31. PubMed doi:10.1097/MLR.0000000000000224
25. Carlson LE, Speca M, Patel KD, et al. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress and levels of cortisol, dehydroepiandrosterone sulfate (DHEAS) and melatonin in breast and prostate cancer outpatients. Psychoneuroendocrinology. 2004;29(4):448-474. PubMed doi:10.1016/S0306-4530(03)00054-4
26. Matousek RH, Dobkin PL, Pruessner J. Cortisol as a marker for improvement in mindfulness-based stress reduction. Complement Ther Clin Pract. 2010;16(1):13-19. PubMed doi:10.1016/j.ctcp.2009.06.004
27. Nykl×Äek I, Mommersteeg PM, Van Beugen S, et al. Mindfulness-based stress reduction and physiological activity during acute stress: a randomized controlled trial. Health Psychol. 2013;32(10):1110-1113. PubMed doi:10.1037/a0032200
28. Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen Hosp Psychiatry. 1982;4(1):33-47. PubMed doi:10.1016/0163-8343(82)90026-3
29. Kabat-Zinn J, Massion AO, Kristeller J, et al. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. Am J Psychiatry. 1992;149(7):936-943. PubMed doi:10.1176/ajp.149.7.936
30. V׸llestad J, Sivertsen B, Nielsen GH. Mindfulness-based stress reduction for patients with anxiety disorders: evaluation in a randomized controlled trial. Behav Res Ther. 2011;49(4):281-288. PubMed doi:10.1016/j.brat.2011.01.007
31. Serpa JG, Taylor SL, Tillisch K. Mindfulness-based stress reduction (MBSR) reduces anxiety, depression, and suicidal ideation in veterans. Med Care. 2014;52(suppl 5):S19-S24. PubMed doi:10.1097/MLR.0000000000000202
32. Marchand WR. Mindfulness meditation practices as adjunctive treatments for psychiatric disorders. Psychiatr Clin North Am. 2013;36(1):141-152. PubMed doi:10.1016/j.psc.2013.01.002
33. Klainin-Yobas P, Cho MA, Creedy D. Efficacy of mindfulness-based interventions on depressive symptoms among people with mental disorders: a meta-analysis. Int J Nurs Stud. 2012;49(1):109-121. PubMed doi:10.1016/j.ijnurstu.2011.08.014
34. Bohlmeijer E, Prenger R, Taal E, et al. The effects of mindfulness-based stress reduction therapy on mental health of adults with a chronic medical disease: a meta-analysis. J Psychosom Res. 2010;68(6):539-544. PubMed doi:10.1016/j.jpsychores.2009.10.005
35. Morone NE, Greco CM, Weiner DK. Mindfulness meditation for the treatment of chronic low back pain in older adults: a randomized controlled pilot study. Pain. 2008;134(3):310-319. PubMed doi:10.1016/j.pain.2007.04.038
36. Szanton SL, Wenzel J, Connolly AB, et al. Examining mindfulness-based stress reduction: perceptions from minority older adults residing in a low-income housing facility. BMC Complement Altern Med. 2011;11:44. PubMed doi:10.1186/1472-6882-11-44
37. Young LA, Baime MJ. Mindfulness-based stress reduction: effect on emotional distress in older adults. Complement Health Pract Rev. 2010;15:59-64. doi:10.1177/1533210110387687
38. Moynihan JA, Chapman BP, Klorman R, et al. Mindfulness-based stress reduction for older adults: effects on executive function, frontal alpha asymmetry and immune function. Neuropsychobiology. 2013;68(1):34-43. PubMed doi:10.1159/000350949
39. Creswell JD, Irwin MR, Burklund LJ, et al. Mindfulness-based stress reduction training reduces loneliness and pro-inflammatory gene expression in older adults: a small randomized controlled trial. Brain Behav Immun. 2012;26(7):1095-1101. PubMed doi:10.1016/j.bbi.2012.07.006
40. Mayer JL, Klumpers L, Maslam S, et al. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalises the corticosterone-induced reduction of adult hippocampal neurogenesis. J Neuroendocrinol. 2006;18(8):629-631. PubMed doi:10.1111/j.1365-2826.2006.01455.x
41. Jha AP, Krompinger J, Baime MJ. Mindfulness training modifies subsystems of attention. Cogn Affect Behav Neurosci. 2007;7(2):109-119. PubMed doi:10.3758/CABN.7.2.109
42. Chiesa A, Calati R, Serretti A. Does mindfulness training improve cognitive abilities? a systematic review of neuropsychological findings. Clin Psychol Rev. 2011;31(3):449-464. PubMed doi:10.1016/j.cpr.2010.11.003
43. Gard T, Hölzel BK, Lazar SW. The potential effects of meditation on age-related cognitive decline: a systematic review. Ann N Y Acad Sci. 2014;1307:89-103. PubMed doi:10.1111/nyas.12348
44. Larouche E, Hudon C, Goulet S. Potential benefits of mindfulness-based interventions in mild cognitive impairment and Alzheimer’s disease: an interdisciplinary perspective. Behav Brain Res. 2015;276:199-212. PubMed doi:10.1016/j.bbr.2014.05.058
45. Wells RE, Kerr CE, Wolkin J, et al. Meditation for adults with mild cognitive impairment: a pilot randomized trial. J Am Geriatr Soc. 2013;61(4):642-645. PubMed doi:10.1111/jgs.12179
46. Lenze EJ, Hickman S, Hershey T, et al. Mindfulness-based stress reduction for older adults with worry symptoms and co-occurring cognitive dysfunction. Int J Geriatr Psychiatry. 2014;29(10):991-1000. PubMed doi:10.1002/gps.4086
47. Gershon RC, Cella D, Fox NA, et al. Assessment of neurological and behavioural function: the NIH Toolbox. Lancet Neurol. 2010;9(2):138-139. PubMed doi:10.1016/S1474-4422(09)70335-7
48. First M, Spitzer R, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID). Washington DC: American Psychiatric Press; 1997.
49. Katzman R, Brown T, Fuld P, et al. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry. 1983;140(6):734-739. PubMed doi:10.1176/ajp.140.6.734
50. Squire LR. Memory and Brain. New York, NY: Oxford University Press; 1987.
51. Storandt M, Botwinick J, Danziger WL, et al. Psychometric differentiation of mild senile dementia of the Alzheimer type. Arch Neurol. 1984;41(5):497-499. PubMed doi:10.1001/archneur.1984.04050170043013
52. Newcomer JW, Craft S, Hershey T, et al. Glucocorticoid-induced impairment in declarative memory performance in adult humans. J Neurosci. 1994;14(4):2047-2053. PubMed
53. Wechsler D. Wechsler Memory Scale-Revised. New York, NY: Harcourt Brace Jovanovich; 1987.
54. Newcomer JW, Selke G, Melson AK, et al. Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Arch Gen Psychiatry. 1999;56(6):527-533. PubMed doi:10.1001/archpsyc.56.6.527
55. Delis DC, Kaplan E, Kramer JH. Delis Kaplan Executive Function System (D-KEFS). San Antonio, TX: The Psychological Corporation; 2001.
56. Delis DC, Kramer JH, Kaplan E, et al. Reliability and validity of the Delis-Kaplan Executive Function System: an update. J Int Neuropsychol Soc. 2004;10(2):301-303. PubMed doi:10.1017/S1355617704102191
57. Holdnack HA. Wechsler Test of Adult Reading: WTAR. San Antonio, TX: The Psychological Corporation; 2001.
58. Randolph C, Tierney MC, Mohr E, et al. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310-319. PubMed doi:10.1076/jcen.20.3.310.823
59. Matthews CG, Klove H. Instruction Manual for the Adult Neuropsychology Test Battery. Madison, WI: University of Wisconsin Medical School; 1964.
60. Hopko DR, Stanley MA, Reas DL, et al. Assessing worry in older adults: confirmatory factor analysis of the Penn State Worry Questionnaire and psychometric properties of an abbreviated model. Psychol Assess. 2003;15(2):173-183. PubMed doi:10.1037/1040-3590.15.2.173
61. Feldman G, Hayes A, Kumar S, et al. Mindfulness and emotion regulation: the development and initial validation of the Cognitive and Affective Mindfulness Scale-Revised (CMS-R). J Psychopathol Behav Assess. 2007;29:177-190. doi:10.1007/s10862-006-9035-8
62. Guy W. ECDEU Assessment Manual for Psychopharmacology. Revised Edition. Dept Health Education and Welfare publication (ADM) 76-338. Rockville, MD: National Institute of Mental Health; 1976:218-222.
63. Pruessner JC, Wolf OT, Hellhammer DH, et al. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61(26):2539-2549. PubMed doi:10.1016/S0024-3205(97)01008-4
64. Mantella RC, Butters MA, Amico JA, et al. Salivary cortisol is associated with diagnosis and severity of late-life generalized anxiety disorder. Psychoneuroendocrinology. 2008;33(6):773-781. PubMed doi:10.1016/j.psyneuen.2008.03.002
65. Wetherell JL, Petkus AJ, White KS, et al. Antidepressant medication augmented with cognitive-behavioral therapy for generalized anxiety disorder in older adults. Am J Psychiatry. 2013;170(7):782-789. PubMed doi:10.1176/appi.ajp.2013.12081104
66. Stahl B, Goldstein E. A Mindfulness-Based Stress Reduction Workbook. Oakland, CA: New Harbinger Press; 2010.
67. Lorig K, Holman H, Sobel D, et al. Living a Healthy Life With Chronic Conditions: Self-Management of Heart Disease, Arthritis, Diabetes, Depression, Asthma, Bronchitis, Emphysema and Other Physical And Mental Health Conditions. 4th ed. Boulder, Colorado: Bull Publishing; 2012.
68. Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry. 2000;31(2):73-86. PubMed doi:10.1016/S0005-7916(00)00012-4
69. Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41(3):237-248. PubMed doi:10.1016/0165-1781(92)90005-N
This PDF is free for all visitors!
Save
Cite