ABSTRACT
Objective: The aims of this review were to describe the relationship between obstructive sleep apnea (OSA) and psychiatric disorders and provide an overview of how to recognize/manage OSA in psychiatric practice.
Data Sources: A literature search of PubMed was conducted (in adults, English language, no limitation on year). Among others, main keywords included “obstructive sleep apnea” AND “psychiatric.”
Study Selection: Articles relevant to the treatment of OSA in psychiatric populations were selected manually.
Data Extraction: No formal data charting was conducted.
Results: A total of 141 articles were included from the literature search. Comorbid OSA is common among patients with psychiatric disorders, particularly depression and posttraumatic stress disorder. Evidence suggests that OSA may be an independent risk factor for the development of psychiatric conditions, as well as for suicidal ideation and attempts in psychiatric populations. Recognizing OSA in patients with psychiatric disorders can be challenging due to the overlap of symptoms (eg, sleep issues, mood changes, and vegetative symptoms) between OSA, psychiatric disorders, and side effects of psychiatric medications. Inadequately treated OSA can affect the severity of psychiatric symptoms and impair response to psychiatric treatment.
Conclusions: Clinicians should not assume that all sleep-related symptoms are consequences of psychiatric illness or medication but should instead be cognizant of the potential for coexisting OSA that requires treatment. Recognizing and managing OSA in patients with psychiatric disorders are critical to improve response to treatment, quality of life, and overall health.
J Clin Psychiatry 2023;84(2):22r14521
To cite: Benca RM, Krystal A, Chepke C, et al. Recognition and management of obstructive sleep apnea in psychiatric practice. J Clin Psychiatry. 2023;84(2):22r14521.
To share: https://doi.org/10.4088/JCP.22r14521
© 2023 Physicians Postgraduate Press, Inc.
aPsychiatry and Behavioral Medicine, Wake Forest University, Winston Salem, North Carolina
bPsychiatry and Behavioral Science, University of California San Francisco, San Francisco, California
cSUNY Upstate Medical University, Syracuse, New York
dExcel Psychiatric Associates, Huntersville, North Carolina
eThomas Jefferson University, Philadelphia, Pennsylvania
*Corresponding author: Ruth M. Benca, MD, PhD, Department of Psychiatry, Wake Forest School of Medicine, Atrium Health Wake Forest Baptist, 791 Jonestown Rd, Winston-Salem, NC 27103 ([email protected]).
See letter by Krakow
Sleep problems are common among patients with psychiatric disorders, and the relationship between sleep and psychiatric pathology is complex and reciprocal in nature.1 Psychiatric disorders may contribute to the development of sleep disturbances, which may be symptoms of psychiatric disorders or side effects of psychiatric medications.1 Conversely, sleep disorders can negatively affect course and treatment response of psychiatric conditions, mood, quality of life, and daily functioning.2,3 Unfortunately, sleep disorders commonly go unrecognized in the setting of psychiatric clinical practice.
Obstructive sleep apnea (OSA) is a sleep disorder characterized by repeated episodes of upper airway obstruction during sleep and is estimated to affect approximately 1 billion adults worldwide.2,4 There is a high prevalence of comorbid OSA with psychiatric disorders.5–8 Many symptoms of OSA, such as excessive daytime sleepiness (EDS), disturbed sleep, impaired cognition, irritability, and mood changes, are also common to psychiatric disorders, rendering recognition of OSA in psychiatric populations challenging. Untreated or suboptimally treated OSA can negatively impact mental and physical health9–13 and impair response to psychiatric treatment.3,14,15 Moreover, some psychiatric medications can increase risk or exacerbate symptoms of OSA (eg, atypical antipsychotics associated with weight gain). Recognizing and managing OSA is therefore critical to improving treatment outcomes, overall health, and quality of life in patients with psychiatric disorders. Importantly, OSA is a risk factor for suicidality in patients with psychiatric disorders, further highlighting the importance of examining this patient population for OSA.16
Psychiatric clinicians are well-positioned to identify many patients with OSA as it is common in their patients; however, greater awareness of the high prevalence of OSA in patients with psychiatric disorders is important to increase recognition and diagnosis of OSA in clinical practice. This review aims to describe the relationship between OSA and psychiatric disorders and provide an overview on how to recognize and manage OSA in psychiatric practice. Assessment and treatment of EDS are highlighted as EDS is not only a common symptom of OSA that can persist despite treatment of the underlying airway obstruction, but also a symptom associated with many psychiatric disorders. Notably, sleep-related symptoms may vary by patient and could include sleep issues other than or in addition to EDS, such as insomnia.
METHODS
Protocol and Registration
No formal review protocol was used for the preparation of this article.
Data Sources and Study Selection
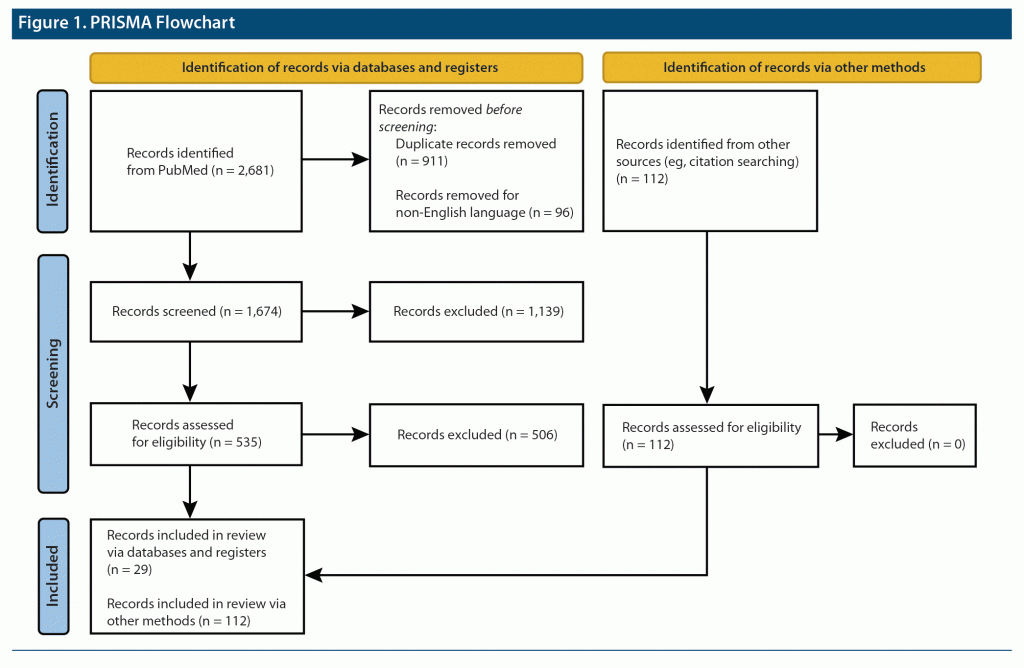
A literature search on PubMed was conducted for relevant journal articles in adult humans, in English, with no limitation on year of publication. The main search keywords included the following: [“obstructive sleep apnea” AND “psychiatric” AND “excessive daytime sleepiness”], [“obstructive sleep apnea” AND “psychiatric”], [“obstructive sleep apnea” AND “depression”], [“obstructive sleep apnea” AND “anxiety”], [“obstructive sleep apnea” AND “bipolar”], [“obstructive sleep apnea” AND “PTSD”], [“obstructive sleep apnea” AND “schizophrenia”], [“obstructive sleep apnea” AND “ADHD”], [“obstructive sleep apnea” AND “attention deficit disorder”], [“obstructive sleep apnea” AND “attention deficit hyperactivity disorder”], [“obstructive sleep apnea” AND “substance abuse disorder”], [“obstructive sleep apnea” AND “cognitive impairment”], [“obstructive sleep apnea” AND “dementia”], [“obstructive sleep apnea” AND “Alzheimer’s”], and [“obstructive sleep apnea” AND “suicidality”]. Additional articles in relation to the treatment of OSA in the psychiatric population were obtained by manually searching relevant guidelines and bibliographies of retrieved review articles (Figure 1). No formal data charting was conducted.
ASSOCIATIONS BETWEEN OSA AND PSYCHIATRIC DISORDERS
Prevalence of OSA in Psychiatric Populations
The overall prevalence of OSA among US adults aged 30 to 70 years has been estimated at 26% (95% confidence interval [CI], 24% to 28%) for mild to severe OSA (apnea-hypopnea index [AHI] ≥ 5) and 10% (95% CI, 8% to 11%) for moderate to severe OSA (AHI ≥ 15), with higher rates among men (33.9% and 13.0%, respectively) than women (17.4% and 5.6%, respectively).17 The prevalence of OSA in psychiatric populations is difficult to pinpoint as a wide range of estimates have been reported (Supplementary Table 1), likely due to differences in study samples (eg, clinic-based or population-based), the criteria used to define OSA, and how psychiatric pathology was defined (diagnosis or reporting of psychiatric symptoms).5–7,18–24 Nonetheless, multiple studies have demonstrated that comorbid OSA is common among patients with psychiatric disorders, particularly depression and posttraumatic stress disorder (PTSD).18,20,25 A 2015 systematic review reported the median prevalence of OSA in studies of clinic-based populations was 48% (range, 0–66) among patients with depression and 43% (range, 1–83) among patients with PTSD,18 both of which are substantially higher than the prevalence of OSA in the general population. A 2017 meta-analysis found the prevalence of OSA among patients with PTSD to be even higher at a pooled prevalence of 55.1% (AHI ≥ 5, 75.7%; AHI ≥ 10, 43.6%).21 A meta-analysis in 2016 reported a pooled prevalence of OSA of 36.3% (95% CI, 19.4% to 57.4%) among patients with depression.7 Other studies have reported lower estimates (14%–20%) among patients with depression.5,6,19,26 Of note, one of these studies,6 which enrolled suicidal patients with insomnia and depression, excluded individuals with a diagnosis or history of OSA; despite the fact that these patients were initially believed to be low risk for OSA, 14% were found to have unsuspected OSA when tested (polysomnography [PSG] or home sleep apnea testing).
Reports on whether the prevalence of OSA is increased among patients with schizophrenia and other disorders, such as anxiety and bipolar disorder, have been less consistent, although a recent meta-analysis estimated that 25% of patients with bipolar disorder and 15% of patients with schizophrenia have OSA.5,7,18
Evidence suggests that psychiatric illness, particularly the presence of depressive symptoms or a diagnosis of depression, may also be a risk factor for the development of OSA. For example, a study in 152 psychiatric inpatients found that 39.5% of patients were at high risk for OSA (defined as positive scores in ≥ 2 of 3 categories on the Berlin Questionnaire) and demonstrated that patients with depressive symptoms were 4 times as likely to be at high risk for OSA than those without depressive symptoms; other factors associated with OSA, including obesity, sex, and age, were also included as variables in this analysis.8 In addition, a cross-sectional study of a large general population sample (> 18,000) from 5 countries found that individuals with depression are 5 times as likely to have a breathing-related sleep disorder (including OSA), even when other factors associated with OSA, such as age, sex, obesity, and hypertension, were taken into account; presence of psychotic features was also associated with a 50% greater likelihood of having a breathing-related sleep disorder in this study.26
Increased Risk of Psychiatric Conditions Associated With OSA
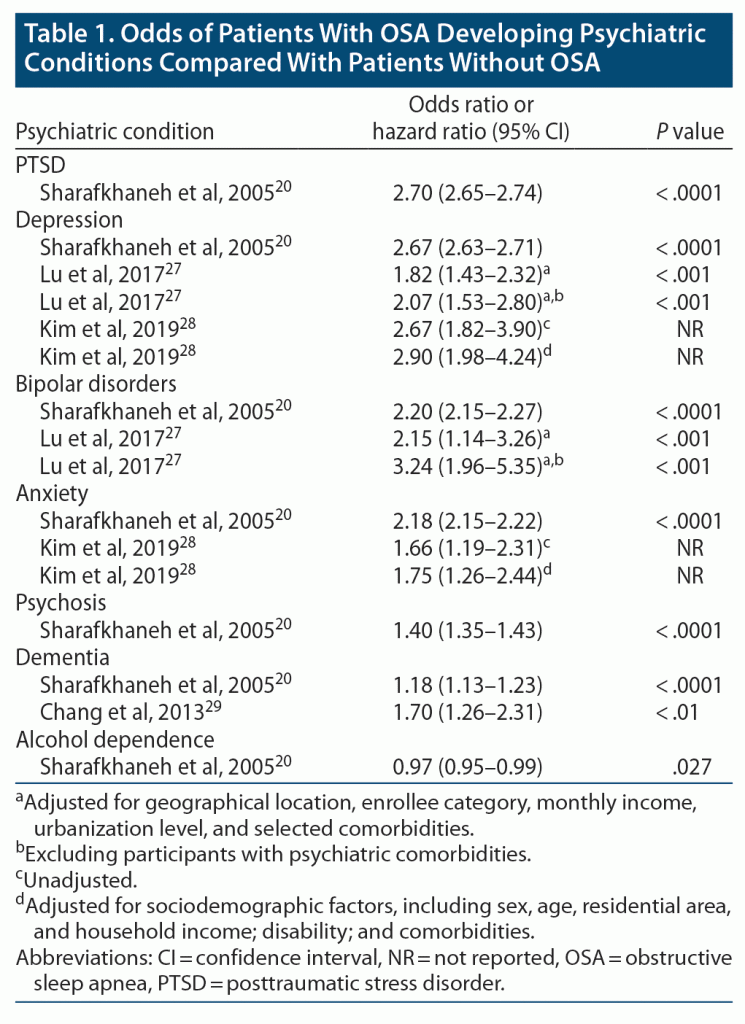
Conversely, evidence suggests that OSA may also be an independent risk factor for the development of psychiatric conditions (Table 1).20,27–29 For example, a longitudinal study found that the presence of sleep apnea was associated with significantly increased risk for depression (adjusted hazard ratio [aHR] = 2.07) and bipolar disorder (aHR = 3.24) after controlling for confounders and other psychiatric comorbidities.27
Additional evidence suggests that the risk of psychiatric disorders increases with the severity of OSA. In a mixed cross-sectional and longitudinal study of 1,408 adults, the presence of sleep-disordered breathing was associated with a higher odds for developing depression, with each escalation in OSA severity category representing progressively greater risk.30 Specifically, patients whose OSA worsened in severity by 1 category (from no OSA [AHI, 0 events/h] to minimal [AHI, 0 to < 5], from minimal to mild [AHI, ≥ 5 to < 15], or from mild to moderate/severe [AHI ≥ 15]) over the 4-year follow-up period had a 1.8-fold increased odds of developing depression, whereas patients whose OSA worsened by 2 categories had a 3.3-fold increased odds of developing depression, even when age, body mass index (BMI), alcohol consumption, and history of cardiovascular disease (CVD) were controlled.30 EDS in OSA specifically has also been found to be a risk factor for depression. In a longitudinal study of 1,137 adults with OSA without depression at baseline, the severity of EDS was a significant predictor of incident depression in both men and women.31
Symptom Overlap
Recognizing OSA in patients with psychiatric disorders can be challenging due to the shared symptoms between OSA and psychiatric disorders and the overlap of symptoms of OSA with side effects of psychiatric medications (eg, EDS). In addition, both sleep issues and mood symptoms are confounded by other factors common to these patient populations, such as medications, obesity, substance use, and other comorbidities. In particular, insomnia commonly co-occurs with OSA, with up to 50% of patients with OSA reporting insomnia symptoms.32 Further, insomnia can result from (ie, be a symptom of) or exacerbate (ie, be a risk factor for) psychiatric conditions, leading to increased psychiatric symptomatology in patients with comorbid OSA and insomnia.33 One study reported that patients with comorbid OSA and insomnia have significantly higher rates of psychiatric disorders compared with those with only OSA (47.8% vs 28.7%, respectively).34 Even when OSA is recognized, it may be difficult to determine whether symptoms, such as EDS or insomnia, are due to the psychiatric condition, a medication side effect, suboptimally treated OSA (or nonadherence to OSA treatment), or other factors.
CLINICAL IMPLICATIONS OF OSA IN PATIENTS WITH PSYCHIATRIC DISORDERS
Impact on Psychiatric Assessments
Diagnostic criteria for OSA include complaints of sleepiness, nonrestorative sleep, fatigue, or insomnia,2 which are also among the diagnostic criteria for many psychiatric disorders, such as major depressive disorder, PTSD, and anxiety disorders.1 Similarly, cognitive symptoms, such as diminished ability to think or concentrate, and mood symptoms, such as irritability, commonly occur with OSA and many psychiatric disorders. Thus, symptoms of OSA can affect scores on commonly used psychiatric assessment scales and hinder the ability to accurately gauge severity of psychiatric symptoms and response to treatment. Many of these scales, such as the Hamilton Depression and Anxiety Rating Scales, Montgomery-Asberg Depression Rating Scale, Patient Health Questionnaire-9, Beck Depression Inventory (BDI), Profile of Mood States, and Minnesota Multiphasic Personality Inventory (MMPI), include items related to sleepiness, sleep disturbance (difficulty falling asleep or maintaining sleep), sleep quality, insomnia, and fatigue.35–38 For example, the BDI asks patients to report if they “wake up more tired in the morning than [they] used to,” “get tired more easily than [they] used to,” or “get too tired to do anything.”39 If a patient is experiencing sleep-related symptoms due to OSA, scores on psychiatric assessments could be artificially inflated. For example, one study found that 58% of patients with OSA have clinically significant elevations in at least 1 scale on the MMPI.40 Severity of oxygen desaturation has also been found to correlate significantly with higher BDI scores in patients with OSA.41 Confounded scores on psychiatric assessments may lead to misdiagnosis or be misinterpreted as a lack of response to psychiatric treatment (or treatment resistance) if OSA is not considered as a potential cause.
Impact on Psychiatric Outcomes
It is critical for psychiatric clinicians to identify and treat, or facilitate the diagnosis and treatment of, OSA because untreated OSA can affect the severity of psychiatric symptoms and/or response to psychiatric treatments. For example, in a study of women with PTSD, the presence of OSA was associated with significantly worse nightmares, sleep quality, anxiety, depression, posttraumatic stress, and impaired quality of life.42 In a study in patients with depression who were treated with 12 weeks of venlafaxine, 44% of patients without OSA responded to treatment, whereas only 28% of patients with OSA responded.3 Similarly, in a study of sertraline, after 10 weeks of treatment, patients with OSA remained significantly more depressed, as measured by the BDI and Hamilton Depression Rating Scale, compared to those without OSA.14 Furthermore, untreated apnea can be worsened by some psychiatric medications, such as sedative-hypnotics, putting patients at increased risk of apnea-related effects on health.43
Considering that the presence of OSA may reduce the response to treatment in psychiatric disorders, it is not surprising that several studies have shown that adequately treating OSA improves psychiatric symptoms and treatment outcomes in patients with comorbid psychiatric disorders. A recent review concluded that in patients with OSA, adequate CPAP treatment reduced depressive symptoms and improved quality of life.9 A longitudinal study of participants with depression and suspected OSA found that symptoms of depression correlated significantly with OSA severity (AHI score) and were markedly improved following the initiation of continuous positive airway pressure (CPAP) in participants identified as having OSA (despite unchanged antidepressant therapy).10 Similarly, another longitudinal study of 2,410 participants with OSA and CVD found that those treated with CPAP plus usual care (education regarding beneficial sleep habits and lifestyle changes to minimize OSA symptoms) had a significantly lower incidence of depression (Hospital Anxiety and Depression Scale—Depression subscale score ≥ 8) and an overall reduction in depressive symptoms compared to those who received usual care alone.11 A prospective study in combat veterans with PTSD and OSA treated with CPAP12 showed dose-dependent response of PTSD Checklist–Military Version scores to duration of CPAP use (r = 0.45; P = .003), and CPAP usage (≥ 4 hours per night for 3 months) was the only significant predictor of overall subjective improvement in PTSD symptoms (odds ratio [OR], 10.5; P = .01); CPAP adherence was associated with a reduction in nightmare distress and frequency, although this was not statistically significant.44 A randomized crossover trial of participants with PTSD and newly diagnosed OSA found that treatment with either CPAP or a mandibular advancement device significantly decreased PTSD severity and improved participants’ quality of life.12 A study of active duty US military personnel with diverse psychiatric disorders found that adherence to CPAP was associated not only with improved sleep quality and reduced sleepiness, but also with improvements in social functioning and depressive symptoms.13 These studies highlight that treatment for OSA can lead to improvement in symptoms of psychiatric conditions. Indeed, the American Psychiatric Association Practice Guideline for the Treatment of Patients with Major Depressive Disorder advises that “clinicians should be alert to the possibility of sleep apnea in patients with depression, particularly those who present with daytime sleepiness, fatigue, or treatment-resistant symptoms.”15
There is some, although more limited, evidence that alternative airway therapies may also improve psychiatric outcomes. For example, a systematic review and meta-analysis of 5 randomized controlled trials (RCTs) concluded that mandibular advancement devices (MAD) were associated with significant improvement in depressive symptoms in patients with OSA and depression.45 Further, a randomized crossover trial found that 12 weeks of MAD improved EDS, PTSD symptomatology, and health-related quality of life measures in veterans with OSA and PTSD. The authors concluded that, although CPAP is more efficacious than MAD at treating OSA (ie, respiratory events), MAD offers a viable alternative for improving psychiatric outcomes for patients who are nonadherent to CPAP.12
Impact on Suicidality
Sleep problems and poor sleep quality are significant risk factors for increased suicidal ideation in mixed clinical and community samples.46 A survey of a large, nationally representative sample of 40,149 US adults found that individuals with sleep apnea were 1.5 (P < .001) and 1.6 (P < .05) times as likely to report suicidal ideation and suicide planning, respectively, compared with those without sleep apnea, even after controlling for key factors, including past-year depressive episode, substance use disorder, or sedative-hypnotic misuse.47 Moreover, OSA imparts an independent risk for suicidality in the context of psychiatric disorders. For example, a recent analysis of data from the National (Nationwide) Inpatient Sample database showed that in patients with depression and OSA, the prevalence of suicidal ideation or attempts was higher compared to a matched cohort of patients with depression without OSA (49.5% vs 41.8%; P < .001).16 Further, a multivariate analysis showed that patients with depression and OSA are 27% more likely to have suicidal ideation and behaviors compared to those with depression but without OSA (OR, 1.27 [95% CI, 1.20 to 1.35]; P < .001). Other factors in the model included severity of illness, which was significant (P < .001), and moderate-to-severe depression, which was not significant (P = .17).16 A study of adults with PTSD found that OSA severity as measured by the respiratory disturbance index was significantly correlated with the level of suicidal ideation as assessed using the Brief Symptom Inventory.48 Similarly, a recent study examining the relationship between OSA and suicidality in bipolar disorder found that among 17,895 patients with bipolar disorder and OSA registered in a national database, the risk of suicidality was significantly higher compared to the 71,575 patients with bipolar disorder who did not have OSA (OR, 1.36).49 Notably, this analysis was not adjusted for bipolar illness severity, which may have confounded results. These findings suggest that OSA may represent an early opportunity for psychiatric clinicians to identify suicide risk in patients.
Additional Consequences of Untreated/Suboptimally Treated OSA
In addition to effects directly related to psychiatric disorders, untreated or suboptimally treated OSA is associated with indirect deleterious consequences that may be relevant to psychiatric populations. Residual EDS in patients with OSA has been associated with structural changes to cerebral white matter and increased cognitive impairment, which has been postulated to be the result of chronic intermittent hypoxia and sleep fragmentation.50–52 Further, studies in patients with OSA have demonstrated that the presence of depressive symptoms is associated with significantly more extensive and different patterns of neural injury53 and that the presence of anxiety is associated with neuronal alterations in areas outside of those affected by OSA alone,54 suggesting processes involved in these psychiatric symptoms are additive to brain injury associated with OSA. A study assessing cognitive performance and biomarkers of Alzheimer’s disease (AD) in a cohort of participants with OSA and healthy controls found that untreated participants with OSA showed significantly greater impairment of cognitive performance and pathological levels of AD biomarkers in cerebrospinal fluid relative to both controls and CPAP-treated participants with OSA.55 Psychiatric disorders, including schizophrenia56 and depression,57 are also associated with an increased risk of dementia and/or Alzheimer’s disease, and severe psychiatric disorders (depression, bipolar disorder, schizophrenia, anxiety, and alcohol dependence) in midlife are significant and independent predictors of late-life dementia.58
OSA is also associated with significant cardiovascular comorbidity, an important consideration for patients with psychiatric disorders that are also associated with increased cardiovascular risk.59-61 Indeed, the severity of sleepiness in OSA is associated with an increased prevalence of CVD and a higher risk of CVD events.62 Several randomized studies have shown that CPAP treatment can reduce blood pressure and improve biomarkers of cardiovascular risk.63
In addition, untreated OSA or the presence of residual EDS in patients using CPAP therapy is associated with impairments in quality of life and daily functioning. OSA leads to deficits in cognitive function and mood problems that can impair work performance, and EDS specifically has been associated with greater deficits in work in patients with OSA.64,65 The presence and severity of OSA and/or sleepiness may be related to quality of life in patients with depression or bipolar disorder.66 In patients with untreated OSA, EDS severity is associated with greater risk for motor vehicle accidents.67 Increased risk of driving accidents has also been associated with psychiatric disorders and their treatments,68,69 so recognizing OSA as a potential additional contributor to this risk is important. In an RCT of CPAP versus conservative medical therapy with/without sham CPAP, CPAP improved several health-related, quality-of-life domains and resulted in significant reductions in EDS.70 Pharmacologic treatment of EDS in patients with OSA has been shown to also improve work productivity and quality of life71,72 and reduce the risk of hospitalization due to motor vehicle accidents.73
In summary, these significant clinical implications underscore the importance of recognizing and treating OSA in patients with psychiatric disorders.
RECOGNIZING OSA IN PATIENTS WITH PSYCHIATRIC DISORDERS
Patient Report of Symptoms
As previously discussed, the overlap in symptoms between OSA and psychiatric disorders can make it difficult to recognize OSA in patients with psychiatric conditions or that OSA is suboptimally treated. Furthermore, the terms patients use to describe their complaints can vary and sometimes add to the difficulty in distinguishing OSA and psychiatric symptoms. For example, when describing EDS, one of the cardinal symptoms of OSA, patients may use terms such as “feeling tired,” “lacking energy,” “lacking motivation,” “irritability,” or “difficulty concentrating.” In fact, patients with OSA are more likely to report fatigue, tiredness, and lack of energy than sleepiness.74 To further complicate matters, depression, anxiety, and negative affect have been found to influence the perception and emotional experience of EDS, which could lead to further variability in subjective reports of this symptom in psychiatric practice.75 Notably, some patients may not complain of EDS, but may instead report insomnia symptoms.33 Inventories may be helpful in identifying OSA in such mixed populations. Indeed, a recent study found that out of 40% of hospitalized psychiatric patients who were determined to have high risk of OSA based on the Berlin sleep questionnaire, only 5% reported that they had been diagnosed with or treated for sleep apnea, suggesting OSA is commonly overlooked in this population.8
Screening for OSA
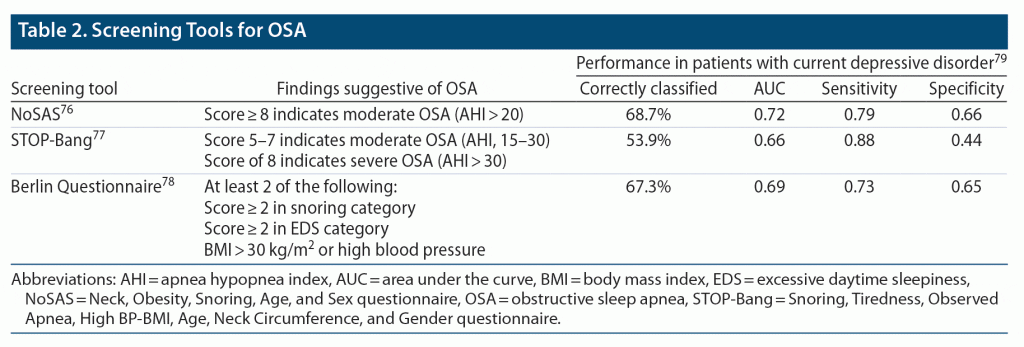
In primary or specialty care settings, clinicians can use validated tools to screen patients for OSA. There are a number of brief, easily accessible screening tools that can be used to identify patients at risk for OSA, including the Neck circumference, Obesity, Snoring, Age, and Sex (NoSAS)76; the Snoring, Tiredness, Observed Apnea, High BP-BMI, Age, Neck Circumference, and Gender (STOP-Bang) questionnaire77; and the Berlin sleep questionnaire78 (Table 2). The NoSAS questionnaire is a 5-item questionnaire based on biometric characteristics that are routinely measured in clinical practice, making it convenient for patients who may have difficulty verbally articulating their symptoms or for situations in which the clinician questions the reliability of subjective reports.76,79 NoSAS scores range from 0 to 17, where scores ≥ 8 indicate clinically significant OSA.76 The STOP-Bang is an 8-item questionnaire based on clinical features of sleep apnea.77 STOP-Bang scores range from 0 to 8, where higher scores (3 or more) indicate an increased risk for OSA.77 The Berlin is a 10-item questionnaire based on 3 OSA symptom categories: snoring, EDS, and BMI or high blood pressure.78 Patients are classified as high or low risk depending on their scores in each symptom category, with high risk for OSA being defined as having a positive score (snoring behavior score ≥ 2, sleepiness or fatigue score ≥ 2, BMI > 30 kg/m2 or high blood pressure) in at least 2 categories.78
All 3 instruments have recently been assessed for identifying patients at increased risk for OSA in patients with current major depressive episodes.79 In patients with depression with OSA, 79% had NoSAS scores ≥ 8, 88% had STOP-Bang scores ≥ 3, and 73% had Berlin scores ≥ 2, compared with 34%, 15%, and 35% of patients with depression without OSA, respectively (P < .001). When comparing the 3 questionnaires’ ability to identify patients with mild, moderate, or severe OSA (ie, based on AHI cutoffs), one did not perform significantly better than the others. Notably, the STOP-Bang had higher sensitivity but lower specificity than the NoSAS and Berlin, meaning it has a low chance of missing a patient at risk for OSA but may result in some unnecessary referrals to sleep clinics. Although future research is needed to validate the NoSAS, STOP-Bang, and Berlin tools in psychiatric populations, these questionnaires offer convenient methods to screen patients for OSA in psychiatric practice. However, diagnosis requires an objective test, either in-laboratory PSG or a more limited home sleep apnea study. If the clinical evaluation raises the suspicion of OSA based on symptoms and examination findings, the gold standard for diagnosing OSA includes objective testing with either home sleep testing or an overnight PSG in a sleep laboratory.
Differential Diagnosis
Once a diagnosis of OSA is confirmed in a patient, it is important to determine whether the symptom of concern, such as EDS or insomnia, is indeed associated with OSA. Detailed suggestions for procedures that can be implemented into routine practice to differentially diagnose EDS associated with OSA have been recently published and, therefore, are only briefly summarized here.80 The clinician should ensure that the OSA is being optimally treated, including assessing adherence to CPAP therapy. The patient’s sleep habits and lifestyle should also be evaluated to determine whether insufficient sleep, diet, or exercise may be contributing to EDS. Finally, other competing etiologies, such as medications, substance abuse, and other medical or sleep disorders, should be ruled out. In most cases, a differential diagnosis can be made by a psychiatric clinician based on the clinical evaluation; however, referral to a sleep specialist may be necessary for more complex cases, such as if another sleep disorder is suspected.80 It is important to note that other sleep disorders that can co-occur with OSA and cause EDS, such as circadian rhythm disorders, idiopathic hypersomnia, narcolepsy, and Kleine-Levin syndrome, also have increased co-occurrence with psychiatric disorders and symptomatology, which may make it particularly challenging to establish the origin of EDS in these populations.81,82
MANAGING PATIENTS WITH PSYCHIATRIC DISORDERS WHO HAVE COMORBID OSA
Considerations for Psychiatric Pharmacotherapy in Patients With OSA
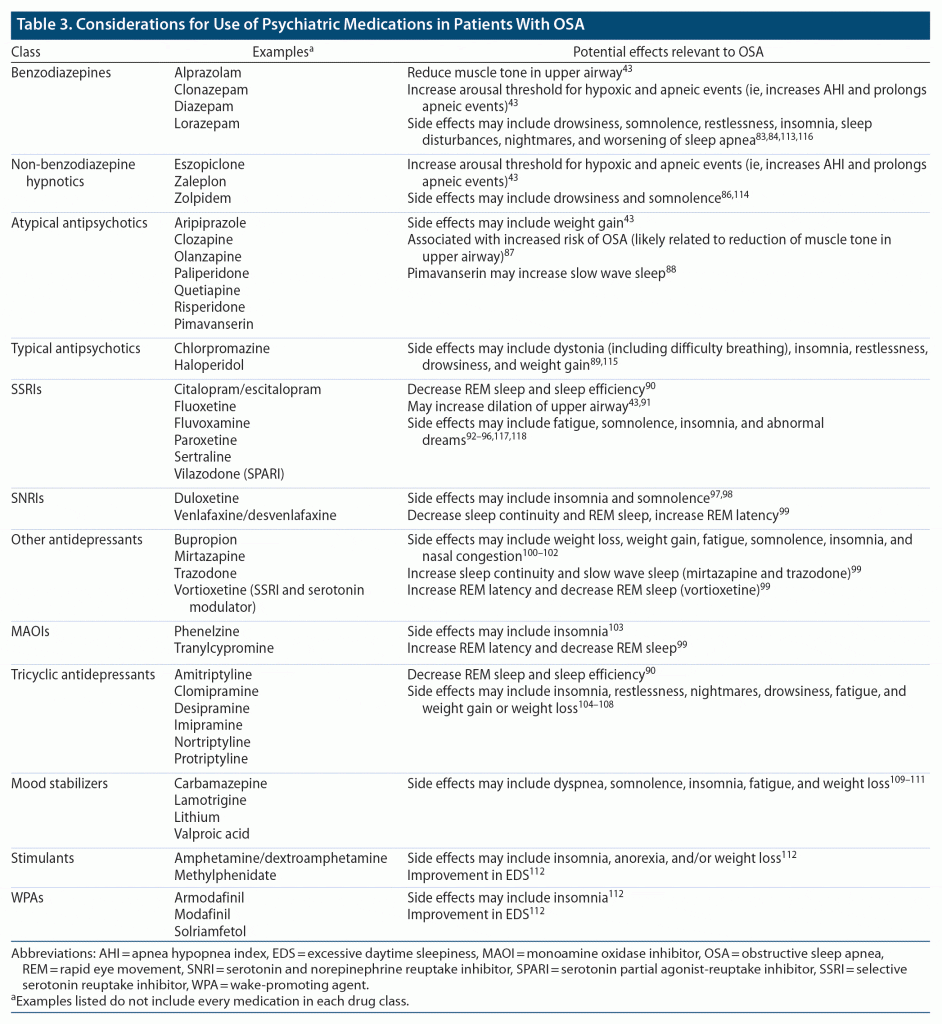
When managing patients with psychiatric disorders who have comorbid OSA, it is important for psychiatric clinicians to consider effects of psychiatric medications that can increase risk or exacerbate symptoms of OSA (Table 3).43,83–118 For instance, benzodiazepines and other hypnotics that have inhibitory effects on the central nervous system can both decrease muscle tone in the upper airway and increase the arousal threshold for hypoxic and apneic events, thereby worsening symptoms of OSA.43 Notably, these effects may not be applicable to all hypnotics (eg, there is no significant evidence that such effects are observed with ramelteon, doxepin, and orexin antagonists [suvorexant and lemborexant] in mild-to-moderate OSA, although they have not been systematically studied in severe OSA). Nonetheless, sedating agents should be used with caution in patients with untreated OSA who are not adequately treated.
Atypical antipsychotics have been associated with a 2-fold increased risk of developing severe OSA.87 One reason for this increased risk is the potential for weight gain associated with most atypical antipsychotics119–124; however, evidence has also suggested that, similar to benzodiazepines, atypical antipsychotics may also reduce muscle tone in the upper airway, suggesting a weight-independent association between atypical antipsychotics and OSA.87 Interestingly, one study125 found that while atypical antipsychotic users with depression had an increased risk of OSA compared to those without depression (OR, 4.53; P < .05), users without depression or with multiple psychiatric diagnoses did not have an elevated risk for OSA.
In general, antidepressant medications have been associated with a reduction in the amount of time spent in rapid eye movement (REM) sleep.90 Since apneas are more likely to occur during REM sleep, it has been hypothesized that reducing the amount of time spent in REM may provide a protective mechanism against sleep-related breathing disorder events.43,90 Evidence suggests that selective serotonin reuptake inhibitors (SSRIs), such as fluoxetine and paroxetine, may reduce the AHI; however, it is unclear whether these agents have a meaningful therapeutic effect on OSA or associated daytime impairments.43,91,126
In summary, psychiatric clinicians should be aware of the potential impacts psychiatric medications can have on OSA symptomatology and sleep so that they can choose the most appropriate agent for an individual patient. For example, for patients with OSA who experience EDS, it may be prudent to choose a medication with alerting effects or the least associated sedation and avoid medications with prominent sedative or muscle-relaxant effects.
Managing OSA in Patients With Psychiatric Disorders
PAP therapy (CPAP, bilevel PAP, or autotitrating PAP) is the primary treatment recommended by the American Academy of Sleep Medicine for the treatment of OSA. PAP therapies warrant monitoring and adjustments to optimize adherence and efficacy. As such, OSA is best managed, at least initially, by a sleep medicine specialist.127 Adherence with CPAP therapy varies by demographics and disease characteristics, as well as technological and psychosocial factors (eg, coping skills, social support).128,129 In people with psychiatric disorders, symptoms may affect patient adherence with PAP therapy. A meta-analysis found that patients with PTSD and OSA had significantly lower adherence compared with patients with OSA alone (regular use: g = −0.658, 95% CI, −0.856 to −0.460; time of average use per night: g = −0.873, 95% CI, −1.550 to −0.196).21 An analysis of data from the Apnea Positive Pressure Long-term Efficacy Study (APPLES) study found that anxiety was related to lower adherence during months 5 and 6 (3.9 ± 2.0 hours versus 4.8 ± 2.1 hours, P = .03) and in a linear regression model was independently associated with lower mean hours of CPAP use (B [95% CI], −0.85 [−1.6 to −0.01]; t = −2.24; P = .02).130 In light of evidence demonstrating improvement in psychiatric outcomes with PAP (discussed above), psychiatric clinicians should strive to achieve effective treatment of psychiatric symptoms as part of efforts to increase adherence to PAP therapy in their patients with OSA. Alternative therapeutic options are also available for patients who cannot tolerate CPAP, such as oral appliances to advance the mandible, positional therapy (ie, avoiding a supine sleep position), hypoglossal nerve stimulation, or surgery for structural issues.131 In addition, lifestyle changes, such as weight loss intervention, should be recommended to all overweight or obese patients, even those who are treated with PAP therapy.
However, where residual EDS persists in the context of optimized treatment of OSA, pharmacotherapy may be considered.132 Even with good adherence to PAP (use > 4 hours per night) and adequate control of AHI, an estimated 9%–22% of patients with OSA continue to experience EDS.133,134 It has been hypothesized that residual EDS may be due to exposure to chronic intermittent hypoxia and sleep fragmentation, resulting in irreversible neuronal damage in wake-promoting brain regions.52 Considering that suboptimally treated OSA can in turn exacerbate psychiatric disorders, identification and management of EDS in patients with OSA are particularly important in this population. Indeed, the management of EDS in OSA has been the subject of several recent reviews.52,80,112,135 Key recommendations include the following:
- Follow-up assessments of EDS as well as tolerability and safety of medications
- Maximization of CPAP therapy adherence
- Referral to a sleep specialist in cases in which the clinician is unable to manage a patient’s EDS.80
Managing Residual EDS in Patients With Psychiatric Disorders
Pharmacologic agents approved for the treatment of EDS in adults with OSA in the US include solriamfetol,136 modafinil,137 and armodafinil.138 Solriamfetol has been shown to reduce EDS in participants with OSA (who were currently using or had previously failed primary OSA therapy)139–141 and is associated with improvements in quality of life, daily functioning, and work productivity,72 but insomnia is a common adverse event.52 Recent evidence has shown that a clinical history of depression does not affect the response to solriamfetol treatment (ie, EDS and common adverse events), relative to placebo, in patients with OSA142; however, research regarding the effects of solriamfetol on current depressive (as well as other psychiatric) symptoms in patients with psychiatric disorders is needed.
Modafinil and armodafinil produce similar improvements in EDS in patients with OSA treated with CPAP and have also been shown to improve quality of life, but may be associated with insomnia as well.52,143 Several studies have shown that modafinil/armodafinil (administered adjunctly with antidepressants) improve EDS and fatigue in adults with major depression who had a partial response to antidepressant treatment (eg, SSRIs).144–146 Some, but not all, studies suggest that modafinil/armodafinil may improve depressive symptoms in patients with unipolar or bipolar disorder.144–148
LIMITATIONS
This narrative review discusses the complex relationship between OSA, psychiatric disorders, and psychiatric medications, highlighting the importance of and challenges associated with identifying and treating OSA in psychiatric populations. Although this review provides a significant contribution to the educational literature for both psychiatrists and sleep specialists, there are limitations that should be noted. Narrative reviews are inherently associated with risk of bias due to lack of explicit criteria for article selection. Although the conclusions presented here are based on evidence, future systematic reviews and/or meta-analyses would help validate these findings. For example, a recent systematic review reported similar findings in terms of the prevalence of OSA among patients with psychiatric disorders; however, the included studies were found to have considerable heterogeneity and high risk of publication and selection bias.18 In addition, available data are primarily concentrated in patients with depression or PTSD; research in patients with OSA and other psychiatric disorders is warranted.
CONCLUSIONS
The overlap of symptoms between OSA and psychiatric disorders can lead to misdiagnosis and contribute to nonresponse to treatment in patients with psychiatric conditions. Given the high comorbidity rate and potential complex interactions between etiology, symptomatology, and treatments, it is critical that psychiatric clinicians not assume that sleep-related symptoms, such as EDS, are simply consequences of psychiatric illness or medication. Instead, psychiatric clinicians should consider the potential for coexisting OSA that requires treatment and take care to identify and manage OSA in patients with psychiatric illness. Increased awareness and recognition of OSA in patients with psychiatric disorders may help to improve patient outcomes, including response to treatment, quality of life, and overall health.
Submitted: May 4, 2021; accepted October 3, 2022.
Published online: January 23, 2023.
Relevant financial relationships: Dr Benca has received grant support from Merck, American Academy of Sleep Medicine Foundation, and NIH and has served as a consultant for Eisai, Idorsia Pharmaceuticals, Janssen, Jazz Pharmaceuticals, Merck, and Sage. Dr Krystal has received research grant support from NIH, Janssen, Jazz Pharmaceuticals, Axsome Therapeutics, and Reveal Biosensors and has served as a consultant for Adare Pharmaceuticals, Eisai Pharmaceuticals, Ferring Pharmaceuticals, Galderma, Idorsia Pharmaceuticals, Jazz Pharmaceuticals, Janssen, Takeda, Merck, Neurocrine, Pernix, and Physician’s Seal. Dr Chepke has served on the advisory boards of AbbVie, Acadia, Alkermes, Eisai, Intracellular, Ironshore, Janssen, Jazz, Lundbeck, Karuna, Neurocrine, Noven, Otsuka, Takeda, and Teva; has served as a consultant for AbbVie, Acadia, Alkermes, Corium, Eisai, Intracellular, Janssen, Jazz, Lundbeck, Neurocrine, Noven, and Otsuka; has received grant/research support from Acadia, Axsome, Harmony, Neurocrine, Teva; and has been on speaker’s bureaus for AbbVie, Acadia, Alkermes, Eisai, Genomind, Intracellular, Ironshore, Janssen, Jazz, Lundbeck, Merck, Neurocrine, Noven, Otsuka, Sunovion, Takeda, and Teva. Dr Doghramji is a consultant for and participates in research that is supported by Jazz Pharmaceuticals.
Funding/support: This review was supported by Jazz Pharmaceuticals and Axsome Therapeutics, Inc. At the time the study was conducted, Jazz Pharmaceuticals had worldwide development, manufacturing, and commercialization rights to solriamfetol, excluding certain jurisdictions in Asia. Jazz Pharmaceuticals completed the divestiture of Sunosi® (solriamfetol) to Axsome Therapeutics, Inc. in the US on May 9, 2022, and ex-US on November 14, 2022. SK Biopharmaceuticals, the discoverer of the compound (also known as SKL-N05), maintains rights in 12 Asian markets, including Korea, China, and Japan.
Role of the sponsor: Jazz Pharmaceuticals and Axsome Therapeutics were involved in the review of the manuscript; however, the content of this manuscript, the ultimate interpretation, and the decision to submit it for publication in The Journal of Clinical Psychiatry were made by the authors independently.
Acknowledgments: Writing and editorial assistance was provided to the authors by Hannah K. Ritchie, PhD, of Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, and was funded by Jazz Pharmaceuticals and Axsome Therapeutics, Inc.
Supplementary material: Available at Psychiatrist.com.
CLINICAL POINTS
- There is a high prevalence of comorbid obstructive sleep apnea (OSA) among patients with psychiatric disorders, and the relationship between OSA and psychiatric disorders is complex.
- Untreated or suboptimally treated OSA can negatively impact mental/physical health and impair response to psychiatric treatment.
- If a patient presents with sleep-related symptoms, psychiatric clinicians should consider the potential for coexisting OSA.
References (148)

- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. American Psychiatric Publishing; 2013.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. American Academy of Sleep Medicine; 2014.
- Waterman L, Stahl ST, Buysse DJ, et al. Self-reported obstructive sleep apnea is associated with nonresponse to antidepressant pharmacotherapy in late-life depression. Depress Anxiety. 2016;33(12):1107–1113. PubMed CrossRef
- Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687–698. PubMed CrossRef
- Hombali A, Seow E, Yuan Q, et al. Prevalence and correlates of sleep disorder symptoms in psychiatric disorders. Psychiatry Res. 2019;279:116–122. PubMed CrossRef
- McCall WV, Benca RM, Rumble ME, et al. Prevalence of obstructive sleep apnea in suicidal patients with major depressive disorder. J Psychiatr Res. 2019;116:147–150. PubMed CrossRef
- Stubbs B, Vancampfort D, Veronese N, et al. The prevalence and predictors of obstructive sleep apnea in major depressive disorder, bipolar disorder and schizophrenia: a systematic review and meta-analysis. J Affect Disord. 2016;197:259–267. PubMed CrossRef
- Talih FR, Ajaltouni JJ, Tamim HM, et al. Risk of obstructive sleep apnea and excessive daytime sleepiness in hospitalized psychiatric patients. Neuropsychiatr Dis Treat. 2017;13:1193–1200. PubMed CrossRef
- Aftab Z, Anthony AT, Rahmat S, et al. An updated review on the relationship of depressive symptoms in obstructive sleep apnea and continuous positive airway pressure. Cureus. 2021;13(6):e15907. PubMed CrossRef
- Edwards C, Mukherjee S, Simpson L, et al. Depressive symptoms before and after treatment of obstructive sleep apnea in men and women. J Clin Sleep Med. 2015;11(9):1029–1038. PubMed CrossRef
- Zheng D, Xu Y, You S, et al. Effects of continuous positive airway pressure on depression and anxiety symptoms in patients with obstructive sleep apnoea: results from the sleep apnoea cardiovascular endpoint randomised trial and meta-analysis. EClinicalMedicine. 2019;11:89–96. PubMed CrossRef
- El-Solh AA, Homish GG, Ditursi G, et al. A randomized crossover trial evaluating continuous positive airway pressure versus mandibular advancement device on health outcomes in veterans with posttraumatic stress disorder. J Clin Sleep Med. 2017;13(11):1327–1335. PubMed CrossRef
- Mysliwiec V, Capaldi VF 2nd, Gill J, et al. Adherence to positive airway pressure therapy in US military personnel with sleep apnea improves sleepiness, sleep quality, and depressive symptoms. Mil Med. 2015;180(4):475–482. PubMed CrossRef
- Roest AM, Carney RM, Stein PK, et al. Obstructive sleep apnea/hypopnea syndrome and poor response to sertraline in patients with coronary heart disease. J Clin Psychiatry. 2012;73(1):31–36. PubMed CrossRef
- Practice Guideline for the Treatment of Patients With Major Depressive Disorder, Third Edition. American Psychiatric Association; 2010.
- Reddy A, Mansuri Z, Vadukapuram R, et al. Increased suicidality and worse outcomes in MDD patients with OSA: a nationwide inpatient analysis of 11 years from 2006 to 2017. J Acad Consult Liaison Psychiatry. 2022;63(1):46–52. PubMed CrossRef
- Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. PubMed CrossRef
- Gupta MA, Simpson FC. Obstructive sleep apnea and psychiatric disorders: a systematic review. J Clin Sleep Med. 2015;11(2):165–175. PubMed CrossRef
- Hein M, Lanquart JP, Loas G, et al. Prevalence and risk factors of moderate to severe obstructive sleep apnea syndrome in major depression: a observational and retrospective study on 703 subjects. BMC Pulm Med. 2017;17(1):165. PubMed CrossRef
- Sharafkhaneh A, Giray N, Richardson P, et al. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. 2005;28(11):1405–1411. PubMed CrossRef
- Zhang Y, Weed JG, Ren R, et al. Prevalence of obstructive sleep apnea in patients with posttraumatic stress disorder and its impact on adherence to continuous positive airway pressure therapy: a meta-analysis. Sleep Med. 2017;36:125–132. PubMed CrossRef
- Tanielian M, Doghramji K, Certa K. Obstructive sleep apnea in psychiatric inpatients. J Nerv Ment Dis. 2020;208(3):190–193. PubMed CrossRef
- Fehr BS, Katz WF, Van Enkevort EA, et al. Obstructive sleep apnea in posttraumatic stress disorder comorbid with mood disorder: significantly higher incidence than in either diagnosis alone. Prim Care Companion CNS Disord. 2018;20(4):18m02281. PubMed CrossRef
- Colvonen PJ, Masino T, Drummond SP, et al. Obstructive sleep apnea and posttraumatic stress disorder among OEF/OIF/OND veterans. J Clin Sleep Med. 2015;11(5):513–518. PubMed CrossRef
- Haddad JM, Chen ES. Identifying psychiatric comorbidities for obstructive sleep apnea in the Biomedical Literature and Electronic Health Record. AMIA Jt Summits Transl Sci Proc. 2017;2017:502–511. PubMed
- Ohayon MM. The effects of breathing-related sleep disorders on mood disturbances in the general population. J Clin Psychiatry. 2003;64(10):1195–1200, quiz, 1274–1276. PubMed CrossRef
- Lu MK, Tan HP, Tsai IN, et al. Sleep apnea is associated with an increased risk of mood disorders: a population-based cohort study. Sleep Breath. 2017;21(2):243–253. PubMed CrossRef
- Kim JY, Ko I, Kim DK. Association of obstructive sleep apnea with the risk of affective disorders. JAMA Otolaryngol Head Neck Surg. 2019;145(11):1020–1026. PubMed CrossRef
- Chang WP, Liu ME, Chang WC, et al. Sleep apnea and the risk of dementia: a population-based 5-year follow-up study in Taiwan. PLoS One. 2013;8(10):e78655. PubMed CrossRef
- Peppard PE, Szklo-Coxe M, Hla KM, et al. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med. 2006;166(16):1709–1715. PubMed CrossRef
- LaGrotte C, Fernandez-Mendoza J, Calhoun SL, et al. The relative association of obstructive sleep apnea, obesity and excessive daytime sleepiness with incident depression: a longitudinal, population-based study. Int J Obes (Lond). 2016;40(9):1397–1404. PubMed CrossRef
- Sweetman A, Lack L, McEvoy RD, et al. Bi-directional relationships between co-morbid insomnia and sleep apnea (COMISA). Sleep Med Rev. 2021;60:101519. PubMed CrossRef
- Luyster FS, Buysse DJ, Strollo PJ Jr. Comorbid insomnia and obstructive sleep apnea: challenges for clinical practice and research. J Clin Sleep Med. 2010;6(2):196–204. PubMed CrossRef
- Krell SB, Kapur VK. Insomnia complaints in patients evaluated for obstructive sleep apnea. Sleep Breath. 2005;9(3):104–110. PubMed CrossRef
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62. https://dcf.psychiatry.ufl.edu/files/2011/05/HAMILTON-DEPRESSION.pdf
- Williams JB, Kobak KA. Development and reliability of a structured interview guide for the Montgomery Asberg Depression Rating Scale (SIGMA). Br J Psychiatry. 2008;192(1):52–58. PubMed CrossRef
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. PubMed CrossRef
- McNair D, Lorr M, Droppleman LF. Manual for the Profile of Mood States. Educational and Industrial Testing Service; 1971. https://www.brianmac.co.uk/poms.htm
- Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4(6):561–571. PubMed CrossRef
- Aikens JE, Caruana-Montaldo B, Vanable PA, et al. MMPI correlates of sleep and respiratory disturbance in obstructive sleep apnea. Sleep. 1999;22(3):362–369. PubMed CrossRef
- Karamanlı H, Kayhan F, Akgedik R. Depressive symptoms in patients with obstructive sleep apnea. Turk Thorac J. 2016;17(3):109–113. PubMed CrossRef
- Krakow B, Melendrez D, Johnston L, et al. Sleep-disordered breathing, psychiatric distress, and quality of life impairment in sexual assault survivors. J Nerv Ment Dis. 2002;190(7):442–452. PubMed CrossRef
- Heck T, Zolezzi M. Obstructive sleep apnea: management considerations in psychiatric patients. Neuropsychiatr Dis Treat. 2015;11:2691–2698. PubMed
- El-Solh AA, Vermont L, Homish GG, et al. The effect of continuous positive airway pressure on post-traumatic stress disorder symptoms in veterans with post-traumatic stress disorder and obstructive sleep apnea: a prospective study. Sleep Med. 2017;33:145–150. PubMed CrossRef
- Povitz M, Bolo CE, Heitman SJ, et al. Effect of treatment of obstructive sleep apnea on depressive symptoms: systematic review and meta-analysis. PLoS Med. 2014;11(11):e1001762. PubMed CrossRef
- Harris LM, Huang X, Linthicum KP, et al. Sleep disturbances as risk factors for suicidal thoughts and behaviours: a meta-analysis of longitudinal studies. Sci Rep. 2020;10(1):13888. PubMed CrossRef
- Bishop TM, Ashrafioun L, Pigeon WR. The association between sleep apnea and suicidal thought and behavior: an analysis of national survey data. J Clin Psychiatry. 2018;79(1):17m11480. PubMed CrossRef
- Gupta MA, Jarosz P. Obstructive sleep apnea severity is directly related to suicidal ideation in posttraumatic stress disorder. J Clin Sleep Med. 2018;14(3):427–435. PubMed CrossRef
- Reddy A, Mansuri Z, Trivedi C, et al. Does obstructive sleep apnea increase suicidality in patients with bipolar disorder? (abstract 760) Sleep. 2021;44(suppl 2):A296. CrossRef
- Zhang J, Weaver TE, Zhong Z, et al. White matter structural differences in OSA patients experiencing residual daytime sleepiness with high CPAP use: a non-Gaussian diffusion MRI study. Sleep Med. 2019;53:51–59. PubMed CrossRef
- Xiong Y, Zhou XJ, Nisi RA, et al. Brain white matter changes in CPAP-treated obstructive sleep apnea patients with residual sleepiness. J Magn Reson Imaging. 2017;45(5):1371–1378. PubMed CrossRef
- Lal C, Weaver TE, Bae CJ, et al. Excessive daytime sleepiness in obstructive sleep apnea: mechanisms and clinical management. Ann Am Thorac Soc. 2021;18(5):757–768. PubMed CrossRef
- Cross RL, Kumar R, Macey PM, et al. Neural alterations and depressive symptoms in obstructive sleep apnea patients. Sleep. 2008;31(8):1103–1109. PubMed
- Kumar R, Macey PM, Cross RL, et al. Neural alterations associated with anxiety symptoms in obstructive sleep apnea syndrome. Depress Anxiety. 2009;26(5):480–491. PubMed CrossRef
- Liguori C, Mercuri NB, Izzi F, et al. Obstructive sleep apnea is associated with early but possibly modifiable Alzheimer’s disease biomarkers changes. Sleep. 2017;40(5):zsx011. PubMed CrossRef
- Cai L, Huang J. Schizophrenia and risk of dementia: a meta-analysis study. Neuropsychiatr Dis Treat. 2018;14:2047–2055. PubMed CrossRef
- Cantón-Habas V, Rich-Ruiz M, Romero-Saldaña M, et al. Depression as a risk factor for dementia and Alzheimer’s disease. Biomedicines. 2020;8(11):457. PubMed CrossRef
- Zilkens RR, Bruce DG, Duke J, et al. Severe psychiatric disorders in mid-life and risk of dementia in late-life (age 65-84 years): a population based case-control study. Curr Alzheimer Res. 2014;11(7):681–693. PubMed CrossRef
- Minhas S, Patel JR, Malik M, et al. Mind-body connection: cardiovascular sequelae of psychiatric illness. Curr Probl Cardiol. 2022;47(10):100959. PubMed CrossRef
- Lévy P, Kohler M, McNicholas WT, et al. Obstructive sleep apnoea syndrome. Nat Rev Dis Primers. 2015;1(1):15015. PubMed CrossRef
- McNicholas WT, Bonsignore MR, Lévy P, et al. Mild obstructive sleep apnoea: clinical relevance and approaches to management. Lancet Respir Med. 2016;4(10):826–834. PubMed CrossRef
- Mazzotti DR, Keenan BT, Lim DC, et al. Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes. Am J Respir Crit Care Med. 2019;200(4):493–506. PubMed CrossRef
- Drager LF, McEvoy RD, Barbe F, et al; INCOSACT Initiative (International Collaboration of Sleep Apnea Cardiovascular Trialists). Sleep apnea and cardiovascular disease: lessons from recent trials and need for team science. Circulation. 2017;136(19):1840–1850. PubMed CrossRef
- Hirsch Allen AJM, Bansback N, Ayas NT. The effect of OSA on work disability and work-related injuries. Chest. 2015;147(5):1422–1428. PubMed CrossRef
- Nena E, Steiropoulos P, Constantinidis TC, et al. Work productivity in obstructive sleep apnea patients. J Occup Environ Med. 2010;52(6):622–625. PubMed CrossRef
- Jermann F, Perroud N, Favre S, et al. Quality of life and subjective sleep-related measures in bipolar disorder and major depressive disorder. Qual Life Res. 2022;31(1):117–124. PubMed
- Ward KL, Hillman DR, James A, et al. Excessive daytime sleepiness increases the risk of motor vehicle crash in obstructive sleep apnea. J Clin Sleep Med. 2013;9(10):1013–1021. PubMed CrossRef
- Hill LL, Lauzon VL, Winbrock EL, et al. Depression, antidepressants and driving safety. Inj Epidemiol. 2017;4(1):10. PubMed CrossRef
- Uvais NA. Mental health conditions and the risk of road traffic accidents. J Family Med Prim Care. 2019;8(6):2166–2167. PubMed CrossRef
- Zhao YY, Wang R, Gleason KJ, et al; BestAIR Investigators. Effect of continuous positive airway pressure treatment on health-related quality of life and sleepiness in high cardiovascular risk individuals with sleep apnea: Best Apnea Interventions for Research (BestAIR) Trial. Sleep. 2017;40(4):zsx040. PubMed CrossRef
- Hirshkowitz M, Black J. Effect of adjunctive modafinil on wakefulness and quality of life in patients with excessive sleepiness-associated obstructive sleep apnoea/hypopnoea syndrome: a 12-month, open-label extension study. CNS Drugs. 2007;21(5):407–416. PubMed CrossRef
- Weaver TE, Drake CL, Benes H, et al. Effects of solriamfetol on quality of life measures from a 12-week phase 3 randomized trial. Ann Am Thorac Soc. 2020;17(8):998–1007. PubMed CrossRef
- Lin YC, Chen TY, Chien WC, et al. Stimulants associated with reduced risk of hospitalization for motor vehicle accident injury in patients with obstructive sleep apnea: a nationwide cohort study. BMC Pulm Med. 2020;20(1):28. PubMed CrossRef
- Chervin RD. Sleepiness, fatigue, tiredness, and lack of energy in obstructive sleep apnea. Chest. 2000;118(2):372–379. PubMed CrossRef
- Smith S, Rossdale J, Serry Y, et al. Multiple dimensions of excessive daytime sleepiness. J Thorac Dis. 2018;10(suppl 1):S170–S176. PubMed CrossRef
- Marti-Soler H, Hirotsu C, Marques-Vidal P, et al. The NoSAS score for screening of sleep-disordered breathing: a derivation and validation study. Lancet Respir Med. 2016;4(9):742–748. PubMed CrossRef
- Chung F, Abdullah HR, Liao P. STOP-Bang questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149(3):631–638. PubMed CrossRef
- Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–491. PubMed CrossRef
- Guichard K, Marti-Soler H, Micoulaud-Franchi JA, et al. The NoSAS score: a new and simple screening tool for obstructive sleep apnea syndrome in depressive disorder. J Affect Disord. 2018;227:136–140. PubMed CrossRef
- Rosenberg R, Schweitzer PK, Steier J, et al. Residual excessive daytime sleepiness in patients treated for obstructive sleep apnea: guidance for assessment, diagnosis, and management. Postgrad Med. 2021;133(7):772–783. PubMed CrossRef
- Sowa NA. Idiopathic hypersomnia and hypersomnolence disorder: a systematic review of the literature. Psychosomatics. 2016;57(2):152–164. PubMed CrossRef
- Barateau L, Lopez R, Franchi JA, et al. Hypersomnolence, hypersomnia, and mood disorders. Curr Psychiatry Rep. 2017;19(2):13. PubMed CrossRef
- Xanax [package insert]. Pfizer Inc; 2011.
- Valium [package insert]. Genentech; 2021.
- Roth T, Dauvilliers Y, Mignot E, et al. Disrupted nighttime sleep in narcolepsy. J Clin Sleep Med. 2013;9(9):955–965. PubMed CrossRef
- Ambien [package insert]. Sanofi-Aventis US; 2008.
- Rishi MA, Shetty M, Wolff A, et al. Atypical antipsychotic medications are independently associated with severe obstructive sleep apnea. Clin Neuropharmacol. 2010;33(3):109–113. PubMed CrossRef
- Ancoli-Israel S, Vanover KE, Weiner DM, et al. Pimavanserin tartrate, a 5-HT(2A) receptor inverse agonist, increases slow wave sleep as measured by polysomnography in healthy adult volunteers. Sleep Med. 2011;12(2):134–141. PubMed CrossRef
- Haldol [package insert]. Ortho-McNeil Pharmaceutical; 2009.
- Smith SS, Dingwall K, Jorgenson G, et al. Associations between the use of common medications and sleep architecture in patients with untreated obstructive sleep apnea. J Clin Sleep Med. 2006;2(2):156–162. PubMed CrossRef
- Kraiczi H, Hedner J, Dahlöf P, et al. Effect of serotonin uptake inhibition on breathing during sleep and daytime symptoms in obstructive sleep apnea. Sleep. 1999;22(1):61–67. PubMed
- Paxil [package insert]. GlaxoSmithKline; 2012.
- Prozac [package insert]. Eli Lilly and Company; 2017.
- Celexa [package insert]. Forest Pharmaceuticals; 2009.
- Viibryd [package insert]. Trovis Pharmaceuticals; 2010.
- Luvox [package insert]. Jazz Pharmaceuticals; 2008.
- Effexor [package insert]. Wyeth Pharmaceuticals; 2008.
- Cymbalta [package insert]. Eli Lilly and Company; 2010.
- Wichniak A, Wierzbicka A, Walęcka M, et al. Effects of antidepressants on sleep. Curr Psychiatry Rep. 2017;19(9):63. PubMed CrossRef
- Desyrel [package insert]. Pragma Pharmaceuticals; 2017.
- Wellbutrin [package insert]. GlaxoSmithKline; 2017.
- Remeron [package insert]. Organon USA; 2007.
- Zelapar [package insert]. Valeant Pharmaceuticals North America; 2008.
- Amitriptyline HCl [package insert]. Sandoz Inc; 2014.
- Protriptyline Hydrochloride [package insert]. West-Ward Pharmaceuticals; 2016.
- Imipramine Hydrochloride [package insert]. Excellium Pharmaceuticals; 2012.
- Norpramin [package insert]. Sanofi-Aventis US; 2014.
- Anafranil [package insert]. Mallinckrodt Inc; 2012.
- Depakene [package insert]. Abbott Laboratories; 2011.
- Tegretol [package insert]. Novartis Pharmaceuticals Corporation; 2009.
- Lamictal [package insert]. GlaxoSmithKline; 2009.
- Sahni AS, Carlucci M, Malik M, et al. Management of excessive sleepiness in patients with narcolepsy and OSA: current challenges and future prospects. Nat Sci Sleep. 2019;11:241–252. PubMed CrossRef
- Klonopin [package insert]. Genentech; 2013.
- Lunesta [package insert]. Sunovion Pharmaceuticals; 2014.
- Largactil [package insert]. Sanofi-Aventis New Zealand Limited; 2013.
- Ativan C-IV [package insert]. Valeant Pharmaceuticals North America; 2016.
- Zoloft [package insert]. Pfizer Inc.; 2016.
- Lexapro [package insert]. Allergan, Inc; 2017.
- Abilify [package insert]. Otsuka Pharmaceuticals; 2014.
- Clozaril [package insert]. Novartis Pharmaceuticals; 2020.
- Zyprexa [package insert]. Eli Lilly and Company; 2021.
- Invega [package insert]. Janssen Pharmaceuticals; 2021.
- Seroquel [package insert]. AstraZeneca Pharmaceuticals; 2016.
- Risperdal [package insert]. Ortho-McNeil-Janssen Pharmaceuticals; 2009.
- Shirani A, Paradiso S, Dyken ME. The impact of atypical antipsychotic use on obstructive sleep apnea: a pilot study and literature review. Sleep Med. 2011;12(6):591–597. PubMed CrossRef
- Hanzel DA, Proia NG, Hudgel DW. Response of obstructive sleep apnea to fluoxetine and protriptyline. Chest. 1991;100(2):416–421. PubMed CrossRef
- Patil SP, Ayappa IA, Caples SM, et al. Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2019;15(2):301–334. PubMed CrossRef
- Rotty MC, Suehs CM, Mallet JP, et al. Mask side-effects in long-term CPAP-patients impact adherence and sleepiness: the InterfaceVent real-life study. Respir Res. 2021;22(1):17. PubMed CrossRef
- Weaver TE, Sawyer AM. Adherence to continuous positive airway pressure treatment for obstructive sleep apnoea: implications for future interventions. Indian J Med Res. 2010;131:245–258. PubMed
- Budhiraja R, Kushida CA, Nichols DA, et al. Impact of randomization, clinic visits, and medical and psychiatric comorbidities on continuous positive airway pressure adherence in obstructive sleep apnea. J Clin Sleep Med. 2016;12(3):333–341. PubMed CrossRef
- Veasey SC, Rosen IM. Obstructive sleep apnea in adults. N Engl J Med. 2019;380(15):1442–1449. PubMed CrossRef
- Epstein LJ, Kristo D, Strollo PJ Jr, et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276. PubMed CrossRef
- Gasa M, Tamisier R, Launois SH, et al; Scientific Council of the Sleep Registry of the French Federation of Pneumology-FFP. Residual sleepiness in sleep apnea patients treated by continuous positive airway pressure. J Sleep Res. 2013;22(4):389–397. PubMed CrossRef
- Pépin JL, Viot-Blanc V, Escourrou P, et al. Prevalence of residual excessive sleepiness in CPAP-treated sleep apnoea patients: the French multicentre study. Eur Respir J. 2009;33(5):1062–1067. PubMed CrossRef
- Chapman JL, Serinel Y, Marshall NS, et al. Residual daytime sleepiness in obstructive sleep apnea after continuous positive airway pressure optimization: causes and management. Sleep Med Clin. 2016;11(3):353–363. PubMed CrossRef
- Sunosi [package insert]. Jazz Pharmaceuticals, Inc; 2021.
- Provigil [package insert]. Teva Pharmaceuticals; 2018.
- Nuvigil [package insert]. Teva Pharmaceuticals; 2018.
- Strollo PJ Jr, Hedner J, Collop N, et al; Tones 4 Study Investigators. Solriamfetol for the treatment of excessive sleepiness in OSA: a placebo-controlled randomized withdrawal study. Chest. 2019;155(2):364–374. PubMed CrossRef
- Schweitzer PK, Rosenberg R, Zammit GK, et al; TONES 3 Study Investigators. Solriamfetol for excessive sleepiness in obstructive sleep apnea (TONES 3): a randomized controlled trial. Am J Respir Crit Care Med. 2019;199(11):1421–1431. PubMed CrossRef
- Malhotra A, Shapiro C, Pepin JL, et al. Long-term study of the safety and maintenance of efficacy of solriamfetol (JZP-110) in the treatment of excessive sleepiness in participants with narcolepsy or obstructive sleep apnea. Sleep. 2020;43(2):zsz220. PubMed CrossRef
- Krystal AD, Benca RM, Rosenberg R, et al. Solriamfetol treatment of excessive daytime sleepiness in participants with narcolepsy or obstructive sleep apnea with a history of depression. J Psychiatr Res. 2022;155:202–210. PubMed CrossRef
- Kuan YC, Wu D, Huang KW, et al. Effects of modafinil and armodafinil in patients with obstructive sleep apnea: a meta-analysis of randomized controlled trials. Clin Ther. 2016;38(4):874–888. PubMed CrossRef
- Fava M, Thase ME, DeBattista C. A multicenter, placebo-controlled study of modafinil augmentation in partial responders to selective serotonin reuptake inhibitors with persistent fatigue and sleepiness. J Clin Psychiatry. 2005;66(1):85–93. PubMed CrossRef
- DeBattista C, Doghramji K, Menza MA, et al; Modafinil in Depression Study Group. Adjunct modafinil for the short-term treatment of fatigue and sleepiness in patients with major depressive disorder: a preliminary double-blind, placebo-controlled study. J Clin Psychiatry. 2003;64(9):1057–1064. PubMed CrossRef
- Fava M, Thase ME, DeBattista C, et al. Modafinil augmentation of selective serotonin reuptake inhibitor therapy in MDD partial responders with persistent fatigue and sleepiness. Ann Clin Psychiatry. 2007;19(3):153–159. PubMed CrossRef
- Goss AJ, Kaser M, Costafreda SG, et al. Modafinil augmentation therapy in unipolar and bipolar depression: a systematic review and meta-analysis of randomized controlled trials. J Clin Psychiatry. 2013;74(11):1101–1107. PubMed CrossRef
- Nunez NA, Singh B, Romo-Nava F, et al. Efficacy and tolerability of adjunctive modafinil/armodafinil in bipolar depression: a meta-analysis of randomized controlled trials. Bipolar Disord. 2020;22(2):109–120. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite