Clinical Problem
Mr C is a 23-year-old student with a 9-month history of schizophrenia. He has responded only partially to antipsychotic drug treatment, developing adverse effects at higher doses of whatever antipsychotic he receives. Mr D is a 41-year-old clerk with a 20-year history of schizophrenia. He is stable on maintenance antipsychotic medication; however, he is withdrawn and shows no interest in leisure and pleasure activities. Are there non-antipsychotic augmentation agents that might improve outcomes in these 2 patients?
Introduction
Typical and atypical antipsychotic drugs are effective treatments for schizophrenia. However, despite best attempts to optimize therapy, fewer than 15% of treated patients recover.1 Most patients continue to exhibit a mix of positive, negative, and cognitive symptoms, and some are considerably impaired by these. Raising (or lowering) the antipsychotic dose, switching antipsychotics, and augmenting with a second antipsychotic are examples of pharmacologic interventions that are antipsychotic-based. For patients in whom antipsychotic approaches fail, and for those who do not tolerate antipsychotic drugs well, are there augmentation approaches involving non-antipsychotic drugs? A previous article in this column suggested that famotidine augmentation may not be of much benefit.2 The present article examines 2 other innovative augmentation treatments: nonsteroidal anti-inflammatory drugs (NSAIDs) and 5-HT3 serotonin receptor antagonists.
Mechanisms
Inflammatory markers have been identified in plasma as well as brain before and during the onset of psychotic symptoms in schizophrenia3-5; if these contribute to the disease process, then anti-inflammatory treatments may attenuate the severity of schizophrenia.6
5-HT3 receptors are widely distributed in the brain, including in limbic and frontal cortical areas. These receptors result in downstream modulation of many neurotransmitters, including dopamine. These receptors also influence auditory gating in schizophrenia.7 5-HT3 receptors probably do not modulate basal dopamine release but facilitate reward-related phasic dopamine release in the nucleus accumbens. 5-HT3 antagonists reduce dopaminergic activity in the nucleus accumbens.8 5-HT3 antagonists have been used to treat different dopaminergic disorders such as tardive dyskinesia,9 Tourette’s disorder,10 and schizophrenia.11 Finally, 5-HT3 antagonists have a calming effect and improve cognition in animal models.12,13 Drugs acting on 5-HT3 receptors may therefore moderate the symptoms of schizophrenia.
NSAIDs for Schizophrenia
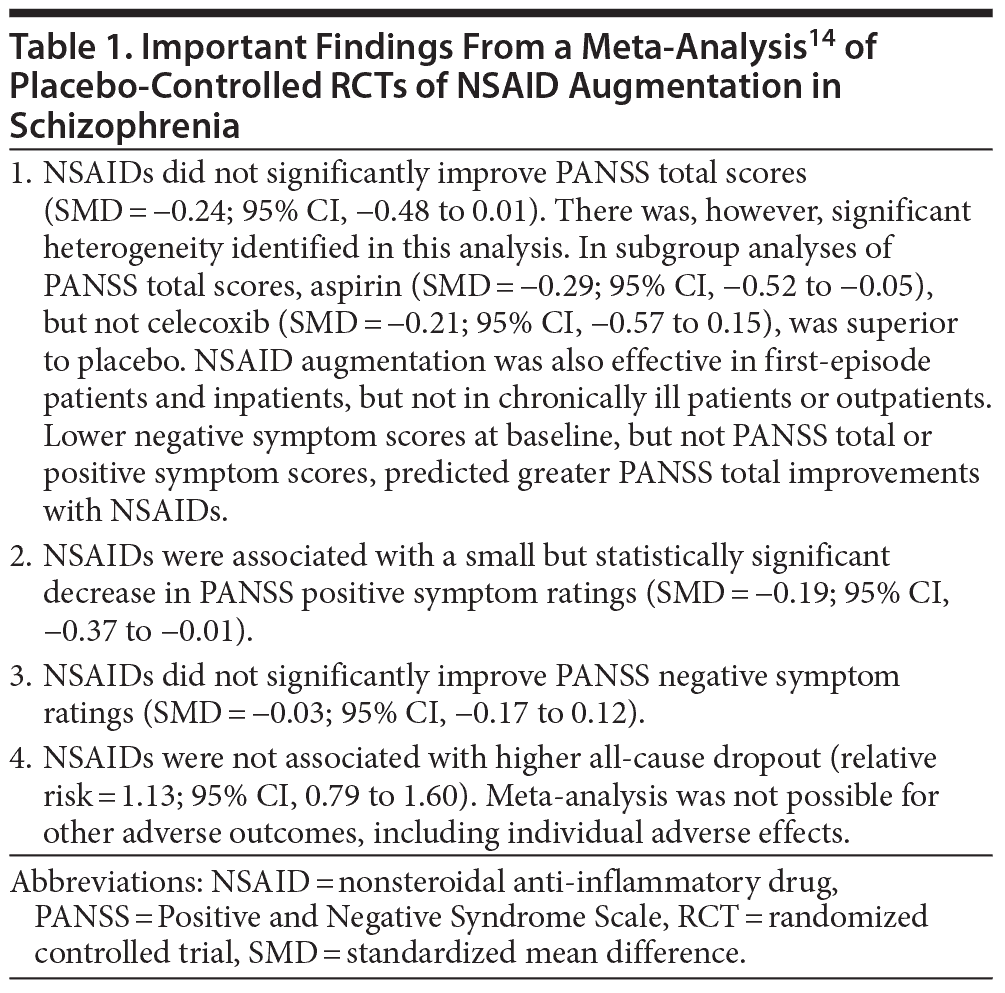
Many randomized controlled trials (RCTs) have examined whether NSAID augmentation is an effective treatment strategy in schizophrenia; not all outcomes were favorable. Nitta et al14 therefore examined the subject in a systematic review and meta-analysis. The authors searched electronic databases, clinical trial registries, reference lists, and other sources and identified 8 relevant placebo-controlled RCTs (pooled N = 774), including 3 that had not been published. These RCTs examined acutely ill schizophrenia patients and those with positive symptoms. The mean age of the pooled sample was about 34 years. The sample was two-thirds male. Risperidone was the commonest antipsychotic that was augmented. Six RCTs examined celecoxib (400 mg/d; N = 504) and 2 examined aspirin (1,000 mg/d; N = 270) augmentation. The trials were 5 to 16 (median = 8) weeks in duration.
Nitta et al14 found that NSAID augmentation of antipsychotic drugs was associated with a statistically significant reduction in positive symptoms scores; however, the standardized mean difference (SMD) was only 0.19, representing an effect size of questionable clinical significance. The SMD for Positive and Negative Syndrome Scale (PANSS) total scores narrowly missed statistical significance; this effect was also small and likely to be of doubtful clinical value. There were no other treatment gains evident. Dropout was not increased by NSAID augmentation.
Many subgroup analyses suggested possible areas of benefit, but these results are best considered preliminary because the analyses were exploratory and not corrected for type II errors. The results of the meta-analysis are summarized in Table 1. In brief, the findings do not encourage the use of anti-inflammatory treatments for schizophrenia unless inflammatory or other biomarkers can be identified that reliably define subpopulations of patients who might benefit from the intervention. In such an event, long-term studies are desirable to determine whether reducing inflammation improves the course of the illness.
5-HT3 Antagonists for Schizophrenia
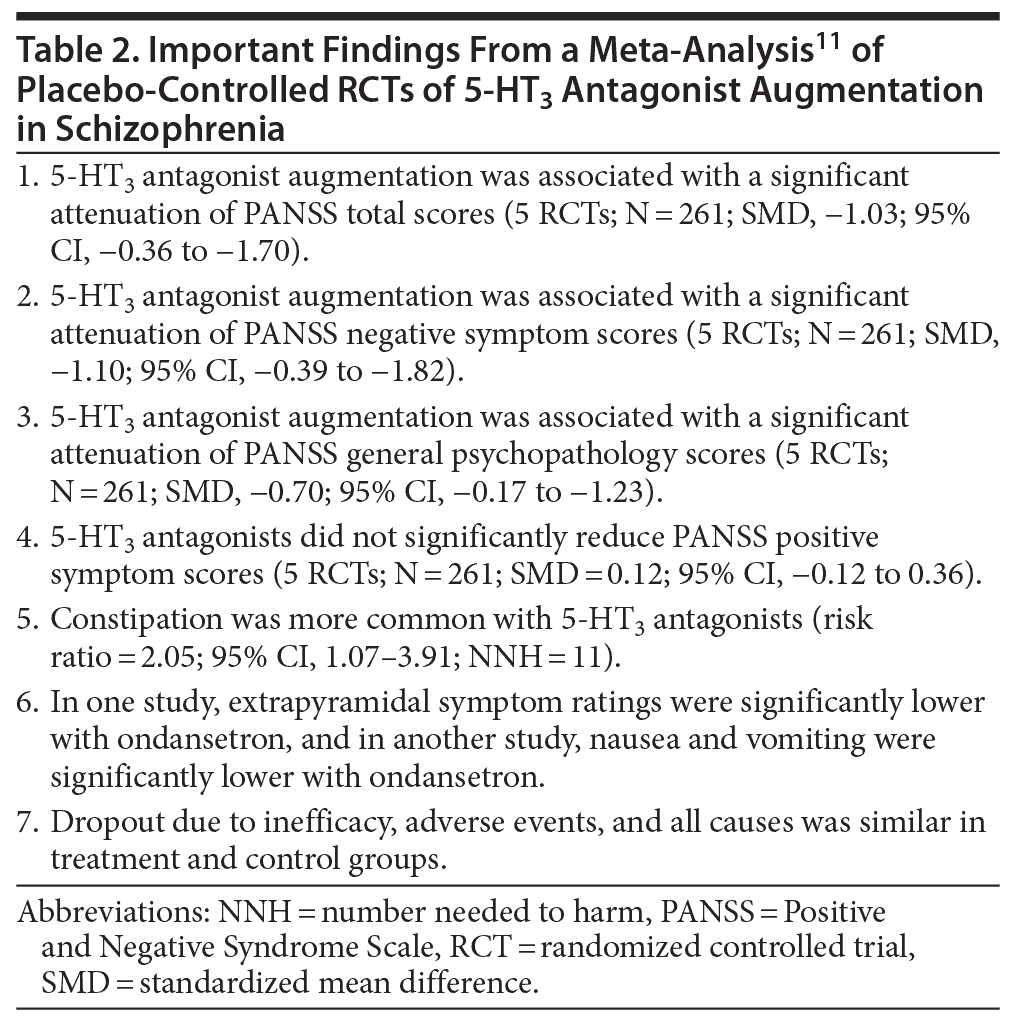
Kishi et al11 presented a systematic review and meta-analysis of selective 5-HT3 antagonist augmentation of antipsychotic medications in schizophrenia. These authors searched electronic databases, clinical trial registries, reference lists, and other sources and identified 6 relevant placebo-controlled RCTs (pooled N = 311), only 5 of which contributed data to efficacy outcomes. The mean age of the sample was about 37 years. Patients in these studies were, in general, clinically stable and on stable doses of antipsychotic medication. The RCTs ranged from 10 days to 12 weeks (median, 8 weeks) in duration. Three RCTs studied tropisetron (5-10 mg/d), 2 studied ondansetron (8 mg/d), and 1 studied granisetron (2 mg/d). One ondansetron RCT augmented haloperidol; the remaining 5 RCTs augmented risperidone. One RCT reported only a completer analysis. None of the RCTs was industry-driven.
The results of the meta-analysis are summarized in Table 2. 5-HT3 antagonists were associated with significant reductions in PANSS total scores, negative symptoms, and general psychopathology scores; the SMDs were medium to large. All 3 analyses, however, were characterized by statistical heterogeneity. 5-HT3 antagonists did not significantly reduce positive symptoms. Dropout rates were not increased by 5-HT3 antagonists.
These results should, at best, be considered preliminary because of the small number of studies, the small size of the pooled sample, and the considerable heterogeneity detected in the analyses. However, the large effect sizes across several outcome measures imply that the field is worthy of further study.
Conclusions
Nonsteroidal anti-inflammatory drugs seem to hold little promise as antipsychotic augmentation agents in acutely ill schizophrenia patients or those with persistent positive symptoms. However, these drugs may merit investigation in samples of patients in whom biomarkers of neuroinflammatory pathology suggest the possibility of benefit. 5-HT3 antagonists may improve negative symptoms, general psychopathology, and overall symptom ratings in schizophrenia patients who are on a stable dose of antipsychotic medication; the field, however, requires stronger evidence before definitive conclusions can be drawn.
Where does this leave the 2 patients who were described at the start of this article? At best, a 5-HT3 antagonist may be trialed in each patient as an experimental, off-label augmenting agent for a period of about 8 weeks. The data suggest that, whereas there can be hopes of improvement, the risks (except for constipation) are no greater than with placebo.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
REFERENCES
1. Jפפskelפinen E, Juola P, Hirvonen N, et al. A systematic review and meta-analysis of recovery in schizophrenia. Schizophr Bull. 2013;39(6):1296-1306. PubMed doi:10.1093/schbul/sbs130
2. Andrade C. Famotidine augmentation in schizophrenia: hope or hype? J Clin Psychiatry. 2013;74(9):e855-e858. PubMed doi:10.4088/JCP.13f08707
3. Smyth AM, Lawrie SM. The neuroimmunology of schizophrenia. Clin Psychopharmacol Neurosci. 2013;11(3):107-117. PubMed doi:10.9758/cpn.2013.11.3.107
4. Feigenson KA, Kusnecov AW, Silverstein SM. Inflammation and the two-hit hypothesis of schizophrenia. Neurosci Biobehav Rev. 2014;38:72-93. PubMed doi:10.1016/j.neubiorev.2013.11.006
5. Zakharyan R, Boyajyan A. Inflammatory cytokine network in schizophrenia. World J Biol Psychiatry. 2014;15(3):174-187. PubMed doi:10.3109/15622975.2013.830774
6. Girgis RR, Kumar SS, Brown AS. The cytokine model of schizophrenia: emerging therapeutic strategies. Biol Psychiatry. 2014;75(4):292-299. PubMed doi:10.1016/j.biopsych.2013.12.002
7. Thompson AJ, Lummis SC. The 5-HT3 receptor as a therapeutic target. Expert Opin Ther Targets. 2007;11(4):527-540. PubMed doi:10.1517/14728222.11.4.527
8. Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther. 2007;113(2):296-320. PubMed doi:10.1016/j.pharmthera.2006.08.004
9. Sirota P, Mosheva T, Shabtay H, et al. Use of the selective serotonin 3 receptor antagonist ondansetron in the treatment of neuroleptic-induced tardive dyskinesia. Am J Psychiatry. 2000;157(2):287-289. PubMed doi:10.1176/appi.ajp.157.2.287
10. Toren P, Weizman A, Ratner S, et al. Ondansetron treatment in Tourette’s disorder: a 3-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2005;66(4):499-503. PubMed doi:10.4088/JCP.v66n0413
11. Kishi T, Mukai T, Matsuda Y, et al. Selective serotonin 3 receptor antagonist treatment for schizophrenia: meta-analysis and systematic review. Neuromolecular Med. 2014;16(1):61-69. PubMed doi:10.1007/s12017-013-8251-0
12. Sridhar N, Veeranjaneyulu A, Arulmozhi DK, et al. 5-HT3 receptors in selective animal models of cognition. Indian J Exp Biol. 2002;40(2):174-180. PubMed
13. Costall B, Naylor RJ. 5-HT3 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3(1):27-37. PubMed doi:10.2174/1568007043482624
14. Nitta M, Kishimoto T, Müller N, et al. Adjunctive use of nonsteroidal anti-inflammatory drugs for schizophrenia: a meta-analytic investigation of randomized controlled trials. Schizophr Bull. 2013;39(6):1230-1241. PubMed doi:10.1093/schbul/sbt070

 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.



