ABSTRACT
Major depressive disorder (MDD) and bipolar depression (BD) can often be difficult to treat. N-acetylcysteine (NAC) is a nutraceutical product that has been trialed in a large number of neuropsychiatric and medical disorders, with mixed results. Many randomized controlled trials (RCTs) have studied NAC augmentation as an intervention in MDD and BD. These RCTs were pooled in 2 recent meta-analyses. One meta-analysis with 7 RCTs (pooled N = 728) conducted in patients with MDD or BD found that NAC was not superior to placebo in the attenuation of depression ratings in either main or sensitivity analyses. The other meta-analysis with 6 RCTs (pooled N = 248) conducted in patients with BD found a small, imprecise effect size for NAC (standardized mean difference, 0.45; 95% confidence interval, 0.06–0.84). The advantage for NAC in this meta-analysis would almost certainly have been lost had the authors excluded from analysis 2 RCTs, both of which had problematic characteristics and findings and both of which also obtained a large and statistically significant advantage for NAC. At present, therefore, evidence does not encourage the use of NAC as an augmentation treatment for patients with MDD or BD. It remains to be seen whether NAC augmentation benefits depressed subpopulations, such as those with higher levels of inflammatory biomarkers at baseline.
J Clin Psychiatry 2021;82(1):21f13891
To cite: Andrade C. N-acetylcysteine augmentation for patients with major depressive disorder and bipolar depression. J Clin Psychiatry. 2021;82(1):21f13891.
To share: https://doi.org/10.4088/JCP.21f13891
© Copyright 2021 Physicians Postgraduate Press, Inc.
Many patients with depression do not respond to or remit with antidepressant drugs. For example, in the Sequenced Treatment Alternatives to Relieve Depression trial, the overall remission rates in patients with major depressive disorder (MDD) were 28%, 25%, 18%, and 10% for pharmacologic treatments at steps 1, 2, 3, and 4, respectively.1 Bipolar depression (BD), a condition for which very few drugs have been approved, can be harder to treat; in 36 placebo-controlled trials (pooled N = 9,485), the pooled (crude) response rates were 54% vs 39% for drug vs placebo.2 Poor outcomes with pharmacologic interventions have resulted in the study of a large number of augmentation agents for both MDD and BD.3–5 N-acetylcysteine (NAC) is one such augmentation agent that has been examined for MDD and BD in randomized controlled trials (RCTs).
NAC is a nutraceutical. It has been trialed as monotherapy or augmentation therapy for a number of clinical indications, including obsessive-compulsive disorder,6 trichotillomania,7 Tourette syndrome,8 autism,9 acetaminophen (paracetamol) overdose,10 cystic fibrosis,11 chronic bronchitis,12 and other conditions,13–15 though not necessarily with favorable results. This article examines 2 recent meta-analyses of NAC in MDD and BD.
What NAC Does in the Brain
NAC is a precursor of the endogenous antioxidant, glutathione. NAC has been shown to modulate glutamatergic and dopaminergic signaling in the central nervous system (CNS). It improves mitochondrial functioning, dampens inflammatory mechanisms in the CNS, and may have neuroprotective action. It is hypothesized that certain of these actions may correct or compensate for the CNS disturbances that underlie depression and other neuropsychiatric disorders.13,15
Meta-Analysis: NAC for Major Depressive Disorder and Bipolar Depression
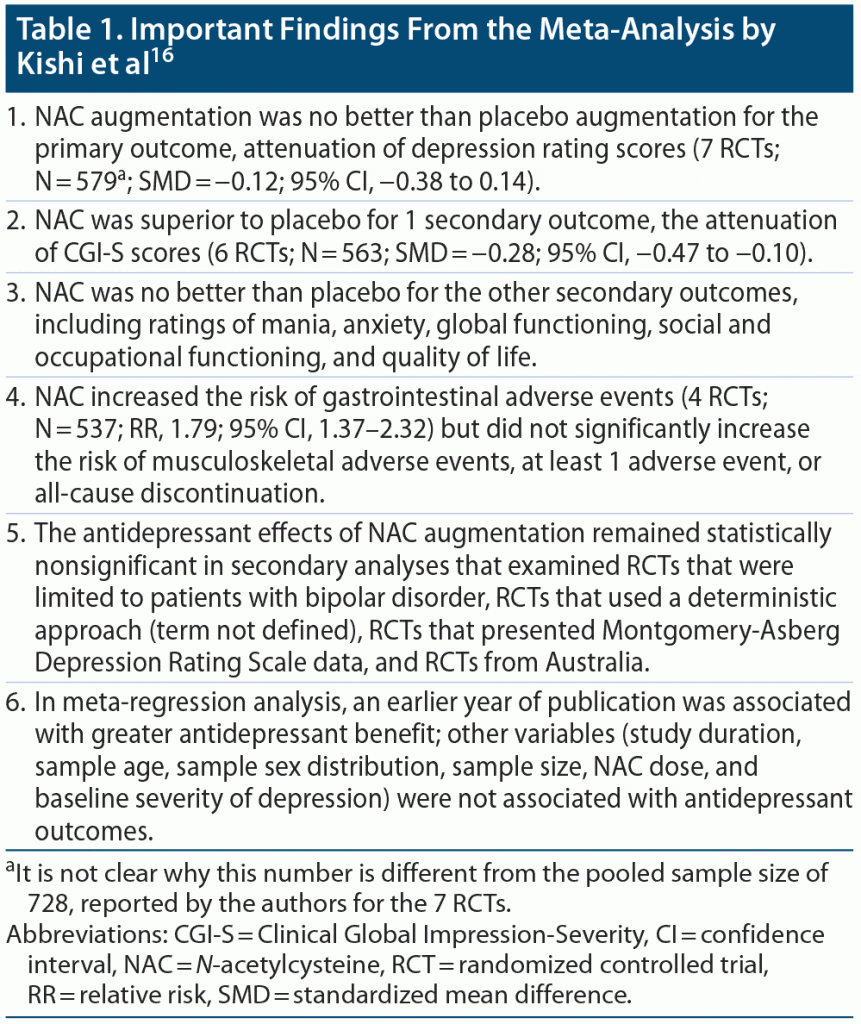
Kishi et al16 described a PRISMA-compliant systematic review and meta-analysis of double-blind, placebo-controlled RCTs of NAC augmentation in patients with MDD and BD. These authors searched electronic databases, including clinical trial registries, as well as reference lists of retrieved publications, and identified 7 RCTs (pooled Nc=c728) that were at least 8 weeks in duration. These RCTs were conducted in Australia, Brazil, Denmark, and the US and were published between 2008 and 2019. Four of the 7 RCTs had been conducted by a single team in Australia.
The mean age of the pooled sample was 46.8 years. The pooled sample was 58.5% female. One study recruited only patients with MDD; 1 recruited patients with MDD and BD; the rest recruited only patients with BD. Almost all studies recruited only or mostly outpatients.
There were 350 patients who had been treated with NAC and 378 who were treated with placebo. At baseline, patients had moderate to severe depression in 3 studies, mild to moderate depression in 3 studies, and very mild depression in 1 study. NAC was dosed at 1–3 g/d (median, 2 g/d). All patients were also receiving other medications, such as antidepressants, atypical antipsychotics, and/or mood stabilizers. The studies ranged from 8 to 24 weeks in duration (median, 16 weeks). Almost all studies were rated at low risk of bias.
Important findings from the meta-analysis are presented in Table 1. In summary, NAC augmentation was no better than placebo augmentation for the primary outcome, attenuation of depression ratings. NAC improved 1 secondary outcome, Clinical Global Impression-Severity, but was no better than placebo for the other secondary outcomes, including ratings of anxiety and mania, ratings of social, occupational, and global functioning, and ratings of quality of life. NAC increased the risk of gastrointestinal but not musculoskeletal adverse effects. NAC did not increase all-cause discontinuation. NAC did not show antidepressant benefit in any of the sensitivity analysis.
Meta-Analysis: NAC for Bipolar Depression
Nery et al17 presented a systematic review and meta-analysis of RCTs of NAC augmentation in patients with BD. These authors identified 6 RCTs (duration, 10–24 weeks), in which 125 patients received NAC (dose, 1–3 g/d) and 123 received placebo. Four of these RCTs were the same as those included by Kishi et al,16 who found no advantage for NAC in a sensitivity analysis of RCTs that specifically addressed a BD sample. Nery et al,17 however, omitted a maintenance therapy RCT18 that Kishi et al16 had perhaps inadvertently included and included an RCT that was published in Chinese.19 An additional strength of the meta-analysis by Nery et al17 is that they wrote to the original authors and obtained data specific to bipolar depression in 2 RCTs that included mixed samples.
Nery et al17 found that NAC augmentation was superior to placebo augmentation (6 RCTs; N = 248; standardized mean difference [SMD], 0.45; 95% confidence interval [CI], 0.06–0.84); heterogeneity was moderate (I2 = 49%). In this meta-analysis, the 10-week Chinese RCT19 (n = 50) with a per-protocol (rather than intent-to-treat) analysis had the largest effect size (SMD, 0.98) and was also the only RCT to be rated with high risk of bias. This study19 was also associated with extraordinarily good results: Hamilton Depression Rating Scale (HDRS) scores fell from a mean of about 26 at baseline to a mean of 1.4 with NAC and 4.1 with placebo. When RCTs were excluded in a leave-one-study-out sensitivity analysis, the advantage for NAC over placebo, as well as the value for heterogeneity, decreased considerably only with the exclusion of the Chinese RCT19 (SMD, 0.27; 95% CI, 0.03–0.58; I2 = 8%); interestingly, whereas the 95% CI, here, indicates statistical significance, Nery et al17 reported the result as nonsignificant (P = .08).
In meta-regression analysis, mean dose of NAC, study duration, and mean baseline depression ratings did not significantly influence antidepressant outcomes.
Critical Comments
There were curiosities in the meta-analysis by Kishi et al.16 For example, the authors did not present a single forest plot in either the main paper or the supplementary data. This makes it hard for the reader to understand which RCTs contributed to or detracted from the statistical significance of the summary measures. Additionally, there were possible errors in the information presented in the table that summarized the characteristics of the RCTs included in the meta-analysis. For example, NAC was stated to outperform placebo in 2 RCTs.20,21 However, in one of these RCTs,20 NAC was actually no better than placebo for the primary outcome and outperformed placebo only in a subset of patients with greater severity of depression at baseline, and in the other RCT,21 NAC outperformed placebo only in patients with higher levels of high sensitivity C-reactive protein at baseline. It is not clear what data Kishi et al16 extracted from these 2 RCTs because the forest plots were not shown; in any case, had an error been made in data extraction, the results obtained with the correct data would be “even more statistically nonsignificant” than the results presented in the paper. Thus, the conclusions of the meta-analysis would not change. As a final and important limitation, Kishi et al16 inappropriately included a maintenance therapy RCT18 along with the acute phase RCTs.
In the Nery et al17 meta-analysis, concerns have already been expressed (in the previous section) over the inclusion of 1 problematic RCT19 with a very large and statistically significant outcome. The only other study21 with a large and statistically significant outcome (SMD, 0.78; 95% CI, 0.03–1.54) was atypical in that mean HDRS scores at baseline were very low: just 11.7 in the NAC group and 9.1 in the placebo group. Both groups in this RCT therefore required very little nudging to drop below 8 and into what would generally be considered as remission. Unfortunately, Nery et al17 did not present a sensitivity analysis that omitted both of these atypical studies. Finally, in this meta-analysis,17 the only other study22 with a large effect size (SMD, 0.95; 95% CI, -0.02 to 1.93) narrowly failed to reach statistical significance in favor of NAC. Readers may also note that all 3 RCTs19,21,22 with outcomes favoring NAC had wide CIs, indicating substantial imprecision.
Concluding Notes
One meta-analysis16 found NAC augmentation ineffective in MDD and BD. Another meta-analysis17 found NAC augmentation superior to placebo augmentation in BD, but the advantage was almost certainly dependent on 2 substantially atypical RCTs. It remains to be seen whether there are special subpopulations in which NAC augmentation is useful; possibilities include depressed patients who are more severely ill at baseline20 or depressed patients with higher levels of inflammatory markers at baseline.21 It is also unclear to what extent duration of illness and treatment-refractoriness at baseline moderate the benefits of NAC, if any. Until more evidence becomes available, a conservative take-home message is that current evidence does not support the use of NAC as an augmentation treatment in patients with MDD or BD.
Published online: February 18, 2021.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
References (22)

- Huynh NN, McIntyre RS. What are the implications of the STAR*D trial for primary care? a review and synthesis. Prim Care Companion J Clin Psychiatry. 2008;10(2):91–96. PubMed CrossRef NLM
- Baldessarini RJ, Vázquez GH, Tondo L. Bipolar depression: a major unsolved challenge. Int J Bipolar Disord. 2020;8(1):1. PubMed CrossRef NLM
- Zhou X, Ravindran AV, Qin B, et al. Comparative efficacy, acceptability, and tolerability of augmentation agents in treatment-resistant depression: systematic review and network meta-analysis. J Clin Psychiatry. 2015;76(4):e487–e498. PubMed CrossRef NLM
- Rafeyan G, Papakostas GI, Jackson WC, et al. Inadequate response to treatment in major depressive disorder: augmentation and adjunctive strategies. J Clin Psychiatry. 2020;81(3):OT19037BR3. PubMed CrossRef NLM
- Bahji A, Ermacora D, Stephenson C, et al. Comparative efficacy and tolerability of adjunctive pharmacotherapies for acute bipolar depression: a systematic review and network meta-analysis [published online ahead of print November 11, 2020]. Can J Psychiatry PubMed CrossRef NLM
- Costa DLC, Diniz JB, Requena G, et al. Randomized, double-blind, placebo-controlled trial of N-acetylcysteine augmentation for treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry. 2017;78(7):e766–e773. PubMed CrossRef NLM
- Bloch MH, Panza KE, Grant JE, et al. N-acetylcysteine in the treatment of pediatric trichotillomania: a randomized, double-blind, placebo-controlled add-on trial. J Am Acad Child Adolesc Psychiatry. 2013;52(3):231–240. PubMed CrossRef NLM
- Bloch MH, Panza KE, Yaffa A, et al. N-acetylcysteine in the treatment of pediatric Tourette syndrome: randomized, double-blind, placebo-controlled add-on trial. J Child Adolesc Psychopharmacol. 2016;26(4):327–334. PubMed CrossRef NLM
- Wink LK, Adams R, Wang Z, et al. A randomized placebo-controlled pilot study of N-acetylcysteine in youth with autism spectrum disorder. Mol Autism. 2016;7(1):26. PubMed CrossRef NLM
- Perry HE, Shannon MW. Efficacy of oral versus intravenous N-acetylcysteine in acetaminophen overdose: results of an open-label, clinical trial. J Pediatr. 1998;132(1):149–152. PubMed CrossRef NLM
- Conrad C, Lymp J, Thompson V, et al. Long-term treatment with oral N-acetylcysteine: affects lung function but not sputum inflammation in cystic fibrosis subjects: a phase II randomized placebo-controlled trial. J Cyst Fibros. 2015;14(2):219–227. PubMed CrossRef NLM
- Johnson K, McEvoy CE, Naqvi S, et al. High-dose oral N-acetylcysteine fails to improve respiratory health status in patients with chronic obstructive pulmonary disease and chronic bronchitis: a randomized, placebo-controlled trial. Int J Chron Obstruct Pulmon Dis. 2016;11:799–807. PubMed NLM
- Berk M, Malhi GS, Gray LJ, et al. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol Sci. 2013;34(3):167–177. PubMed CrossRef NLM
- Deepmala SJ, Slattery J, Kumar N, et al. Clinical trials of N-acetylcysteine in psychiatry and neurology: a systematic review. Neurosci Biobehav Rev. 2015;55:294–321. PubMed CrossRef NLM
- Minarini A, Ferrari S, Galletti M, et al. N-acetylcysteine in the treatment of psychiatric disorders: current status and future prospects. Expert Opin Drug Metab Toxicol. 2017;13(3):279–292. PubMed CrossRef NLM
- Kishi T, Miyake N, Okuya M, et al. N-acetylcysteine as an adjunctive treatment for bipolar depression and major depressive disorder: a systematic review and meta-analysis of double-blind, randomized placebo-controlled trials. Psychopharmacology (Berl). 2020;237(11):3481–3487. PubMed CrossRef NLM
- Nery FG, Li W, DelBello MP, et al. N-acetylcysteine as an adjunctive treatment for bipolar depression: a systematic review and meta-analysis of randomized controlled trials [published online ahead of print December 22, 2020]. Bipolar Disord. 2020;bdi.13039. PubMed CrossRef NLM
- Berk M, Dean OM, Cotton SM, et al. Maintenance N-acetyl cysteine treatment for bipolar disorder: a double-blind randomized placebo controlled trial. BMC Med. 2012;10(1):91. PubMed CrossRef NLM
- Hu CC, Xie J. N-acetylcysteine add on treatment for depressive symptoms in bipolar disorder: a comparative trial. Clin Educ Gen Pract. 2012;10:515–517.
- Berk M, Dean OM, Cotton SM, et al. The efficacy of adjunctive N-acetylcysteine in major depressive disorder: a double-blind, randomized, placebo-controlled trial. J Clin Psychiatry. 2014;75(6):628–636. PubMed CrossRef NLM
- Porcu M, Urbano MR, Verri WA Jr, et al. Effects of adjunctive N-acetylcysteine on depressive symptoms: modulation by baseline high-sensitivity C-reactive protein. Psychiatry Res. 2018;263:268–274. PubMed CrossRef NLM
- Berk M, Copolov DL, Dean O, et al. N-acetyl cysteine for depressive symptoms in bipolar disorder: a double-blind randomized placebo-controlled trial. Biol Psychiatry. 2008;64(6):468–475. PubMed CrossRef NLM
Save
Cite
Advertisement
GAM ID: sidebar-top