ABSTRACT
Evidence suggests that women with schizophrenia are less likely to be screened for breast cancer, more likely to suffer from breast cancer, and more likely to die of breast cancer than women without schizophrenia or general population controls. Antipsychotic drugs, and especially prolactin-raising antipsychotic drugs, have been suggested to increase the breast cancer risk, but the evidence has so far been inconclusive. Against this background, a recent, large, nationwide, case-control study in Finland examined the odds of previous prolonged exposure to prolactin-raising and prolactin-sparing antipsychotic drugs in women with schizophrenia who were (cases) versus were not (controls) diagnosed with breast cancer. The study found that, relative to < 1 year of antipsychotic exposure, breast cancer was associated with significantly increased odds of previous, prolonged (> 5 years) exposure to prolactin-raising antipsychotics. The associations were not statistically significant for prolactin-sparing antipsychotics. The study is critically examined from the perspective of interpretation of the odds ratio and its limitations in order to help readers understand how to better evaluate and generalize findings in case-control studies. This is necessary because results in case-control studies are often incorrectly interpreted, and the limitations of the odds ratios derived in such studies are often not recognized. It is concluded that the design and findings of the reviewed study could not allow readers to judge whether or not prolactin-sparing antipsychotics are associated with lower breast cancer risk than prolactin-raising antipsychotics. In contexts other than breast cancer risk, adverse consequences associated with prolactin elevation are well known, and avoidance or management of hyperprolactinemia is therefore desirable.
J Clin Psychiatry 2021;82(6):21f14319
To cite: Andrade C. Understanding the limitations of the odds ratio in a case-control study of the association between breast cancer and exposure to antipsychotic drugs. J Clin Psychiatry. 2021;82(6):21f14319.
To share: https://doi.org/10.4088/JCP.21f14319
© Copyright 2021 Physicians Postgraduate Press, Inc.
Cancer is a leading contributor to the global burden of disease. In 2017, across the world, there were 24.5 million new cases of cancer and 9.6 million deaths due to cancer. In women, breast cancer was the second most common category of cancer with 1.9 million incident cases; breast cancer was also the leading cause of cancer deaths and disability-adjusted life-years (DALYs) with 601,000 deaths and 17.4 million DALYs.1
Breast Cancer and Schizophrenia
A systematic review and meta-analysis of 37 studies found that cancer screening was less frequent in persons with mental illness than in the general population (odds ratio [OR], 0.76; 95% confidence interval [CI], 0.72–0.79). This was true in 27 studies of breast cancer, as well (OR, 0.65; 95% CI, 0.60–0.71).2 In further systematic review and meta-analysis, women with schizophrenia were found to be significantly less likely to receive mammography screening than women without schizophrenia (11 studies; OR, 0.50; 95% CI, 0.38–0.64)3; this is of concern because schizophrenia has been associated with increased breast cancer incidence (12 studies; standardized incidence ratio, 1.31; 95% CI, 1.14–1.50).4
In other meta-analyses, mortality from cancer was found to be higher in patients with schizophrenia than in controls (15 studies; standardized mortality ratio, 1.40; 95% CI, 1.29–1.52, and 4 studies; hazard ratio, 1.51; 95% CI, 1.13–2.03),5 and this was true for breast cancer in women with schizophrenia, as well (7 studies; relative risk [RR], 1.97; 97% CI, 1.38–2.83).6
Antipsychotic Drugs and Breast Cancer
There are theoretical reasons why antipsychotic drugs may increase the risk of breast cancer. For example, antipsychotic drugs may be sedating and they may cause weight gain; lack of physical exercise associated with sedentariness and obesity are both known risk factors for breast cancer. Many antipsychotic drugs raise serum prolactin. On the one hand, if estrogen levels are decreased by raised prolactin levels, the risk of estrogen receptor positive breast cancer may also decrease. On the other hand, specific mechanisms have been identified whereby prolactin may directly raise breast cancer risk.7
Clinical data suggest that higher prolactin levels are associated with increased risk. In a meta-analysis of 7 observational studies, relative to the lowest levels of plasma prolactin, the highest levels were associated with an increased risk of breast cancer (RR, 1.16; 95% CI, 1.04–1.29); this relationship, though, was statistically significant only in postmenopausal and not in premenopausal women.8 However, a review of the relationship between antipsychotics, prolactin, and breast cancer risk in patients with schizophrenia yielded inconclusive results.9
Against this background, Taipale et al10 described a large, nationwide, nested case-control study of the relationship between breast cancer and antipsychotic use in Finnish women with schizophrenia. The study specifically examined risks associated with prolactin-raising and prolactin-sparing antipsychotic drugs. The study is important because it was large, spanned a long duration, and had data on a long period of cumulative antipsychotic drug use. It was published in an important journal, was disseminated by several academic alert services, and was summarized at a popular educational website. However, the authors interpreted and used the ORs (estimated in their analyses) in a way that is problematic. These statistical issues are discussed in some detail because an understanding will improve the ability of the reader to critically read and interpret the findings of case-control studies.
The Finland Study
Taipale et al10 used linked nationwide registers in Finland to identify a cohort of 30,785 women, aged 16 years or older, who were diagnosed with schizophrenia during 1972–2014. Within this cohort, they identified 1,069 women (cases) histologically diagnosed with invasive breast cancer during 2000–2014. All cases had at least 5 years of data on antipsychotic use before the cancer diagnosis. Women with biases such as a previous cancer diagnosis were excluded. Each case was matched with up to 5 controls (n = 5,339) without breast cancer, based on age, time since first diagnosis of schizophrenia, and absence of cancer diagnosis before matching.
The mean age of the sample was 62 years. The mean time since schizophrenia diagnosis was 24 years. Ductal adenocarcinoma accounted for 73% of cases, and lobular adenocarcinoma for an additional 20%. Cancer was localized in 39% of the cases. About 28% of women had used hormone replacement therapy. In cases and controls, risperidone and perphenazine were each used by about 30% of women. Thioridazine, olanzapine, and levomepromazine were each used in about a quarter of the women (the same woman may have used more than 1 antipsychotic). Curiously, no information was provided about aripiprazole, which was an important drug under study. Cases and controls were closely similar on almost all important background variables.
Cases and controls were compared using conditional logistic regression, adjusted for about 20 illness- and medication-related confounding variables. The principal outcome of interest was the odds of antipsychotic exposure in different exposure bands, expressed (in most analyses) in terms of years of antipsychotic exposure with the reference group being < 1 year of exposure or nil exposure. Analyses were run separately for prolactin-sparing (clozapine, quetiapine, aripiprazole) and prolactin-raising (risperidone, perphenazine, thioridazine, olanzapine, levomepromazine, chlorprothixene, chlorpromazine, haloperidol, and zuclopenthixol) antipsychotics. A large number of subgroup and sensitivity analyses were also run.
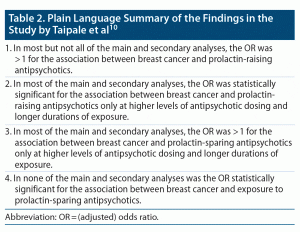
Important findings from the study are presented in Table 1, and the overall findings are summarized in Table 2.
Strengths and Limitations
The study10 had many strengths. As already stated, it linked national registers and was therefore a population-based study. It spanned several decades. The data on cumulative antipsychotic use were substantial in terms of both dosing and duration of treatment. Exposures were thoughtfully defined; for example, simultaneous use of prolactin-sparing and prolactin-raising drugs was coded as prolactin-raising. Many subgroup and sensitivity analyses were performed. Several analyses were thoughtfully planned; for example, sensitivity analyses contrasted the effects of prolactin-raising antipsychotics when aripiprazole was versus was not concurrently prescribed (this is important because concurrently prescribed aripiprazole can lower serum prolactin).
However, the study10 also had limitations. For example, the authors could not adjust their analyses for breast cancer risk factors such as physical activity levels, body mass index, smoking, alcohol intake, oral contraceptive use, family history of breast cancer, estrogen receptor status of the cancer, and presence of risk-related genes. Adjustment for these variables could have swung the results of the analysis in either direction. There were 2 other limitations, both related to the way in which the results were interpreted. These are considered in the sections that follow.
Absence of Direct Comparison
From the summary in Table 2, it is evident that (a) breast cancer was associated with OR values that were significantly > 1 in relation to prolactin-raising antipsychotics, but that this was so only at higher levels of antipsychotic exposure; and that (b) breast cancer was associated with OR values that were nonsignificantly > 1 in relation to prolactin-sparing antipsychotics, and that this was so only at higher levels of antipsychotic exposure. Based on these findings, Taipale et al10 argued strongly that prolactin-raising (but not prolactin-sparing) antipsychotic drugs raise the risk of breast cancer; in fact, they went so far as to state at the end of their abstract that “monitoring prolactinemia and addressing hyperprolactinemia is paramount in women with schizophrenia being treated with prolactin-increasing antipsychotics.” And, in the last paragraph of their Discussion, they stated, “Therefore, antipsychotics without prolactin-increasing properties should be considered as a first-line long-term treatment for women with schizophrenia.”
Such an interpretation of the findings is premature. All that the authors found was that the ORs associated with prolactin-raising antipsychotics crossed the threshold for statistical significance whereas the ORs associated with prolactin-sparing antipsychotics did not. If A is superior to controls and if B is not superior to controls, one cannot draw conclusions about the relative merits of A and B unless one directly compares A and B. Taipale et al10 did not directly compare prolactin-raising with prolactin-sparing antipsychotics, as would have been necessary to formally support a recommendation for prolactin-sparing over prolactin-raising drugs. To perform such a comparison would have required a rather different approach to analysis from that presented in the study; alternately, as a rough approximation, the authors could have examined the extent to which the CIs overlapped between the groups, or they could test if the ORs for the two groups were different by converting them to log ORs and performing a Wald test on the difference.
Interpretation of the OR
Taipale et al10 interpreted and applied their ORs in ways that are logically and mathematically problematic. In their abstract, they concluded that long-term exposure to prolactin-increasing (but not prolactin-sparing) antipsychotics was associated with significantly increased odds of breast cancer. The wording here is logically problematic.
In the first paragraph of their Discussion, they stated:
-
- Exposure to prolactin-increasing drugs for 5 or more years (adjusted OR 1.56) was associated with 56% higher odds of developing breast cancer than shorter exposure, and no significant association was found with cumulative exposure to prolactin-sparing antipsychotics. Conservatively, if we subtract the 19% non-significantly increased odds with prolactin-sparing antipsychotics from the 56% significantly increased odds with prolactin-increasing antipsychotics, we obtain a 37% relative increase in odds.
They went on to state that, assuming a lifetime incidence of 12% for breast cancer among women in the general population, this 37% relative increase in odds would translate into a 37% × 12%, or an approximately 4%, clinically meaningful increase in absolute breast cancer odds with prolactin-increasing antipsychotic treatment. Their calculations here are mathematically problematic.
There are 3 important reasons why what they wrote and did is problematic. First, had the authors performed a cohort study to compare, looking forward, how many women with schizophrenia who had prolonged versus brief antipsychotic exposure did versus did not develop breast cancer, they could validly conclude that prolonged exposure to prolactin-raising antipsychotics was or was not associated with increased risk of breast cancer. However, what they actually performed was a case-control study in women with schizophrenia, where they identified breast cancer cases and controls and then looked backward to assess how many cases and controls did versus did not have prolonged exposure, in the past, to antipsychotic drugs. This means that they cannot generalize the study findings to women with schizophrenia who are exposed to antipsychotics because they did not sample women with schizophrenia who were exposed to antipsychotics. Rather, they can only generalize their findings to women with schizophrenia who also have breast cancer because such women (and matched controls) comprised their sample. In short, to be logically correct, all that the authors can validly conclude from this case-control study is that breast cancer in women with schizophrenia was associated with an increased odds of previous prolonged exposure to prolactin-raising antipsychotics.
At this juncture, readers may be puzzled; does it really matter how the conclusions are worded? The answer is yes, and this leads to the second point, because wording makes a material difference to the way in which one understands how data are accessed and processed in cohort versus case-control studies, as described above and explained below.
Ordinarily, in a 2-by-2 contingency table, the rows and columns can be interchanged when computing the OR, and it does not matter how the association between variables is interpreted. For example, we may look at a contingency table and its OR and say that men are more likely than women to develop cardiovascular disease or, equally correctly, that cardiovascular disease is more likely to develop in men than in women. However, when there is a difference in what data are accessed, how the OR is interpreted becomes important for a mathematical reason. When looking forward in a cohort study, we can capture data from the entire population of exposed subjects. That is, we can count how many women did versus did not have prolonged exposure to antipsychotic drugs and, among these, how many women did versus did not develop breast cancer in those specified time windows. In such a situation, the OR for breast cancer as an outcome has a fixed value. When looking backward, as in a case-control design, we do not have access to the entire population of exposed subjects. That is, we cannot know how many women did versus did not have prolonged exposure, and so the OR cannot be directly calculated. Furthermore, the value of the OR, as estimated in the logistic regression, is not fixed; it varies with what confounds it is adjusted for.11 In fact, the very direction of association can be reversed after adjustment, and an OR that was < 1 can become > 1.12 This means that the study value of 1.56 for the OR was accurate only for the analysis that was conducted, and not necessarily so for the sample (because the value could change with additional adjustment for unmeasured confounds), let alone for the population (because the population of interest is not women with schizophrenia who have breast cancer but women with schizophrenia who are receiving antipsychotic medication). So wording, and how it alters our understanding, makes a very big difference, indeed.
As an interlude, it is important for readers to understand that the 2 points explained above apply not just to the study by Taipale et al10 but to all case-control studies. The third point is specific to the study by Taipale et al.10 These authors subtracted the OR for prolactin-sparing antipsychotics (OR, 1.19) from the OR for prolactin-raising antipsychotics (OR, 1.56) and concluded that there was a 37% relative increase in odds associated with the use of prolactin-raising antipsychotics. This conclusion is mathematically incorrect. Odds ratios cannot be added or subtracted. To understand why, consider what risk and odds mean in mathematical terms. The “risk” that a tossed coin will fall heads is 0.5 (50%). The risk that it will fall tails is 0.5 (50%). Therefore, the risk that it will fall either heads or tails, as obtained by simple addition, is 0.5 + 0.5 (50% + 50%), or 1.0 (100%). In other words, we’re 100% sure that if a coin is tossed, it will fall either heads or tails. That makes sense. In contrast, the odds that a tossed coin will fall heads is 1:1 (commonly stated as 50-50) or 1.0 because the ratio of 1 to 1 is 1.0. The odds that it will fall tails is likewise 1:1 or 1.0 (note that in both cases 1.0 does not mean 100%; it means 1.0 against a reference value of 1). So, what are the odds that the coin will fall either heads or tails? The answer does not emerge from adding the individual odds, as we added the individual risks. In fact, the question does not even make sense; odds of either heads or tails relative to what as the reference? So, to reiterate, odds cannot be added or subtracted. Readers may wish to refer to an earlier article in this column that explained RRs and ORs in simple terms13 and to an article that explained the limitations of ORs in logistic regression.11
Concluding Notes
This study found that in women with schizophrenia, breast cancer was associated with significantly increased odds of previous, > 5 years exposure to prolactin-raising antipsychotic drugs. The study design and findings could not indicate whether or not the risk of breast cancer was significantly lower in women with schizophrenia who were treated with prolactin-sparing as compared with prolactin-raising antipsychotic drugs. Therefore, no firm recommendations can be made for favoring prolactin-sparing over prolactin-raising antipsychotic drugs, based on this study. However, importantly, in contexts other than breast cancer risk, the undesirable consequences of antipsychotic-associated serum prolactin elevation are well known, and avoidance or management of hyperprolactinemia is therefore desirable.14,15
Published online: November 23, 2021.
Acknowledgments: Dr Andrade thanks Prof David Streiner, PhD, CPsych, Department of Psychiatry and Behavioural Neurosciences, McMaster University, and Department of Psychiatry, University of Toronto, for his careful reading of a previous version of this manuscript and his suggestions for its improvement.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
References (15)

- Fitzmaurice C, Abate D, Abbasi N, et al; Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5(12):1749–1768. PubMed CrossRef
- Solmi M, Firth J, Miola A, et al. Disparities in cancer screening in people with mental illness across the world versus the general population: prevalence and comparative meta-analysis including 4 717 839 people. Lancet Psychiatry. 2020;7(1):52–63. PubMed CrossRef
- Hwong A, Wang K, Bent S, et al. Breast cancer screening in women with schizophrenia: a systematic review and meta-analysis. Psychiatr Serv. 2020;71(3):263–268. PubMed CrossRef
- Zhuo C, Triplett PT. Association of schizophrenia with the risk of breast cancer incidence: a meta-analysis. JAMA Psychiatry. 2018;75(4):363–369. PubMed CrossRef
- Zhuo C, Tao R, Jiang R, et al. Cancer mortality in patients with schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2017;211(1):7–13. PubMed CrossRef
- Ni L, Wu J, Long Y, et al. Mortality of site-specific cancer in patients with schizophrenia: a systematic review and meta-analysis. BMC Psychiatry. 2019;19(1):323. PubMed CrossRef
- Johnston AN, Bu W, Hein S, et al. Hyperprolactinemia-inducing antipsychotics increase breast cancer risk by activating JAK-STAT5 in precancerous lesions. Breast Cancer Res. 2018;20(1):42. PubMed CrossRef
- Wang M, Wu X, Chai F, et al. Plasma prolactin and breast cancer risk: a meta- analysis. Sci Rep. 2016;6(1):25998. PubMed CrossRef
- De Hert M, Peuskens J, Sabbe T, et al. Relationship between prolactin, breast cancer risk, and antipsychotics in patients with schizophrenia: a critical review. Acta Psychiatr Scand. 2016;133(1):5–22. PubMed CrossRef
- Taipale H, Solmi M, Lähteenvuo M, et al. Antipsychotic use and risk of breast cancer in women with schizophrenia: a nationwide nested case-control study in Finland. Lancet Psychiatry. 2021;8(10):883–891. PubMed CrossRef
- Norton EC, Dowd BE, Maciejewski ML. Odds ratios-current best practice and use. JAMA. 2018;320(1):84–85. PubMed CrossRef
- Andrade C. Odd odds. J Clin Psychiatry. 2011;72(11):1558–1559, author reply 1559. PubMed CrossRef
- Andrade C. Understanding relative risk, odds ratio, and related terms: as simple as it can get. J Clin Psychiatry. 2015;76(7):e857–e861. PubMed CrossRef
- Henderson DC. Managing weight gain and metabolic issues in patients treated with atypical antipsychotics. J Clin Psychiatry. 2008;69(suppl 1):e04. PubMed CrossRef
- Peuskens J, Pani L, Detraux J, et al. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs. 2014;28(5):421–453. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top