Abstract
Acute and long-term objectives must be linked early in the treatment of schizophrenia. Maintenance therapy is pivotal in relapse prevention. Relapses are serious events that alter disease trajectory and are most often related to nonadherence. Patients with schizophrenia have a substantial risk of relapse, especially when they are nonadherent to antipsychotics. Because relapses are accompanied by structural brain changes, worsening symptoms, and increased treatment resistance when medication is resumed, clinicians must monitor nonadherence and offer strategies to avoid or improve it. Long-acting injectable (LAI) antipsychotics have the potential to reduce nonadherence, relapse, rehospitalization, and mortality, even among patients with first-episode schizophrenia and with comorbid substance use disorder. Long-acting formulations can be a very powerful strategy in helping to ensure that patients get the benefit of the medication they have been prescribed, as the use of LAIs is more easily monitored than oral medications due to the nature of their administration to patients. However, prescribers tend to believe that patients have negative attitudes about LAIs and avoid prescribing them. Patients should be offered the option of LAI antipsychotic treatment and should understand the logistics and the potential benefits of the regimen. LAIs differ regarding their initiation strategy, duration, and flexibility of injection intervals, in addition to the differences of the antipsychotic that the LAI formulation is based on. Presentation matters for LAI treatments to be accepted by patients and family members. Clinicians can use certain communication tools to improve their dialogue with patients about the benefits of switching from oral agents.
See letter by Hirakawa and Ishii and reply by Kane and Correll

CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation.
This Academic Highlights activity is derived from the planning teleconference series “Optimizing Treatment Choices to Improve Adherence and Outcomes in Schizophrenia,” which was held in March and April 2019. This report was prepared and independently developed by the CME Institute of Physicians Postgraduate Press, Inc., and was supported by an educational grant from Indivior Inc.
The teleconference was chaired by John M. Kane, MD, Department of Psychiatry, The Zucker Hillside Hospital, Glen Oaks, New York. The faculty was Christoph U. Correll, MD from the Department of Psychiatry, The Zucker Hillside Hospital, Glen Oaks, New York.
CME Objectives
After studying this article, you should be able to:
- Monitor adherence to treatment in patients with schizophrenia
- Identify patients who would benefit from LAI treatment
- Address somatic and psychiatric comorbidities in the care of patients with schizophrenia
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Release, Expiration, and Review Dates
This educational activity was published in September 2019 and is eligible for AMA PRA Category 1 Creditâ„¢ through October 31, 2021. The latest review of this material was August 2019.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Marlene P. Freeman, MD, Editor in Chief, has received research funding from JayMac and Sage; has been a member of the advisory boards for Otsuka, Alkermes, and Sunovion; has been a member of the Independent Data Safety and Monitoring Committee for Janssen; and, as a Massachusetts General Hospital (MGH) employee, works with the MGH National Pregnancy Registry, which is sponsored by Teva, Alkermes, Otsuka, Actavis, and Sunovion, and works with the MGH Clinical Trials Network and Institute, which receives research funding from multiple pharmaceutical companies and the National Institute of Mental Health. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
J Clin Psychiatry 2019;80(5):IN18031AH1C
To cite: Kane JM, Correll CU. Optimizing treatment choices to improve adherence and outcomes in schizophrenia. J Clin Psychiatry. 2019;80(5):IN18031AH1C
To share: https://doi.org/10.4088/JCP.IN18031AH1C
© Copyright 2019 Physicians Postgraduate Press, Inc.
Schizophrenia is a serious, lifelong mental illness. Recovery is possible with consistent, supportive treatment, but relapse is common. A frequent contributor to relapse is treatment nonadherence. Long-acting injectable (LAI) antipsychotics can improve treatment adherence and continuity, potentially improving outcomes for patients, but these agents are underused. Experts John M. Kane, MD, and Christoph U. Correll, MD, described the frequency and consequences of nonadherence and strategies to improve adherence and overall patient outcomes.
MONITORING ADHERENCE IN PATIENTS WITH SCHIZOPHRENIA AND IDENTIFYING CANDIDATES FOR LAI ANTIPSYCHOTICS
Medication adherence is a major problem in all areas of medicine, not just psychiatry, whether patients have heart disease, diabetes, asthma, epilepsy, or any other condition that requires daily medication.1,2 According to a report by the World Health Organization, poor medication adherence for chronic diseases is a worldwide problem of enormous importance—the rate of adherence averages 50% in developed countries and is lower in developing countries.3 As many as two-thirds of patients with schizophrenia are estimated to be at least partially nonadherent to oral antipsychotic treatment.4 Clinicians need to address the problem of nonadherence with patient-tailored interventions and education. In his presentation, Dr Kane offered insight into communication with patients about adherence and about interventions to diminish nonadherence.
Factors in Nonadherence
Prescribers would like for patients to believe that their medicine is beneficial, makes them feel better, and is worth the cost. However, many factors contribute to nonadherence. It may be related to patient issues, specific treatments (eg, side effects, lack of efficacy), or the patient’s overall condition.3 Individuals with schizophrenia face special challenges in taking medication as prescribed. The illness is often complicated by poor insight and cognitive dysfunction; poor symptom control can contribute to nonadherence; patients are less likely to take medicine if they cannot see the value in taking it; adverse effects may be intolerable; patients may have poor social support; and substance abuse is common and can contribute to nonadherence.5-7 Social, economic, and health system factors may interfere as well.
Keeping up with daily medications can be difficult, as one person shared about her brother with schizophrenia:
 Family Perspectives
Family Perspectives
“It seems that deciding to take medication is one thing for diagnosed people, but deciding to ensure that it is taken exactly as prescribed at the exact right time is another issue entirely. My brother is proof that it is extremely difficult to keep track of many different medications simultaneously. . . . A single mismanaged dose can potentially lead to weeks of hardship. . . . Going without has caused a lot of unnecessary hardship for my brother and it’s possible that some of his visits to the emergency room for psychological distress could have been avoided entirely.”8
Assessing Nonadherence
Unfortunately, many physicians overestimate treatment adherence.9,10 Dr Kane said that clinicians’ perceptions of their own patients can be biased and may reflect the view that nonadherence is a problem that other clinicians have. Physicians may think that their patients are better with compliance or are more likely to listen to them than the patients reported in the literature,11 even though this may not necessarily be the case.
Expert consensus recommendations are to assess adherence via not only self-report but also objective tools.12 For example, the Medication Adherence Rating Scale can be used with patients who have schizophrenia.13,14 Pill counts and informant ratings can also be useful.15
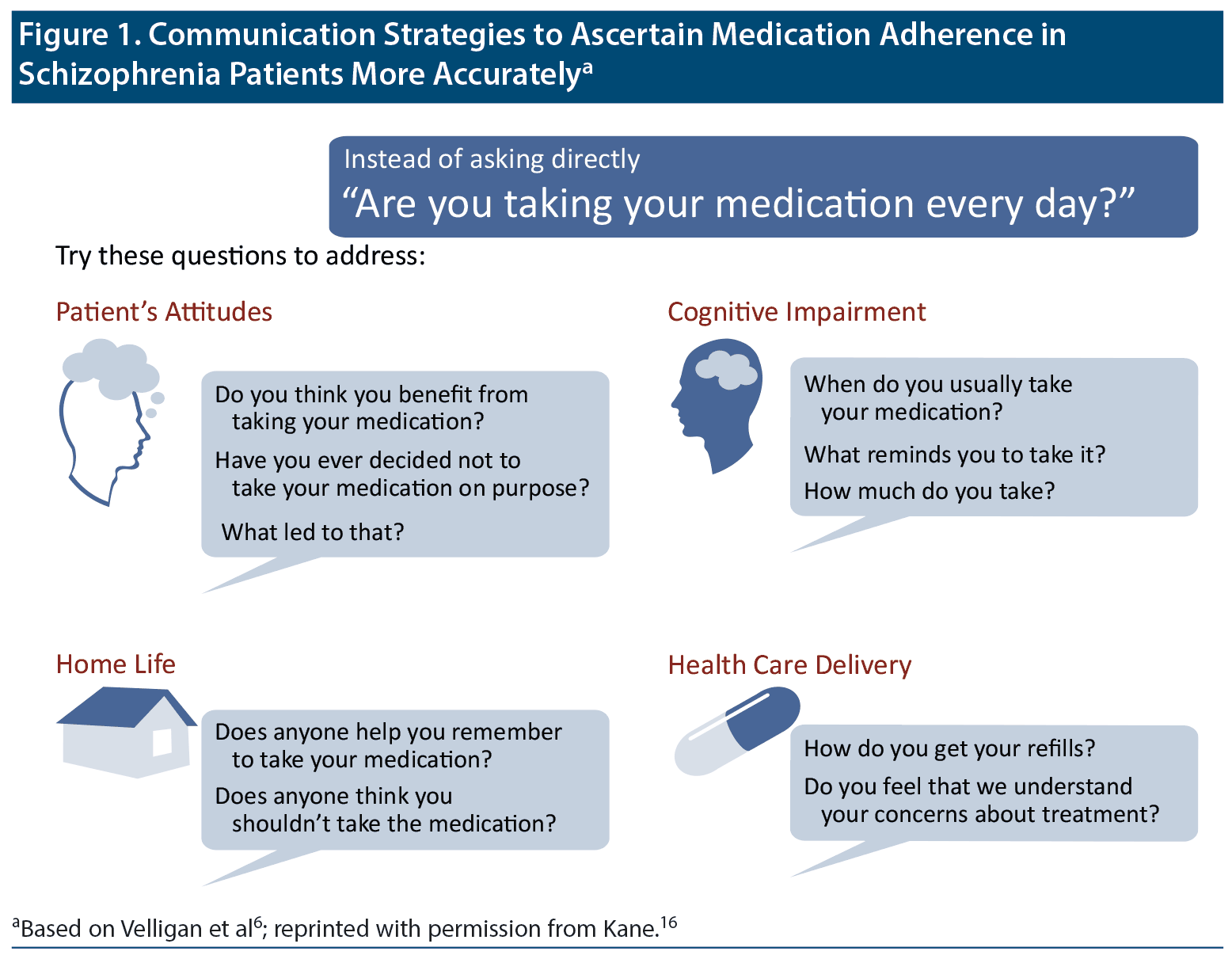
To improve the accuracy of patients’ self-report, asking about their experiences taking medication may be more useful than asking a direct question about nonadherence (Figure 1).6,16 Dr Kane recommended that clinicians avoid conveying an attitude that patients who miss or stop taking medications are “bad patients”; instead, they should communicate that nonadherence is human nature and happens frequently and that doctors want to help patients benefit from their medications as much as possible.
Relationship Between Nonadherence and Relapse
Poor adherence to oral antipsychotics is the most common cause of relapse.4 A summary of 5 studies compared continuous antipsychotic treatment with intermittent treatment (ie, patients were treated only when clinicians thought they were experiencing early signs of relapse).17 All 5 studies showed a significantly lower rate of relapse in patients with continuous treatment than in those with intermittent treatment.
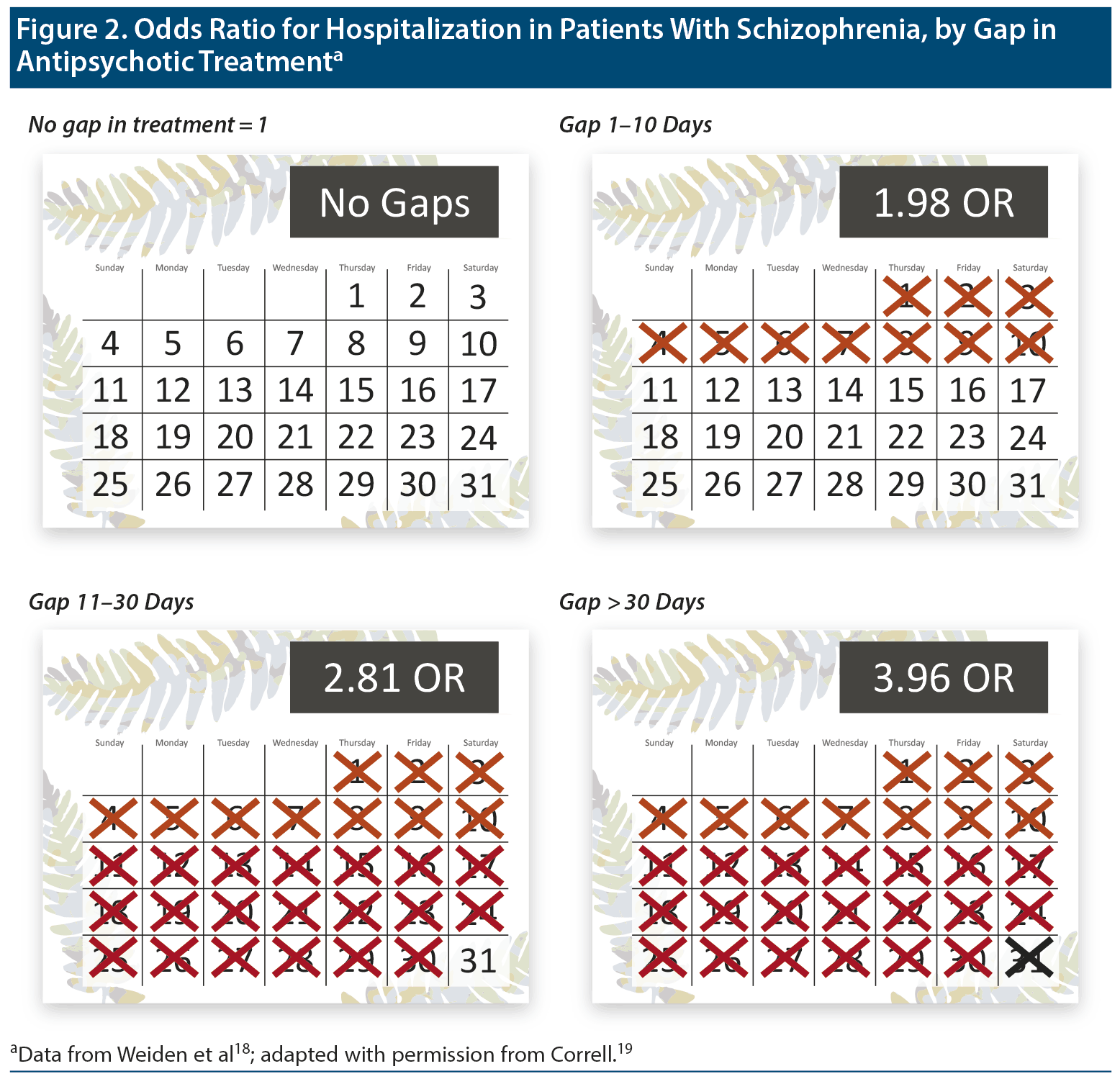
Patients with schizophrenia are at risk for relapse and hospitalization after intervals of missed medication as short as 10 days or less (Figure 2).18,19
A systematic review20 of 6 studies assessing the risk of relapse after treatment discontinuation found that 77% of patients relapsed within 1 year. The rate of relapse among these patients jumped to 90% after 2 years. In comparison, the rate of relapse among patients with good adherence has been reported as 18%.21
A study22 by Robinson and colleagues followed 104 first-episode patients and found that after 5 years, 82% of the cohort had experienced at least one relapse. Stopping medication was found to be the most powerful predictor of relapse; patients who stopped were 5 times more likely to relapse than patients who continued with their medication.
Consequences of Relapse
Dr Kane noted that, with each relapse, the onset of therapeutic effect of medication may be slower, treatment may be less effective, and patients can experience greater hardships and illness burden. Relapses have been associated with progressive decline in brain volume.23 Ramifications of relapse among patients with schizophrenia include the following24:
- Increased risk of self-harm
- Increased risk of harm to others
- Increased distress for patients and families
- Increased strain on friendships and relationships
- Disruptions in education
- Disruptions in vocational functioning
- Decreased patient autonomy
- Increased social stigma
- Increased economic burden associated with treatment
- Increased risk of not returning to baseline functioning
- Increased resistance to treatment
A study25 that followed 130 patients showed that reaching the same degree of improvement after a relapse took longer with the second episode than the first. The illness, in other words, became less responsive to treatment.
A Dutch study26 of 603 patients with schizophrenia investigated the relationship between an interruption in medication of at least 30 days and suicide. After adjusting for age and gender, the relative risk of suicide attempt was found to increase approximately 4-fold among patients with a 30-day or more interruption compared with patients who did not have an interruption.
Cooperation between patients and clinicians is necessary to minimize relapses and their consequences such as lost jobs, as illustrated by a patient who experienced multiple relapses:
 Patient Perspectives
Patient Perspectives
“I was diagnosed as schizophrenic when I was 13 years old. I spent the better part of my adolescence and young adulthood in hospitals. . . . I fought my disease and the stigma of mental illness in my struggle for employment. I learned from my mistakes which cost me several jobs, and along with my psychiatrist, we experimented with different medications. Fortunately, we found a combination of medications which kept me out of hospitals and I kept employment.”27
Using Long-Acting Injectable Antipsychotics
Long-acting injectable (LAI) antipsychotics can play a role in helping patients with schizophrenia remain adherent.4,28,29 Research indicates that patients treated with LAI antipsychotics have decreased hospitalization and use of emergency services compared with those treated with oral antipsychotics.30-32 A real-world comparative effectiveness trial33 that included almost 30,000 patients found that LAIs and clozapine were the most effective treatments for preventing schizophrenia relapse; rehospitalization rates among patients receiving LAIs were 20%-30% lower than for patients receiving equivalent oral formulations. However, this treatment modality is not prescribed as often as oral antipsychotics.4,34
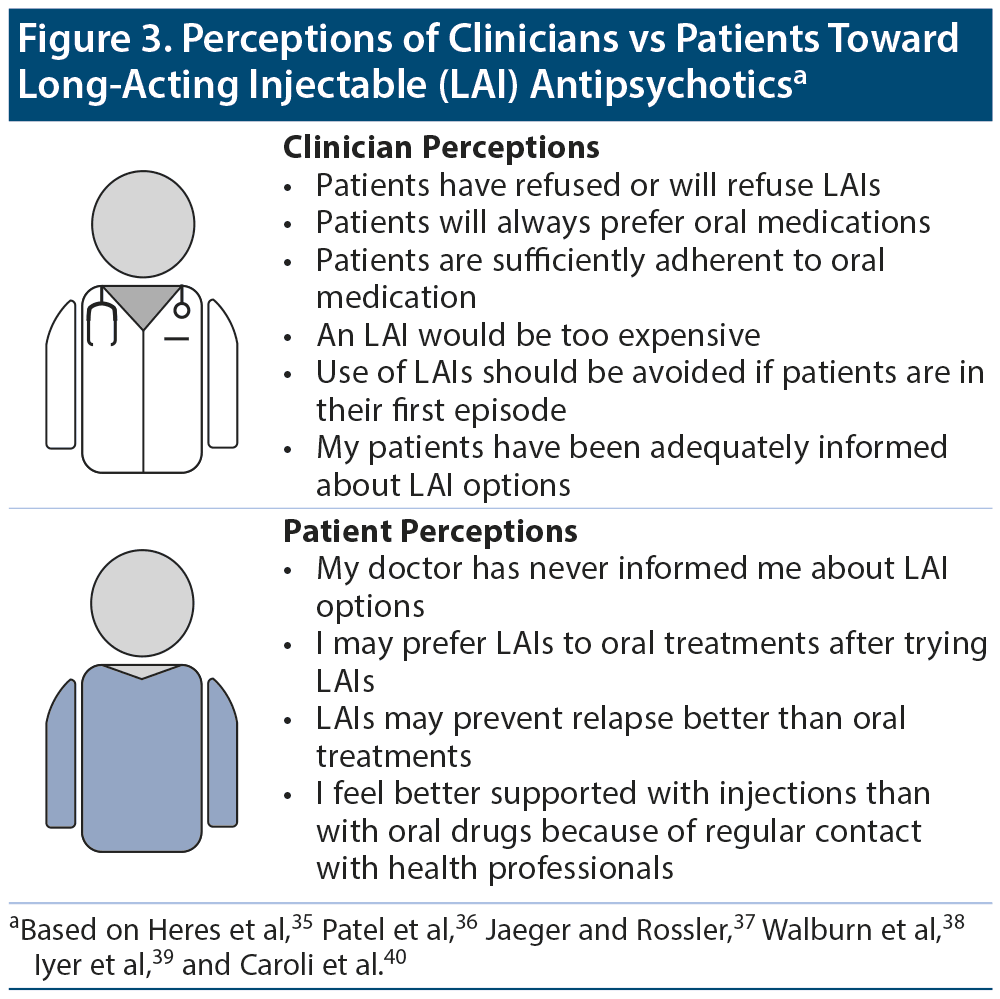
Many factors play a role in the underuse of LAIs. Clinicians may have inaccurate perceptions about the medications and their patients’ attitudes toward them (Figure 3).35-40 Additionally, clinicians may think that they have clearly explained the treatment options to patients, but the conversation may be interpreted differently by patients. A survey37 of patients being treated for schizophrenia and having no prior experience with LAIs, as well as psychiatrists, highlighted the disconnect between patients and clinicians. Only 21% of the patients reported that they had been informed of LAI options by their psychiatrist. Yet, 75% of the psychiatrists surveyed said that they had informed their patients about different formulations of antipsychotics, including LAI options.
Patient attitudes toward LAIs are frequently positive.38 In a survey of patients with > 3 months of experience with an LAI formulation, LAIs were preferred, and 70% of patients felt better supported in their illness (compared with oral medication) because of regular contact with the clinician who administered their injection.40
What Can Clinicians Do to Improve Adherence?
The LAIs used to be considered appropriate for only a subgroup of patients with schizophrenia who had major nonadherence problems and frequent relapses.41 Recent guidelines,41,42 however, have noted the potential benefit of using second-generation LAIs following the first episode; LAIs have been found to be effective to prevent relapse in patients experiencing their first episode43 as well as in those who have already experienced multiple relapses.44,45 These agents should be “systematically proposed to any patients for whom maintenance antipsychotic treatment is indicated.”41
Communication issues between clinicians and patients need to be overcome, noted Dr Kane. If clinicians are inadequately aware of data or are ambivalent about the message that LAIs are useful, then the dialogue will be ineffective. Clinicians should reflect on their own beliefs and recognize any negative assumptions about patient preferences. Clinicians with negative assumptions about LAIs will most likely present the option with a pessimistic tone that patients notice.39
Dr Kane stated that clinicians should consider the use of LAI formulations for any patient and start the conversation at the beginning of treatment. If the option of LAIs is introduced at a later date, it might be confusing or viewed as a negative response to a patient’s hospitalization. Clinicians also need to make sure that their staff members and patients’ family members understand why LAI formulations are being offered. A single team member who does not support the use of LAIs can undermine the treatment strategy with the patient.
Recent research showed that, even among first-episode and early-phase patients, the overwhelming majority of patients would consider the use of LAI formulations—only 14.4% of outpatients who were approached declined LAI treatment.46 Staff education, which was successfully provided for this research, has the potential to substantially enhance the use of LAI antipsychotics. Staff members were trained on the role of nonadherence in relapse and hospitalization and the rationale for using an LAI formulation in patients with early-phase psychosis.46 They also received education about shared decision-making, communication strategies, and patients’ frequently asked questions; role-playing was used to develop their communication skills. Solutions were also devised to overcome logistical barriers to LAI use, and study prescribers received training on prescribing guidelines.
Dr Kane concluded that, if clinicians can employ these strategies for assessment, communication, and education, then far more patients could be engaged in treatment with LAI antipsychotics, which may provide enormous benefit to the patients, their families, and public health.
 Case Practice Question
Case Practice Question
Discussion of the best response can be found at the end of the activity.
Case 1. John is a 33-year-old man who is diagnosed with schizophrenia and in his third hospitalization for psychosis. What should the clinical team consider in terms of preventing future relapses?
- John has refused LAI antipsychotics in the past, but the options may not have been communicated in the best way.
- John would be more likely to relapse if his oral medication is switched to an LAI than if he keeps the same regimen.
- Nonadherence is probably not why John has had relapses, as he says he takes his tablets daily.
TREATING THE WHOLE PATIENT: INCORPORATING THE PHARMACOLOGY OF TREATMENTS FOR SCHIZOPHRENIA AND SPECIAL CONSIDERATIONS FOR COMORBID CONDITIONS
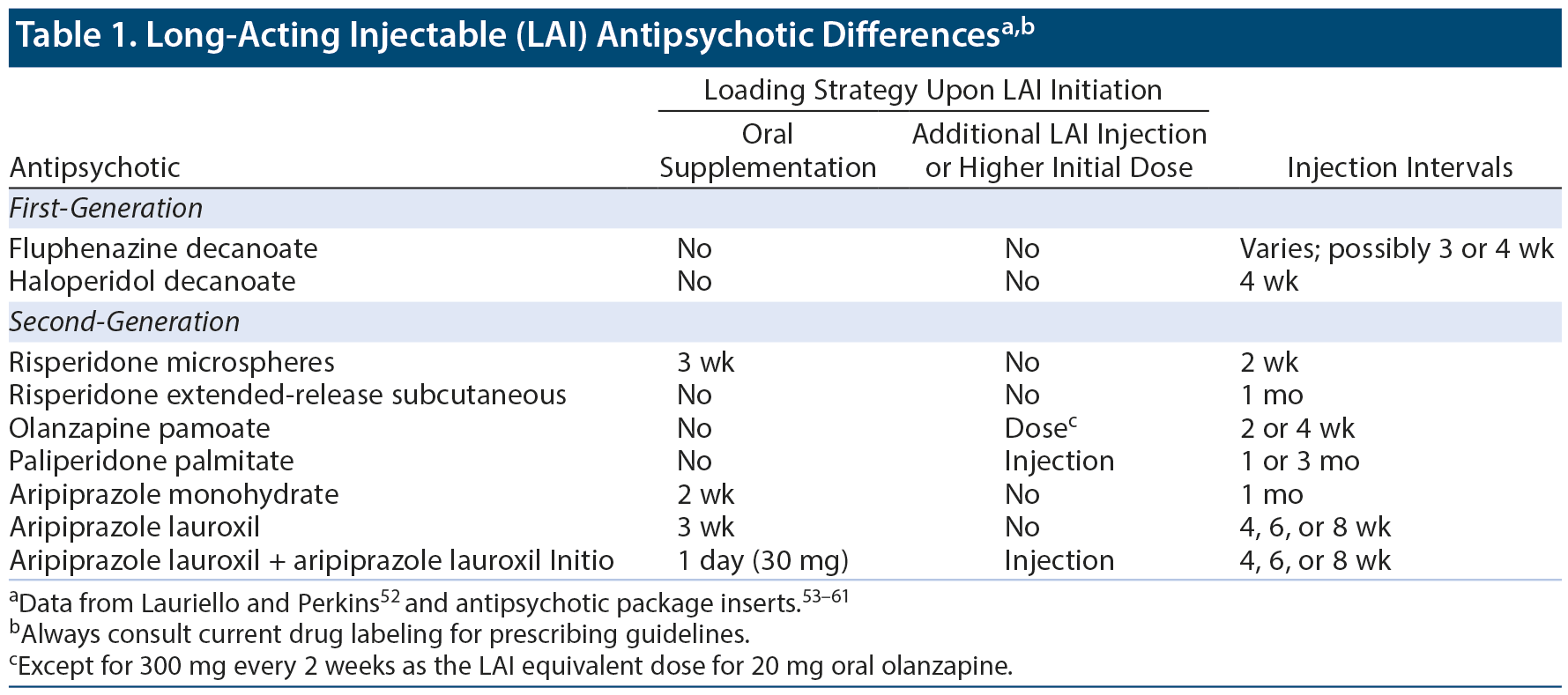
While acute treatment efficacy and early remission of symptoms are important, effective maintenance treatment is crucial in keeping patients with schizophrenia stable.47,48 Effective maintenance management of schizophrenia starts with efficacious and tolerable treatment. In general, efficacy differences among antipsychotics are relatively small, while tolerability differences are larger, which is true both for acute49,50 and maintenance51 treatment. Several LAI antipsychotics are available and differ based on starting dose, maintenance dose, and whether or not oral cotreatment is necessary at initiation (Table 1).52-61 They also differ in that first-generation LAIs are oil-based, while second-generation LAIs are water-based, which leads to less injection pain and reaction.
In his presentation, Dr Correll reviewed evidence on LAIs and described situations in which LAIs might be especially useful.
Evidence on Discontinuation and Relapse With LAI Antipsychotics
A large meta-analysis62 of randomized controlled trials (RCTs) was not able to demonstrate LAI superiority over oral agents, but this finding appears to be due to biased populations enrolled in RCTs.63 Patients in double-blind RCTs tend to be more adherent, tend to have better illness insight, and might not be as severely ill as patients in the “real world.”64 Consistent with this interpretation, in meta-analyses of mirror image64 and cohort65 studies, which reflect more real-world patient populations, LAIs have been shown to prevent treatment discontinuation and reduce hospitalizations better than oral treatments.
One reason for the consistent results of LAI superiority over oral antipsychotics in 23 of the 25 meta-analyzed mirror image studies64 may be improved medication possession. A study66 of medication possession ratios based on pharmacy records reported that patients were in possession of oral medications only 40% and 42% of the time during a 12-month period, for commercially insured and Medicare-insured groups, respectively. However, when these patients were switched to LAIs for 12 months, the medication possession ratios rose to 67% and 68%.
A meta-analysis67 showed that maintenance treatment with either oral or LAI antipsychotics has a strong relapse-prevention effect, translating into in a number-needed-to-treat of 3 versus placebo; however, the injectable formulations were associated with a greater reduction in relapse than oral drugs (P = .03).
Unlike with oral medication, clinicians have clear information when a patient misses a dose of an LAI. Furthermore, the window of opportunity to get the patient back into care before a relapse is extended due to the long-acting nature of LAIs. A study68 indirectly compared relapse rates from separate RCTs, in which patients were switched to placebo following stabilization on 1 of 3 formulations of paliperidone: oral, once-monthly, and every 3 months. The duration until half of the patients relapsed was 59 days after the last dose of oral paliperidone treatment, 200 days after the last dose in patients who had received once-monthly paliperidone, and 479 days among the group that had received every-3-months paliperidone.
Special Populations
In the past, LAI antipsychotics were reserved for patients who had had multiple relapses, showed poor insight into the illness, and demonstrated nonadherence, but, as Dr Kane noted, guidelines now suggest considering LAIs as an initial treatment for any patient. Dr Correll stated that certain subgroups who would especially benefit from this change are those in the early stage of illness, who have substance abuse, and/or who are in contact with the legal system.43,69-75 Although comorbid depression76,77 and sleep disruption78-80 are common in patients with schizophrenia and negatively affect outcome,81 studies of LAIs in patients with these comorbidities are still lacking.
Early-illness patients. A study43 of patients hospitalized between 2000 and 2007 with first-episode schizophrenia who were discharged on either oral or LAI treatments showed that the LAI treatment group had a 59% decreased risk for discontinuation and a 64% decreased risk for rehospitalization. A 12-month trial71 of patients with first-episode schizophrenia (N = 86) found that oral treatment was associated with an observed relapse rate of 33% compared with 5% in the group receiving the LAI. In fact, in survival analyses, the relapse rates were estimated to be 50% vs 8.5% if all patients had been followed for an entire year, and at the end of the study, only 33% of orally treated patients, compared with 95% of LAI treated patients, had “excellent” adherence. Finally, in a study of patients with a recent diagnosis of schizophrenia (within 1-5 years),69 LAI treatment reduced relapse risk by 29.4% versus oral treatment.
Patients with substance use or legal involvement. Substance abuse occurs more frequently in individuals with schizophrenia than in the general population and is associated with antipsychotic nonadherence.74 A mother described her son’s current situation:
 Family Perspectives
Family Perspectives
“My son is 21 and has been living with this horrible disease since he was 18. We have had 4 hospitalizations, each over a month in duration. We seem to be in a rut right now. He is non-med-compliant, refuses to go to treatment. . . . Before his hospitalization he was drinking heavily.”82
A naturalistic 3-year study75 of patients treated for first-episode schizophrenia who had substance use disorder found that, despite worse prognostic factors in the group receiving LAIs (eg, history of homelessness), these patients fared better than those taking oral antipsychotics. The LAI group experienced a lower relapse rate (68% vs 77%) and a longer time to relapse (694 days vs 447 days).
A 15-month, open-label randomized study70 of patients with schizophrenia and a history of incarceration demonstrated a lower rate of treatment failure (arrest/incarceration, psychiatric hospitalization, suicide, treatment discontinuation or supplementation, or increased service use to prevent hospitalization) in the LAI treatment group (39.8%) than in the oral treatment group (53.7%).
Patients experiencing breakthrough psychosis. Patients with breakthrough psychotic symptoms while on LAI treatment need special care.72,83 Dr Correll recommended that clinicians should rule out or address medical illness or substance use, identify and address stressors, optimize nonpharmacologic treatments, and treat comorbidities that could contribute to the exacerbation. Clinicians must also review whether the LAI administration was properly handled. The medication could have been mixed improperly or not injected deeply enough, reducing the rate of distribution.
If doses have been missed or delayed, the patient’s next injection could be scheduled earlier than usual while staying within the recommended interval.72 Clinicians could also increase LAI doses if not at the maximum. If doses are at the maximum, a possible solution is to add an extra oral dose of the same medication and monitor for 2-4 weeks. If the patient tolerates the extra dose and responds well, then his or her injection interval could be shortened (an off-label strategy).
The site of injection may also need to be moved because deltoid injections reach higher peak levels but result in a shorter half-life, while gluteal injections reach lower peak levels but result in a longer half-life.84-86 If the symptoms still persist, clinicians may consider switching from one LAI to another or implementing different oral treatment, possibly clozapine for treatment resistance.72
 Case Practice Question
Case Practice Question
Discussion of the best responses can be found at the end of the activity.
Case 2. Jamal is a 24-year-old man with first-episode schizophrenia who has responded well to an oral second-generation antipsychotic. Which statement about next steps is most applicable?
- Oral treatment that works will have the best chance to limit Jamal’s risk of relapse.
- Because Jamal has had only a first episode of schizophrenia, it is too early to start LAI treatment.
- An LAI antipsychotic would have the best chance of reducing Jamal’s relapse risk.
 Case Practice Question
Case Practice Question
Case 3. Mateo is a 37-year-old man who has schizophrenia and is abusing marijuana and alcohol. During several inpatient stays, Mateo’s schizophrenia stabilized, and he was unable to use drugs. After each discharge, he resumed his drug use and intermittent nonadherence with oral antipsychotics, leading to relapse. Mateo was recently involved in minor criminal activity, arrested, and briefly incarcerated. You need to devise a treatment plan; which statement reflects the most evidence-based strategy for Mateo?
- Because Mateo uses alcohol and marijuana and has had problems with the law, an LAI antipsychotic will not be better than oral treatment for relapse prevention.
- Relapse prevention with an LAI antipsychotic will most likely be more effective for Mateo than it would if the same LAI antipsychotic were prescribed for a patient without drug or alcohol use.
- Relapse prevention with an LAI antipsychotic will most likely be similarly effective for Mateo as when the same LAI antipsychotic is prescribed for a patient without drug or alcohol use.
- Relapse prevention with an LAI antipsychotic will most likely be less effective for Mateo than it would when prescribed for a patient without drug or alcohol use, but it may be more effective than the oral agents for Mateo.
Adverse Effects
When devising a treatment plan, it is very important to consider tolerability,87 which can affect the likelihood that patients will continue treatment. Dr Correll stated that clinicians should bear in mind that antipsychotic side effect differences are larger and easier to predict than efficacy differences.50
A survey of patients with schizophrenia ranked the 5 most commonly occurring adverse effects as weight gain, somnolence or insomnia, problems with concentration, memory loss, and disorganized thoughts.88 Relatives have reported that particularly unpleasant side effects for patients include sedation, weight gain, and extrapyramidal symptoms.89 A meta-analysis90 of RCTs that compared 119 adverse effects found no significant differences among LAI and oral versions of the same antipsychotics for 115 of them. Only 3 adverse effects occurred significantly more frequently with LAIs than with oral treatments: akinesia, higher low-density lipoprotein cholesterol, and anxiety. Prolactin change/hyperprolactinemia occurred significantly less frequently with LAIs than oral medications. Iatrogenic effects—including weight gain, hyperprolactinemia, diabetes, and extrapyramidal side effects—have been reported to reduce patients’ medication adherence91 and quality of life.88,92
Risk of mortality. In a recent, 30-year Finnish study93 of mortality, the mean age at death was found to have increased among patients with schizophrenia, and the rate of suicide had decreased. However, a gap still exists, with the average age at death in the general population being 77.5 years and in those with schizophrenia being 70.1 years. Increases were found in deaths from cardiovascular diseases and cancer. In a Swedish study,94 the risk of death was found to be 56% lower among individuals with schizophrenia who were taking antipsychotics than among those taking no antipsychotics. The use of LAI antipsychotics was associated with a 33% lower risk of death compared with equivalent oral formulations.94
Offering LAIs
Dr Correll agreed with Dr Kane that prescribers appear to have misconceptions about attitudes their patients may have toward LAI treatment. In a survey of opinions about LAIs among patients and health professionals,95 the health professionals overestimated many concerns they believed their patients taking oral antipsychotics had about LAIs, including fear of pain at the injection site, embarrassment, reduced autonomy, and stigma. The only concern that patients had to a greater extent than imagined by clinicians was related to the amount of time they would be required to stay in the clinic for the mandatory observation after an injection of olanzapine pamoate.
Clinicians who offer LAIs may require training in communication. In a 2015 study,96 psychiatrists were recorded while offering LAIs to their patients. In 33 conversations, only 9% of the speech time was dedicated to explaining positive aspects of LAI treatment (eg, no need for daily medication); 91% of the time was spent on the modality (“a shot”) or neutral aspects. Two-thirds of patients expressed either a neutral or positive response to the offer of LAI therapy (with 33% indicating a potential willingness to try it). When another physician trained in delivering a positive message about LAIs and their potential advantages went back a few days later to speak to the patients, as many as 96% stated that they were open to try.
Patients may hear “injection” and not understand what is meant, as the following qualitative study’s comments indicate:
 Patient Perspectives
Patient Perspectives
“A patient . . . feared that he might get ‘ addicted’ to a[n] LAI as it was administered by an injection. This view related to him associating ‘ addiction’ with injectable ‘ street drugs.’ Another patient queried whether a[n] LAI needed to be injected daily ‘ like insulin.’ “97
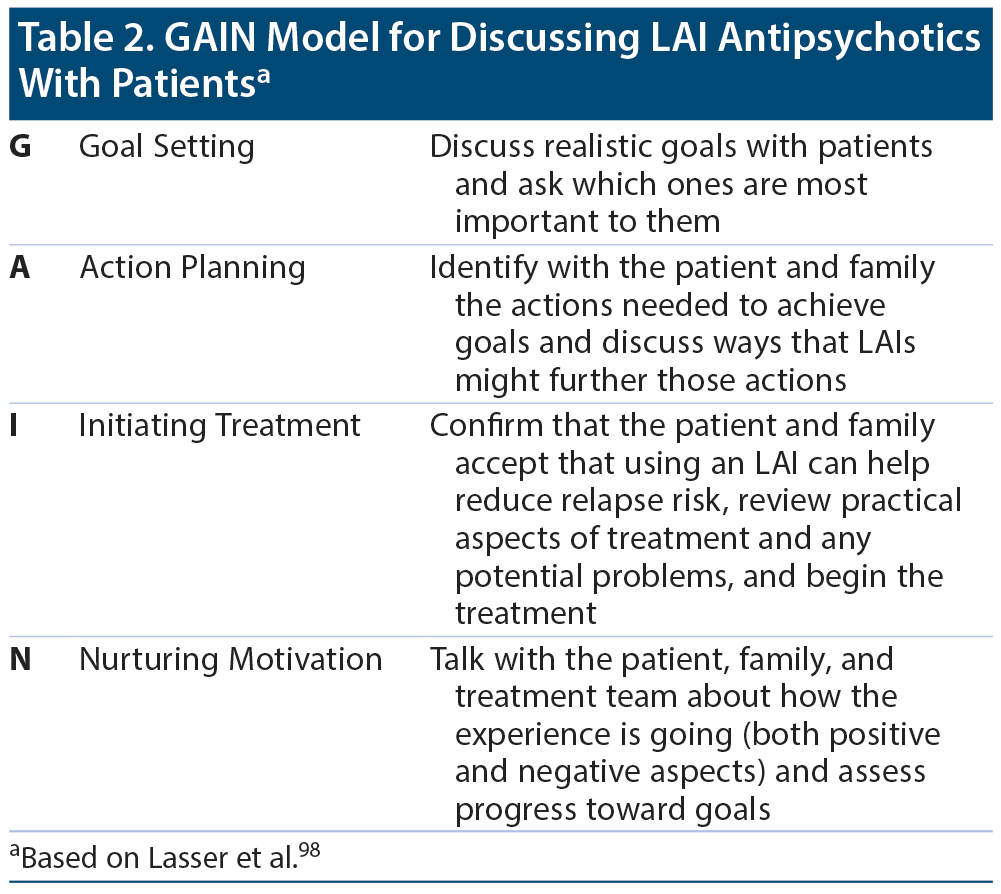
Dr Correll suggested that clinicians practice motivational interviewing by listening to and understanding the patient’s values and fears. Clinicians should be nonjudgmental, respectful, collaborative, flexible, and empathic. The GAIN model98 to facilitate change is based on the LEAP communication model,99 in which the steps are Listen, Empathize, Agree, and Partner. The GAIN model can be used to discuss LAI antipsychotics with patients (Table 2).98 Dr Correll concluded that clinicians should identify patient goals and should help patients recognize the disparity between where they are now and where they would like to be ideally, with medication being one tool to help them reach their goals.
CONCLUSIONS
Acute and long-term objectives must be linked early in the treatment of schizophrenia. Maintenance therapy is pivotal in relapse prevention. Relapses are serious events that alter disease trajectory and are most often related to nonadherence, which can be prevented in many cases with LAI treatments. Long-acting injectable treatments are still underused. Presentation matters for LAI treatments to be accepted by patients and family members. Communication strategies can be improved with clinician/staff training. As always, the risk-benefit ratio of treatment must be considered when choosing among available options.
Clinical Points
- Antipsychotic medication is critical in the prevention of relapse and rehospitalization.
- Rates of nonadherence are enormously high among patients taking antipsychotic medication.
- Long-acting formulations can be a very powerful strategy in helping to ensure that patients get the benefit of the medication they have been prescribed.
- Patients should be offered the option of LAI antipsychotic treatment and should understand the logistics and the potential benefits of the regimen.
- Communication strategies can be used to improve clinicians’ dialogue with patients about LAI treatment
 Discussion of Case Practice Questions
Discussion of Case Practice Questions
Case 1: Preferred response is a.
Clinicians may not communicate effectively with patients about LAI options, especially if they harbor negative opinions of the treatment modality or of patients’ willingness to try LAIs. John’s clinical team should use nonjudgmental questions and objective tools to assess whether John has a problem with adherence and, if so, offer education and patient-tailored interventions. He may be more willing to try an LAI now if his treatment team describes it in a positive way, answers his questions, and helps him understand that it might help him to avoid relapses and achieve his own, personalized goals.
Case 2: Preferred response is c.
See explanation below.
Case 3: Preferred response is d.
Guidelines now suggest considering the use of LAIs following the first episode of schizophrenia to reduce the risk of relapse (as in Case 2). Studies have also indicated benefits of LAIs versus oral treatment among patients who have substance abuse and contact with the legal system (as in Case 3).
Published online: September 17, 2019.
Disclosure of off-label usage: The chair has determined that, to the best of his knowledge, no investigational information about pharmaceutical agents that is outside US Food and Drug Administration-approved labeling has been presented in this article.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Creditâ„¢. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Academy of Physician Assistants (AAPA) accepts certificates of participation for educational activities certified for AMA PRA Category 1 Creditâ„¢ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 1 hour of Category I credit for completing this program.
Financial Disclosure
Dr Kane is a consultant for and has received honoraria from Alkermes, Allergan, Genentech, Lundbeck, Intracellular Therapies, Janssen, Johnson & Johnson (J&J), Merck, Neurocrine, Otsuka, Pierre Fabre, Reviva, Roche, Sunovion, Takeda, and Teva; has received grant/research support from Otsuka, Lundbeck, and Janssen; is a member of the speakers/advisory boards for Alkermes, Intracellular Therapies, Lundbeck, Neurocrine, Otsuka, Pierre Fabre, Roche, Sunovion, Takeda, Teva, and Reviva; and is a stock shareholder of Vanguard Research Group and LB Pharma. Dr Correll is a consultant for and has received honoraria from Alkermes, Allergan, Angelini, Boehringer-Ingelheim, Gerson Lehrman Group, Indivior, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Merck, Neurocrine, Noven, Otsuka, Pfizer, Rovi, Servier, Sumitomo Dainippon, Sunovion, Supernus, Takeda, and Teva; has received grant/research support from Janssen and Takeda; is a member of the advisory boards for Alkermes, Allergan, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, Merck, Neurocrine, Noven, Otsuka, Sunovion, Supernus, Takeda, and Teva; is a stock shareholder of LB Pharma; and has received other financial support from Janssen and Otsuka (expert testimony) and UpToDate (royalties).
Review Process
The faculty member(s) agreed to provide a balanced and evidence-based presentation and discussed the topic(s) and CME objective(s) during the planning sessions. The faculty’s submitted content was validated by CME Institute staff, and the activity was evaluated for accuracy, use of evidence, and fair balance by the Chair and a peer reviewer who is without conflict of interest.
The opinions expressed herein are those of the faculty and do not necessarily reflect the opinions of the CME provider and publisher or the commercial supporter.
This CME activity is expired. For more CME activities, visit cme.psychiatrist.com.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCES
1. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487-497. PubMed CrossRef
2. Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry. 2013;12(3):216-226. PubMed CrossRef
3. World Health Organization. In: Sabaté E, ed. Adherence to Long-Term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization; 2003. WHO website. https://www.who.int/chp/knowledge/publications/adherence_full_report.pdf.
4. Kaplan G, Casoy J, Zummo J. Impact of long-acting injectable antipsychotics on medication adherence and clinical, functional, and economic outcomes of schizophrenia. Patient Prefer Adherence. 2013;7:1171-1180. PubMed CrossRef
5. Achtyes ED, Simmons A, Skabeev A, et al. 21 patient preferences concerning the efficacy and side-effect profile of schizophrenia medication: a survey of patients living with schizophrenia. CNS Spectr. 2019;24(1):184. PubMed CrossRef
6. Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(suppl 4):1-46, quiz 47-48. PubMed CrossRef
7. Werner F-M, Covenas R. Long-term administration of antipsychotic drugs in schizophrenia and influence of substance and drug abuse on the disease outcome. Curr Drug Abuse Rev. 2017;10(1):19-24. PubMed CrossRef
8. Treatment plans: lessons learned. Family and Caregiver Schizophrenia Discussion Forum. https://family.schizophrenia.com/. Published May 2019. Accessed July 2, 2019.
9. Stephenson JJ, Tunceli O, Gu T, et al. Adherence to oral second-generation antipsychotic medications in patients with schizophrenia and bipolar disorder: physicians’ perceptions of adherence vs pharmacy claims. Int J Clin Pract. 2012;66(6):565-573. PubMed CrossRef
10. Byerly MJ, Thompson A, Carmody T, et al. Validity of electronically monitored medication adherence and conventional adherence measures in schizophrenia. Psychiatr Serv. 2007;58(6):844-847. PubMed CrossRef
11. Kane JM, Leucht S, Carpenter D, et al; Expert Consensus Panel for Optimizing Pharmacologic Treatment of Psychotic Disorders. The Expert Consensus Guideline series, optimizing pharmacologic treatment of psychotic disorders: introduction: methods, commentary, and summary. J Clin Psychiatry. 2003;64(suppl 12):5-19. PubMed
12. Velligan DI, Weiden PJ, Sajatovic M, et al. Assessment of adherence problems in patients with serious and persistent mental illness: recommendations from the Expert Consensus Guidelines. J Psychiatr Pract. 2010;16(1):34-45. PubMed CrossRef
13. Jaeger S, Pfiffner C, Weiser P, et al. Adherence styles of schizophrenia patients identified by a latent class analysis of the Medication Adherence Rating Scale (MARS): a six-month follow-up study. Psychiatry Res. 2012;200(2-3):83-88. PubMed CrossRef
14. Zemmour K, Tinland A, Boucekine M, et al; French Housing First Study Group. Validation of the Medication Adherence Rating Scale in homeless patients with schizophrenia: Results from the French Housing First experience. Sci Rep. 2016;6(1):31598. PubMed CrossRef
15. Brain C, Sameby B, Allerby K, et al. Twelve months of electronic monitoring (MEMS) in the Swedish COAST-study: a comparison of methods for the measurement of adherence in schizophrenia. Eur Neuropsychopharmacol. 2014;24(2):215-222. PubMed CrossRef
16. Kane JM. Attitudinal barriers to prescribing LAI antipsychotics in the outpatient setting: communicating with patients, families, and caregivers. J Clin Psychiatry. 2014;75(12):e33. PubMed CrossRef
17. Kane JM. Schizophrenia. N Engl J Med. 1996;334(1):34-41. PubMed CrossRef
18. Weiden PJ, Kozma C, Grogg A, et al. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv. 2004;55(8):886-891. PubMed CrossRef
19. Correll CU. Recognition of patients who would benefit from LAI antipsychotic treatment: how to assess adherence. J Clin Psychiatry. 2014;75(11):e29. PubMed CrossRef
20. Zipursky RB, Menezes NM, Streiner DL. Risk of symptom recurrence with medication discontinuation in first-episode psychosis: a systematic review. Schizophr Res. 2014;152(2-3):408-414. PubMed CrossRef
21. Morken G, Widen JH, Grawe RW. Non-adherence to antipsychotic medication, relapse and rehospitalisation in recent-onset schizophrenia. BMC Psychiatry. 2008;8(1):32. PubMed CrossRef
22. Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241-247. PubMed CrossRef
23. Andreasen NC, Liu D, Ziebell S, et al. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: a prospective longitudinal MRI study. Am J Psychiatry. 2013;170(6):609-615. PubMed CrossRef
24. Emsley R, Chiliza B, Asmal L. The evidence for illness progression after relapse in schizophrenia. Schizophr Res. 2013;148(1-3):117-121. PubMed CrossRef
25. Takeuchi H, Siu C, Remington G, et al. Does relapse contribute to treatment resistance? antipsychotic response in first- vs second-episode schizophrenia. Neuropsychopharmacology. 2019;44(6):1036-1042. PubMed CrossRef
26. Herings RM, Erkens JA. Increased suicide attempt rate among patients interrupting use of atypical antipsychotics. Pharmacoepidemiol Drug Saf. 2003;12(5):423-424. PubMed CrossRef
27. Schizophrenia and Related Disorders Alliance of America. Member Stories. SARDAA website. https://sardaa.org/schizophrenia-alliance/member-stories/. Published 2018. Accessed February 27, 2018.
28. Greene M, Yan T, Chang E, et al. Medication adherence and discontinuation of long-acting injectable versus oral antipsychotics in patients with schizophrenia or bipolar disorder. J Med Econ. 2018;21(2):127-134. PubMed CrossRef
29. Yoshimatsu K, Elser A, Thomas M, et al. Recovery-oriented outcomes associated with long-acting injectable antipsychotics in an urban safety-net population [published online May 17, 2019]. Community Ment Health J. PubMed CrossRef
30. Parro-Torres C, Ros-Cucurull E, Arques-Egea S. 12 impact of aripiprazole long-acting injectable (ALAI) initiation on hospitalizations and visits to emergency. CNS Spectr. 2019;24(1):179-180. PubMed CrossRef
31. Marcus SC, Zummo J, Pettit AR, et al. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768. PubMed CrossRef
32. Nielsen RE, Hessellund KB, Valentin JB, et al. Second-generation LAI are associated to favorable outcome in a cohort of incident patients diagnosed with schizophrenia. Schizophr Res. 2018;202:234-240. PubMed CrossRef
33. Tiihonen J, Mittendorfer-Rutz E, Majak M, et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29,823 patients with schizophrenia. JAMA Psychiatry. 2017;74(7):686-693. PubMed CrossRef
34. Patel MX, Taylor M, David AS. Antipsychotic long-acting injections: mind the gap. Br J Psychiatry suppl. 2018;195(S52):s1-s4. PubMed CrossRef
35. Heres S, Hamann J, Kissling W, et al. Attitudes of psychiatrists toward antipsychotic depot medication. J Clin Psychiatry. 2006;67(12):1948-1953. PubMed CrossRef
36. Patel MX, Haddad PM, Chaudhry IB, et al. Psychiatrists’ use, knowledge and attitudes to first- and second-generation antipsychotic long-acting injections: comparisons over 5 years. J Psychopharmacol. 2010;24(10):1473-1482. PubMed CrossRef
37. Jaeger M, Rossler W. Attitudes towards long-acting depot antipsychotics: a survey of patients, relatives and psychiatrists. Psychiatry Res. 2010;175(1-2):58-62. PubMed CrossRef
38. Walburn J, Gray R, Gournay K, et al. Systematic review of patient and nurse attitudes to depot antipsychotic medication. Br J Psychiatry. 2001;179(4):300-307. PubMed CrossRef
39. Iyer S, Banks N, Roy M-A, et al. A qualitative study of experiences with and perceptions regarding long-acting injectable antipsychotics, part I: patient perspectives. Can J Psychiatry. 2013;58(suppl 1):14S-22S. PubMed CrossRef
40. Caroli F, Raymondet P, Izard I, et al. Opinions of French patients with schizophrenia regarding injectable medication. Patient Prefer Adherence. 2011;5:165-171. PubMed CrossRef
41. Llorca PM, Abbar M, Courtet P, et al. Guidelines for the use and management of long-acting injectable antipsychotics in serious mental illness. BMC Psychiatry. 2013;13(1):340. PubMed CrossRef
42. Remington G, Addington D, Honer W, et al. Guidelines for the pharmacotherapy of schizophrenia in adults. Can J Psychiatry. 2017;62(9):604-616. PubMed CrossRef
43. Tiihonen J, Haukka J, Taylor M, et al. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011;168(6):603-609. PubMed CrossRef
44. Kirson NY, Weiden PJ, Yermakov S, et al. Efficacy and effectiveness of depot versus oral antipsychotics in schizophrenia: synthesizing results across different research designs. J Clin Psychiatry. 2013;74(6):568-575. PubMed CrossRef
45. Leucht C, Heres S, Kane JM, et al. Oral versus depot antipsychotic drugs for schizophrenia: a critical systematic review and meta-analysis of randomised long-term trials. Schizophr Res. 2011;127(1-3):83-92. PubMed CrossRef
46. Kane JM, Schooler NR, Marcy P, et al. Patients with early-phase schizophrenia will accept treatment with sustained-release medication (long-acting injectable antipsychotics): results from the recruitment phase of the PRELAPSE Trial. J Clin Psychiatry. 2019;80(3):18m12546. PubMed CrossRef
47. Correll CU, Rubio JM, Kane JM. What is the risk-benefit ratio of long-term antipsychotic treatment in people with schizophrenia? World Psychiatry. 2018;17(2):149-160. PubMed CrossRef
48. Carbon M, Correll CU. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin Neurosci. 2014;16(4):505-524. PubMed
49. Zhu Y, Li C, Huhn M, et al. How well do patients with a first episode of schizophrenia respond to antipsychotics: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2017;27(9):835-844. PubMed CrossRef
50. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962. PubMed CrossRef
51. Kishimoto T, Hagi K, Nitta M, et al. Long-term effectiveness of oral second-generation antipsychotics in patients with schizophrenia and related disorders: a systematic review and meta-analysis of direct head-to-head comparisons. World Psychiatry. 2019;18(2):208-224. PubMed CrossRef
52. Lauriello J, Perkins DO. Enhancing the treatment of patients with schizophrenia through continuous care. J Clin Psychiatry. 2019;80(1):AL18010AH2C. PubMed CrossRef
53. Fluphenazine decanoate [package insert]. Rockford, IL: Mylan Institutional LLC; 2018.
54. Haloperidol decanoate [package insert]. Rockford, IL: Mylan Institutional LLC; 2019.
55. Risperidone long-acting injection [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2007.
56. Risperidone extended-release injectable suspension, for subcutaneous use [package insert]. North Chesterfield, VA: Indivior Inc; 2018.
57. Olanzapine pamoate [package insert]. Indianapolis, IN: Elly Lilly and Company; 2018.
58. Paliperidone palmitate injection [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2019.
59. Paliperidone palmitate injection, suspension, extended release [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2018.
60. ARISTADA (aripiprazole lauroxil) extended-release injectable suspension, for intramuscular use [package insert]. Waltham, MA: Alkermes, Inc; 2018.
61. ARISTADA INITIO (aripiprazole lauroxil) extended-release injectable suspension, for intramuscular use [package insert]. Waltham, MA: Alkermes, Inc; 2018.
62. Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192-213. PubMed CrossRef
63. Kane JM, Kishimoto T, Correll CU. Assessing the comparative effectiveness of long-acting injectable vs oral antipsychotic medications in the prevention of relapse provides a case study in comparative effectiveness research in psychiatry. J Clin Epidemiol. 2013;66(suppl):S37-S41. PubMed CrossRef
64. Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957-965. PubMed CrossRef
65. Kishimoto T, Hagi K, Nitta M, et al. Effectiveness of long-acting injectable vs oral antipsychotics in patients with schizophrenia: a meta-analysis of prospective and retrospective cohort studies. Schizophr Bull. 2018;44(3):603-619. PubMed CrossRef
66. Offord S, Wong B, Mirski D, et al. Healthcare resource usage of schizophrenia patients initiating long-acting injectable antipsychotics vs oral. J Med Econ. 2013;16(2):231-239. PubMed CrossRef
67. Leucht S, Tardy M, Komossa K, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379(9831):2063-2071. PubMed CrossRef
68. Weiden PJ, Kim E, Bermak J, et al. Does half-life matter after antipsychotic discontinuation? a relapse comparison in schizophrenia with 3 different formulations of paliperidone. J Clin Psychiatry. 2017;78(7):e813-e820. PubMed CrossRef
69. Schreiner A, Aadamsoo K, Altamura AC, et al. Paliperidone palmitate versus oral antipsychotics in recently diagnosed schizophrenia. Schizophr Res. 2015;169(1-3):393-399. PubMed CrossRef
70. Alphs L, Benson C, Cheshire-Kinney K, et al. Real-world outcomes of paliperidone palmitate compared to daily oral antipsychotic therapy in schizophrenia: a randomized, open-label, review board-blinded 15-month study. J Clin Psychiatry. 2015;76(5):554-561. PubMed CrossRef
71. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829. PubMed CrossRef
72. Correll CU, Sliwa JK, Najarian DM, et al. Practical considerations for managing breakthrough psychosis and symptomatic worsening in patients with schizophrenia on long-acting injectable antipsychotics. CNS Spectr. 2018;1-17. PubMed CrossRef
73. Lynn Starr H, Bermak J, Mao L, et al. Comparison of long-acting and oral antipsychotic treatment effects in patients with schizophrenia, comorbid substance abuse, and a history of recent incarceration: an exploratory analysis of the PRIDE study. Schizophr Res. 2018;194:39-46. PubMed CrossRef
74. Koola MM, Wehring HJ, Kelly DL. The potential role of long-acting injectable antipsychotics in people with schizophrenia and comorbid substance use. J Dual Diagn. 2012;8(1):50-61. PubMed CrossRef
75. Abdel-Baki A, Thibault D, Medrano S, et al. Long-acting antipsychotic medication as first-line treatment of first-episode psychosis with comorbid substance use disorder [published online ahead of print May 24, 2019]. Early Interv Psychiatry. PubMed CrossRef
76. Hou C-L, Ma X-R, Cai M-Y, et al. Comorbid moderate-severe depressive symptoms and their association with quality of life in Chinese patients with schizophrenia treated in primary care. Community Ment Health J. 2016;52(8):921-926. PubMed CrossRef
77. van Rooijen G, Vermeulen JM, Ruhé HG, et al. Treating depressive episodes or symptoms in patients with schizophrenia. CNS Spectr. 2019;24(2):239-248. PubMed CrossRef
78. Chung K-F, Poon YPY, Ng T-K, et al. Correlates of sleep irregularity in schizophrenia. Psychiatry Res. 2018;270:705-714. PubMed CrossRef
79. Monti JM, Torterolo P, Pandi Perumal SR. The effects of second generation antipsychotic drugs on sleep variables in healthy subjects and patients with schizophrenia. Sleep Med Rev. 2017;33:51-57. PubMed CrossRef
80. Baglioni C, Nanovska S, Regen W, et al. Sleep and mental disorders: a meta-analysis of polysomnographic research. Psychol Bull. 2016;142(9):969-990. PubMed CrossRef
81. Siu M-W, Chong CS-Y, Lo WT-L. Prevalence and clinicians’ awareness of psychiatric comorbidities among first-episode schizophrenia. Early Interv Psychiatry. 2018;12(6):1128-1136. PubMed CrossRef
82. Unable to live with my son. Family and Caregiver Schizophrenia Discussion Forum. https://family.schizophrenia.com/t/unable-to-live-with-my-son/7794. Published May 20, 2019. Accessed June 24, 2019.
83. Rubio JM, Taipale H, Correll CU, et al. Psychosis breakthrough on antipsychotic maintenance: results from a nationwide study [published online ahead of print June 13, 2019]. Psychol Med. PubMed CrossRef
84. Rossenu S, Cleton A, Hough D, et al. Pharmacokinetic profile after multiple deltoid or gluteal intramuscular injections of paliperidone palmitate in patients with schizophrenia. Clin Pharmacol Drug Dev. 2015;4(4):270-278. PubMed CrossRef
85. Yin J, Collier AC, Barr AM, et al. Paliperidone palmitate long-acting injectable given intramuscularly in the deltoid versus the gluteal muscle: are they therapeutically equivalent? J Clin Psychopharmacol. 2015;35(4):447-449. PubMed CrossRef
86. Ravenstijn P, Samtani M, Russu A, et al. Paliperidone palmitate long-acting injectable given intramuscularly in the deltoid versus the gluteal muscle. J Clin Psychopharmacol. 2016;36(6):744-745. PubMed CrossRef
87. Correll CU. What are we looking for in new antipsychotics? J Clin Psychiatry. 2011;72(suppl 1):9-13. PubMed CrossRef
88. McIntyre RS. Understanding needs, interactions, treatment, and expectations among individuals affected by bipolar disorder or schizophrenia: the UNITE global survey. J Clin Psychiatry. 2009;70(suppl 3):5-11. PubMed CrossRef
89. Angermeyer MC, Matschinger H. Attitude of family to neuroleptics [German]. Psychiatr Prax. 1999;26(4):171-174. PubMed
90. Misawa F, Kishimoto T, Hagi K, et al. Safety and tolerability of long-acting injectable versus oral antipsychotics: a meta-analysis of randomized controlled studies comparing the same antipsychotics. Schizophr Res. 2016;176(2-3):220-230. PubMed CrossRef
91. Dibonaventura M, Gabriel S, Dupclay L, et al. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12(1):20. PubMed CrossRef
92. Briggs A, Wild D, Lees M, et al. Impact of schizophrenia and schizophrenia treatment-related adverse events on quality of life: direct utility elicitation. Health Qual Life Outcomes. 2008;6(1):105. PubMed CrossRef
93. Tanskanen A, Tiihonen J, Taipale H. Mortality in schizophrenia: 30-year nationwide follow-up study. Acta Psychiatr Scand. 2018;138(6):492-499. PubMed CrossRef
94. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2017;S0920-9964(17)30762-4. PubMed
95. Cahling L, Berntsson A, Bröms G, et al. Perceptions and knowledge of antipsychotics among mental health professionals and patients. BJPsych Bull. 2017;41(5):254-259. PubMed CrossRef
96. Weiden PJ, Roma RS, Velligan DI, et al. The challenge of offering long-acting antipsychotic therapies: a preliminary discourse analysis of psychiatrist recommendations for injectable therapy to patients with schizophrenia. J Clin Psychiatry. 2015;76(6):684-690. PubMed CrossRef
97. Das AK, Malik A, Haddad PM. A qualitative study of the attitudes of patients in an early intervention service towards antipsychotic long-acting injections. Ther Adv Psychopharmacol. 2014;4(5):179-185. PubMed CrossRef
98. Lasser RA, Schooler NR, Kujawa M, et al. A new psychosocial tool for gaining patient understanding and acceptance of long-acting injectable antipsychotic therapy. Psychiatry (Edgmont). 2009;6(4):22-27. PubMed
99. Amador X. LEAP Foundation For Research To Practice. LFRP website. https://lfrp.org/about-leap. Published 2018. Accessed July 3, 2019.
Save
Cite
Advertisement
GAM ID: sidebar-top