Abstract
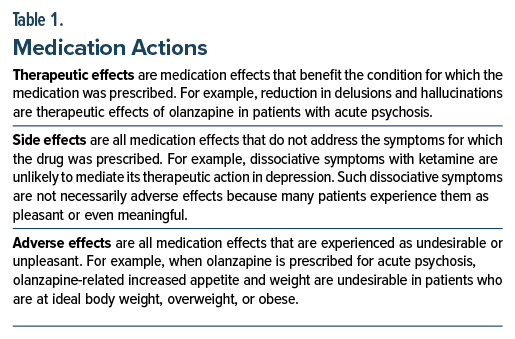
Drugs have actions that may be classified as therapeutic effects and side effects; side effects are actions that do not contribute to therapeutic benefit. Some side effects are neutral; others, experienced as undesirable or unpleasant, are recorded as adverse effects. Some drug actions are therapeutic for some disorders and adverse for others; or therapeutic during acute illness and adverse during maintenance treatment. As an example, anticholinergic action may be adverse when a tricyclic antidepressant is used to treat depression but therapeutic when the drug is used to treat irritable bowel syndrome with diarrhea. In clinical practice, side or adverse effects of a drug may be leveraged to manage troublesome symptoms. As an example, the sedative effect of a low dose of trazodone may be useful for some patients with insomnia. With this background, studies have examined whether the increase in appetite and weight associated with olanzapine and mirtazapine may be effective against anorexia and cachexia associated with cancer and cancer chemotherapy. The subject is important because cachexia may be present in 30%–50% of patients with cancer (with higher prevalence in patients with more advanced cancer) and because the presence of cachexia is associated with a higher risk of disease progression and mortality. Many randomized controlled trials (RCTs) have examined pharmacologic interventions such as progestins, corticosteroids, anamorelin, and medical cannabis for cancer related cachexia; most results have been disappointing. A recent RCT found that olanzapine (2.5 mg/d for 12 weeks) improved appetite, weight, other nutritional parameters, and quality of life in patients with locally advanced or metastatic cancer treated with chemotherapy. Another RCT, however, found that mirtazapine (30 mg/d for 8 weeks) brought no nutritional or anthropometric gain in patients with cancer and anorexia. It is concluded that olanzapine but not mirtazapine merits further investigation in patients with cancer who have anorexia and cachexia.
J Clin Psychiatry 2024;85(3):24f15532
Author affiliations are listed at the end of this article.
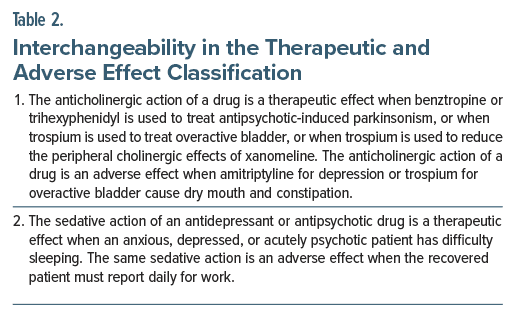
Medications have actions that can be classified as therapeutic effects, side effects, and adverse effects (Table 1). The classification of these actions for a particular drug is not set in stone; what is therapeutic and adverse depends on the context. Here, context can refer to either indication for treatment or phase of treatment (Table 2).
Side or adverse effects of drugs are sometimes leveraged to provide treatment benefits. As example, low doses of trazodone or quetiapine may be used off-label to treat insomnia. Both pharmacokinetic and pharmacodynamic actions can be taken advantage of, as with bupropion and trospium in the dextromethorphan bupropion and the xanomeline-trospium combinations for depression and schizophrenia, respectively. Bupropion inhibits cytochrome P450 (CYP) 2D6, the enzyme that metabolizes dextromethorphan. Inhibition or induction of CYP enzymes is generally considered disadvantageous because of the increased risk of adverse drug interactions. However, in the dextromethorphan bupropion combination, the CYP2D6 inhibition is advantageous because it increases the blood levels and especially the duration of action of dextromethorphan, which otherwise has a short half-life. The anticholinergic actions of trospium are undesirable except to the extent that they reduce the symptoms of overactive bladder, but in the xanomeline-trospium combination, the anticholinergic actions are desirable because they reduce the problematic peripheral cholinergic actions of xanomeline without interfering with the desired central cholinergic actions of the drug.
Increased appetite and weight are regarded as adverse effects of olanzapine and mirtazapine and with good reason: Weight gain is associated with an increased risk of a wide range of medical disorders. In some situations, however, weight gain may be a desired outcome. This article examines the potential exploitation of increased appetite and weight with olanzapine and mirtazapine in patients with cancer.1,2
Cancer, Anorexia, and Weight Loss
Loss of appetite has long been recognized in cancer and appears to be driven by many mechanisms that include cytokines, hormones, other chemical messengers, and their targets.3 Depending on how it was assessed, the prevalence of anorexia was found to range from 40% to 65% in 438 patients at first cancer diagnosis, identified at 7 cancer centers across the world.4 Loss of appetite in cancer patients is associated with loss of weight that can be so severe as to result in sarcopenia and cachexia.
At the time of diagnosis, cancer-associated weight loss was observed in 34.1% of 3,180 consecutive patients with gastrointestinal (including liver and pancreas) or lung cancer; the prevalence was higher in patients with more advanced stages of cancer. The prevalence of wasting ranged from 27.3% in colorectal cancer to 53% in gastroesophageal cancer.5 In a systematic review and meta-analysis of 10 studies of 7,186 patients with gastroesophageal cancer posted for curative resection surgery, the pooled prevalence of pretreatment cachexia was 35% (95% CI, 24%–47%).6 In a systematic review and meta-analysis of 26 studies of 5,919 patients with non–small cell lung cancer, the pooled prevalence of cachexia was 39% (range, 19%–64%).7 These studies5–7 also found that weight loss or cachexia predicted poorer overall survival. Low body mass index (BMI) was associated with an increased risk of cancer progression and death in metastatic colorectal cancer, as well.8 It is therefore important to address anorexia and weight loss in cancer patients in addition to addressing the disease, itself.
Current Approaches to Treating Anorexia and Weight Loss in Cancer Patients
The American Society of Clinical Oncology Guideline9 suggests progesterone analogs and short-term corticosteroids as possible pharmacologic approaches toward improving appetite and weight in patients with cancer-related cachexia. However, systematic review and meta-analysis data10 suggest no benefits from low- or high-dose megestrol acetate (pooled weight gain, 0.75 kg; 95% CI, −1.64 to 3.15 kg; 8 studies; 576 patients). Evidence from randomized controlled trials (RCTs) and meta-analyses found that medicinal cannabis also did not improve appetite and weight in patients with cancer.11–13 One meta-analysis14 of 7 RCTs conducted in 1944 patients with cancer-related anorexia/cachexia syndrome found that anamorelin, a ghrelin receptor agonist, increased total body weight by 1.73 (95% CI, 1.34–2.13) kg and lean body mass by 1.06 (95% CI, 0.30–1.81) kg; quality of life was also marginally improved (standardized mean difference [SMD], 0.16; 95% CI, 0.04–0.27). However, appetite, hand grip strength, and overall survival did not improve. Anamorelin did not increase all adverse events or serious adverse events.
Effects of Olanzapine and Mirtazapine on Appetite and Weight
With the possible exception of drugs such as cariprazine, brexpiprazole, aripiprazole, lurasidone, and lumateperone, most antipsychotic drugs increase appetite and weight; the effect may be dose dependent.15–17 Among the antipsychotics, weight gain can be substantial with drugs such as clozapine and olanzapine.18–21 Olanzapine has already demonstrated nutritional benefits in anorexic subjects. For example, in a systematic review and meta-analysis of 230 anorexia nervosa patients studied in 4 RCTs,22 olanzapine improved BMI to a greater extent than did placebo; the pooled mean difference was 0.67 (95% CI, 0.15–1.18) kg/m2.
Bupropion is generally regarded to be weight-neutral. In contrast, many antidepressants, especially tricyclics and mirtazapine, increase appetite and weight. Mirtazapine is more likely to increase appetite and cause weight gain than selective serotonin reuptake inhibitors.23–25 These findings encourage the consideration of olanzapine and mirtazapine for cancer related anorexia and weight loss.
Olanzapine for Cancer Chemotherapy-Related Anorexia
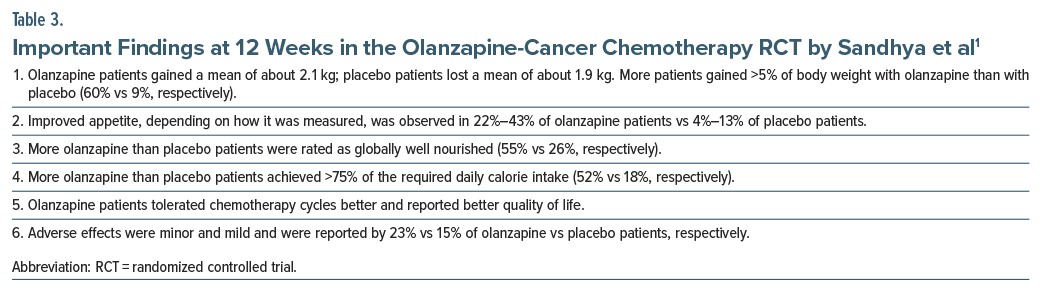
Many RCTs have shown that olanzapine (5 mg/d), in combination with other antiemetics, protects against chemotherapy-induced or chemotherapy-unrelated nausea and vomiting in cancer patients.26–28 One RCT found that olanzapine, combined with megestrol acetate, was superior to megestrol alone in patients with cancer related anorexia.29 Against this background, Sandhya et al1 described a 12-week RCT of olanzapine (2.5 mg/d) vs placebo for anorexia associated with chemotherapy cycle initiation in adults (n = 124) diagnosed with locally advanced or metastatic gastric, hepatopancreaticobiliary, or lung cancer.
The median age of the sample was 55 years. The sample was 64% male. Most patients had gastric (55%) or lung (35%) cancer. Cancer was advanced (stage IV) in about 80% of the sample. About 90% of patients reported anorexia at baseline, and nearly a third were underweight (BMI <18.5). Chemotherapy cycles were given once in 2–3 weeks based on clinical decision. All patients received dietary advice in addition to their study medication. Adherence to study medication was >90%. There were 58 vs 54 evaluable patients in olanzapine vs placebo arms, respectively. Dropout and reasons for dropout were similar in the 2 groups.
Important findings from the study1 are presented in Table 3. In summary, relative to placebo patients, olanzapine patients gained more weight, were more likely to gain >5% body weight, had better appetite, were more likely to become globally well nourished, were more likely to achieve >75% of required calorie intake, tolerated chemotherapy better, and had better quality of life. Olanzapine was also well tolerated.
The study1 had many limitations, such as the statement of many primary outcomes and the use of rudimentary cross-sectional analyses at the study end point rather than longitudinal, intent-to-treat analyses. Nevertheless, the advantage of olanzapine over placebo was evident and hard to dispute.
Mirtazapine for Anorexia Related to Lung Cancer
Although mirtazapine increases appetite and weight in patients with depression,23–25 it was found to be no better than placebo30 or megestrol acetate31 in the treatment of anorexia and cachexia in patients with cancer. Against this background, Arrieta et al2 described an 8-week RCT of mirtazapine (30 mg/d) with the expectation that the treatment would improve appetite and other nutritional parameters in patients (n = 86) with anorexia associated with advanced non–small cell lung cancer. The authors highlighted, as findings, that mirtazapine increased energy intake, including protein, carbohydrate, and fat intake, and that it reduced sarcopenia. They projected their findings with optimism in their abstract, discussion, and conclusions.
Their optimism was ill-founded. In cross-sectional between-group analyses, the trial2 failed on the primary outcome stated in the title of the paper: improvement in appetite ratings. In between-group analyses, the trial also failed on almost all of a dozen analyses also described as primary outcomes. Furthermore, the trial failed on almost all of the secondary outcomes, also numbering a dozen and more. Most of the favorable findings reported with mirtazapine emerged from within-group rather than from between-group analyses. In the very few statistically significant between-group comparisons, differences observed may have been false-positive findings related to the large number of cross-sectional comparisons performed at different time points during the trial. In one particularly ill-advised interpretation, a between-groups advantage for mirtazapine for the sarcopenia outcome appeared to be driven by baseline differences between treatment arms; within-group changes in sarcopenia were small. Last but not least, intent-to-treat longitudinal analyses, expected in RCTs, were not performed. In summary, this RCT failed to make a case for the use of mirtazapine (30 mg/d) to improve 8-week nutritional and anthropometric outcomes in patients with cancer.
Take-Home Message
Anorexia, weight loss, and cachexia are common in patients with cancer and in those who receive cancer chemotherapy. Olanzapine reduces nausea associated with cancer chemotherapy. Olanzapine also shows promise for improving appetite, weight, and quality of life in cancer patients receiving chemotherapy. Mirtazapine does not appear to improve nutritional and anthropometric outcomes in patients with cancer and anorexia.
Parting Notes
Antiemetics are routinely administered to address nausea and vomiting, and the associated anorexia, in cancer patients undergoing chemotherapy. This does not suffice to improve nutritional outcomes because anorexia, weight loss, sarcopenia, and cachexia can complicate cancer independently of the chemotherapy; many mechanisms are suggested.3 In the study by Sandhya et al,1 patients in both groups received olanzapine (5 mg/d), along with short-term steroids, for 4 days, as the antiemetic standard of care at their center. Maintenance treatment with olanzapine (2.5 mg/d) nevertheless outperformed maintenance treatment with placebo.
Article Information
Published Online: August 21, 2024. https://doi.org/10.4088/JCP.24f15532
© 2024 Physicians Postgraduate Press, Inc.
To Cite: Andrade C. Therapeutic effects, side effects, and adverse effects of neuropsychiatric drugs in the context of treating cancer-related anorexia with olanzapine and mirtazapine.
J Clin Psychiatry. 2024;85(3):24f15532.
Author Affiliations: Department of Psychiatry, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, India; Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India. ([email protected]).
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
References (31)

- Sandhya L, Devi Sreenivasan N, Goenka L, et al. Randomized double-blind placebo-controlled study of olanzapine for chemotherapy-related anorexia in patients with locally advanced or metastatic gastric, hepatopancreaticobiliary, and lung cancer. J Clin Oncol. 2023;41(14):2617–2627. PubMed CrossRef
- Arrieta O, Cárdenas-Fernández D, Rodriguez-Mayoral O, et al. Mirtazapine as appetite stimulant in patients with non-small cell lung cancer and anorexia: a randomized clinical trial. JAMA Oncol. 2024;10(3):305–314. PubMed CrossRef
- Hariyanto TI, Kurniawan A. Appetite problem in cancer patients: pathophysiology, diagnosis, and treatment. Cancer Treat Res Commun. 2021;27:100336. PubMed CrossRef
- Molfino A, de van der Schueren MAE, Sánchez-Lara K, et al. Cancer-associated anorexia: validity and performance overtime of different appetite tools among patients at their first cancer diagnosis. Clin Nutr. 2021;40(6):4037–4042. PubMed CrossRef
- Gannavarapu BS, Lau SKM, Carter K, et al. Prevalence and survival impact of pretreatment cancer-associated weight loss: a tool for guiding early palliative care. J Oncol Pract. 2018;14(4):e238–e250. PubMed CrossRef
- Brown LR, Sayers J, Yule MS, et al. The prognostic impact of pre-treatment cachexia in resectional surgery for oesophagogastric cancer: a meta-analysis and meta-regression. Br J Surg. 2023;110(12):1703–1711. PubMed CrossRef
- Zhang J, Tang X, Zhang W, et al. Cancer cachexia as a predictor of adverse outcomes in patients with non-small cell lung cancer: a meta-analysis. Clin Nutr. 2024;43(7):1618–1625. PubMed CrossRef
- Renfro LA, Loupakis F, Adams RA, et al. Body mass index is prognostic in metastatic colorectal cancer: pooled analysis of patients from first-line clinical trials in the ARCAD database. J Clin Oncol. 2016;34(2):144–150. PubMed
- Roeland EJ, Bohlke K, Baracos VE, et al. Management of cancer cachexia: ASCO guideline. J Clin Oncol. 2020;38(21):2438–2453. PubMed
- Lim YL, Teoh SE, Yaow CYL, et al. A systematic review and meta-analysis of the clinical use of megestrol acetate for cancer-related anorexia/cachexia. J Clin Med. 2022;11(13):3756. PubMed CrossRef
- Razmovski-Naumovski V, Luckett T, Amgarth-Duff I, et al. Efficacy of medicinal cannabis for appetite-related symptoms in people with cancer: a systematic review. Palliat Med. 2022;36(6):912–927. PubMed CrossRef
- Simon L, Baldwin C, Kalea AZ, et al. Cannabinoid interventions for improving cachexia outcomes in cancer: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2022;13(1):23–41. PubMed
- Hammond S, Erridge S, Mangal N, et al. The effect of cannabis-based medicine in the treatment of cachexia: a systematic review and meta-analysis. Cannabis Cannabinoid Res. 2021;6(6):474–487. PubMed CrossRef
- Taniguchi J, Mikura S, da Silva Lopes K. The efficacy and safety of anamorelin for patients with cancer-related anorexia/cachexia syndrome: a systematic review and meta-analysis. Sci Rep. 2023;13(1):15257. PubMed CrossRef
- Stogios N, Smith E, Bowden S, et al. Metabolic adverse effects of off-label use of second-generation antipsychotics in the adult population: a systematic review and meta-analysis. Neuropsychopharmacology. 2022;47(3):664–672. PubMed CrossRef
- Wu H, Siafis S, Hamza T, et al. Antipsychotic-induced weight gain: dose-response meta-analysis of randomized controlled trials. Schizophr Bull. 2022;48(3):643–654. PubMed CrossRef
- Sabé M, Pallis K, Solmi M, et al. Comparative effects of 11 antipsychotics on weight gain and metabolic function in patients with acute schizophrenia: a dose response meta-analysis. J Clin Psychiatry. 2023;84(2):22r14490. PubMed
- Andrade C. Cardiometabolic risks in schizophrenia and directions for intervention, 1: magnitude and moderators of the problem. J Clin Psychiatry. 2016;77(7):e844–e847. PubMed CrossRef
- Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939–951. PubMed CrossRef
- Leucht S, Schneider-Thoma J, Burschinski A, et al. Long-term efficacy of antipsychotic drugs in initially acutely ill adults with schizophrenia: systematic review and network meta-analysis. World Psychiatry. 2023;22(2):315–324. PubMed CrossRef
- Burschinski A, Schneider-Thoma J, Chiocchia V, et al. Metabolic side effects in persons with schizophrenia during mid- to long-term treatment with antipsychotics: a network meta-analysis of randomized controlled trials. World Psychiatry. 2023;22(1):116–128. PubMed CrossRef
- Han R, Bian Q, Chen H. Effectiveness of olanzapine in the treatment of anorexia nervosa: a systematic review and meta-analysis. Brain Behav. 2022;12(2):e2498. PubMed CrossRef
- Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71(10):1259–1272. PubMed CrossRef
- Watanabe N, Omori IM, Nakagawa A, et al. Safety reporting and adverse-event profile of mirtazapine described in randomized controlled trials in comparison with other classes of antidepressants in the acute-phase treatment of adults with depression: systematic review and meta-analysis. CNS Drugs. 2010;24(1):35–53. PubMed CrossRef
- Petimar J, Young JG, Yu H, et al. Medication-induced weight change across common antidepressant treatments: a target trial emulation study. Ann Intern Med. Published online July 2, 2024. doi:10.7326/M23-2742. CrossRef
- Tan L, Liu J, Liu X, et al. Clinical research of olanzapine for prevention of chemotherapy-induced nausea and vomiting. J Exp Clin Cancer Res. 2009;28(1):131. PubMed CrossRef
- Navari RM, Pywell CM, Le-Rademacher JG, et al. Olanzapine for the treatment of advanced cancer-related chronic nausea and/or vomiting: a randomized pilot trial. JAMA Oncol. 2020;6(6):895–899. PubMed CrossRef
- Shen J, Zhao J, Jin G, et al. A prospective randomized controlled clinical trial investigating the efficacy of low-dose olanzapine in preventing nausea and vomiting associated with oxaliplatin-based and irinotecan-based chemotherapy. J Cancer Res Clin Oncol. 2024;150(5):283. PubMed CrossRef
- Navari RM, Brenner MC. Treatment of cancer-related anorexia with olanzapine and megestrol acetate: a randomized trial. Support Care Cancer. 2010;18(8):951–956. PubMed CrossRef
- Hunter CN, Abdel-Aal HH, Elsherief WA, et al. Mirtazapine in cancer-associated anorexia and cachexia: a double-blind placebo-controlled randomized trial. J Pain Symptom Manage. 2021;62(6):1207–1215. PubMed CrossRef
- Chowdhury IH, Rahman MS, Chowdhury MNK, et al. Mirtazapine versus megestrol acetate in treatment of anorexia-cachexia in advanced cancer patients: a randomized, double-blind trial. Jpn J Clin Oncol. 2024;54(5):530–536. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite