ABSTRACT
Background: The use of electroconvulsive therapy (ECT) in children and adolescents is based on a limited evidence base in the medical literature. We report outcomes of a cohort of youth treated with ECT at a single US academic medical center.
Methods: We conducted a retrospective chart review and analysis of all patients aged 18 years and younger who received ECT at the University of Utah from 1985 through 2016. For each patient record, 3 short-term clinical outcomes were assessed: response on the Clinical Global Impressions–Improvement scale, number of treatments administered, and reported side effects. Baseline characteristics were tested as predictors of clinical outcomes.
Results: One hundred seven youth (aged 10–18 years, 46% female) received ECT for a mood disorder, psychotic disorder, catatonia, or neuroleptic malignant syndrome. The most common diagnoses (DSM-IV-TR or DSM-5) were major depressive disorder (76 patients) and bipolar disorder (23 patients). The rate of response (much improved or very much improved) for the entire cohort was 77%. The mean number of treatments administered was 10.5. The most commonly reported side effects were headache (75%) and memory problems (65%). One patient experienced tardive seizures. There were no deaths or serious injuries. Clinical response was not predicted by age, sex, or clinical features (all P > .05).
Conclusions: These data suggest that ECT is a safe and effective treatment for children and adolescents with certain severe psychiatric illnesses. ECT outcomes and side effects were similar to those reported in adults, particularly for patients aged 15–18 years, for whom there are the most data.
J Clin Psychiatry 2021;82(2):19m13164
To cite: Pierson MD, Mickey BJ, Gilley LB, et al. Outcomes of youth treated with electroconvulsive therapy: a retrospective cohort study. J Clin Psychiatry. 2021;82(2):19m13164.
To share: https://doi.org/10.4088/JCP.19m13164
© Copyright 2021 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, University of Utah, Salt Lake City, Utah
*Corresponding author: Matthew D. Pierson, MD, 501 Chipeta Way, Salt Lake City, UT 84108 ([email protected]).
Electroconvulsive therapy (ECT) is a well-established treatment for adults with certain severe mental disorders. A large body of research1–3 demonstrates response rates ranging from 50%–90% for adults with severe mood disorders, with the higher response rates expected in patients who receive ECT as a first-line treatment or have shorter duration of illness. Catatonia,4 psychosis,5–7 and older age8,9 in an affective disorder may also predict response. In adult patients with primary psychotic disorders, ECT response reported in the literature is more variable. When ECT is used as monotherapy, response is usually inferior to that of antipsychotic medications used as monotherapy, but in combination with antipsychotic medications, ECT seems to confer an additional benefit.10–12 Higher response rates are predicted among patients with shorter episode duration or acute psychotic exacerbations.13 In adults, ECT is relatively well tolerated, with typical side effects being transient cognitive problems, headache, nausea, and muscle soreness.14
ECT use in youth is much less common than in adults, for multiple reasons. Prior studies15,16 have shown that a majority of child and adolescent psychiatrists have little knowledge of the use of ECT in youth and would not be confident in providing a second opinion. Few ECT providers are trained in child and adolescent psychiatry, and few child and adolescent psychiatry training programs provide experience on an ECT service.17 Among child psychiatrists who reported they had never used ECT, the most common reason was the limited evidence base for its use.15 In a study16 of child psychiatrists and psychologists, respondents believed that ECT was safer in adults than in youth and safer in adolescents than in children. Finally, some states have specific legislation that prohibits the use of this treatment under certain ages. Studies18,19 have shown that youth represent only about 1% of the patients who receive ECT.
The evidence supporting the use of ECT in youth is growing, but it remains based primarily on case reports and case series. Four relatively recent retrospective studies,20–23 with sample sizes ranging from 13 to 51 (n = 111 total), suggest that ECT is safe and effective for youth with severe affective and psychotic disorders. What is not well described, however, is the value of demographic and baseline clinical data for predicting outcomes for youth treated with ECT. Here we assess outcomes of a cohort of children and adolescents treated with ECT at a single academic institution in the United States. We also examine associations between baseline features and clinical outcome variables.
METHODS
Data Source
The protocol for this study was approved by the institutional review board at the University of Utah. We reviewed the charts of all patients 18 years and younger who received ECT at the University Neuropsychiatric Institute in Salt Lake City, Utah, from 1985 through 2016. Diagnoses at admission, time of ECT, and discharge were collected. When the primary diagnosis was not clear, the authors (L.B.G. and H.R.W.) independently reviewed the charts to come to a consensus. DSM-IV-TR24 criteria were used for cases prior to 2013 and DSM-525 criteria were used for cases from 2013 onward. Only youth who received ECT for an affective, psychotic, or catatonic diagnosis were included for analysis, because these are the best-established indications for ECT in adults. Global Assessment of Functioning (GAF)24 scores at time of ECT, when available, were collected. We collected demographic information, including age, sex, and ethnicity, when available.
Children and adolescents who received ECT were evaluated by at least 2 board-eligible/board-certified child and adolescent psychiatrists and, as per hospital policy, approved for ECT by the medical director of the hospital. Patients and their legal guardians were provided with information about the diagnosis, treatment options, and the risks and benefits of ECT. Written informed consent from the patients’ guardians was obtained prior to starting ECT.
We collected data on ECT treatments, including number of treatments and electrode placement. For patients who received more than 1 treatment series during their adolescence, we analyzed data on the first ECT series only. ECT was administered using the spECTrum 5000Q device (MECTA Corporation, Tualatin, Oregon). Treatments were typically done every other day, 3 times per week. Motor seizure duration was measured using a blood pressure cuff on the forearm, and seizure adequacy was defined as a motor seizure of at least 30 seconds. All patients received hyperventilation before the stimulus was administered. During a course of ECT, patients who no longer had adequate seizure duration at maximum stimulus typically received caffeine intravenously, though on a few instances theophylline or aminophylline was used when caffeine was unavailable at the institution. The typical anesthetic agent for induction was methohexital, and succinylcholine was the muscle paralytic for all patients.
Outcome Measures
Response to ECT was determined by independent reviews of the charts by 2 authors (L.B.G. and H.R.W.). All patients were assigned a Clinical Global Impressions–Improvement scale (CGI-I) score.26 If there was disagreement between the authors about the CGI-I score, they reviewed the chart together for consensus. Response was defined as a score of 1 (very much improved) or 2 (much improved) on the CGI-I. In addition, we recorded all side effects that patients endorsed or psychiatrists observed during the ECT course and the number of treatments administered in the ECT series. Generally, patients were treated until symptoms resolved, so the number of treatments in the series was considered as a proxy for speed of response.
Statistical Analyses
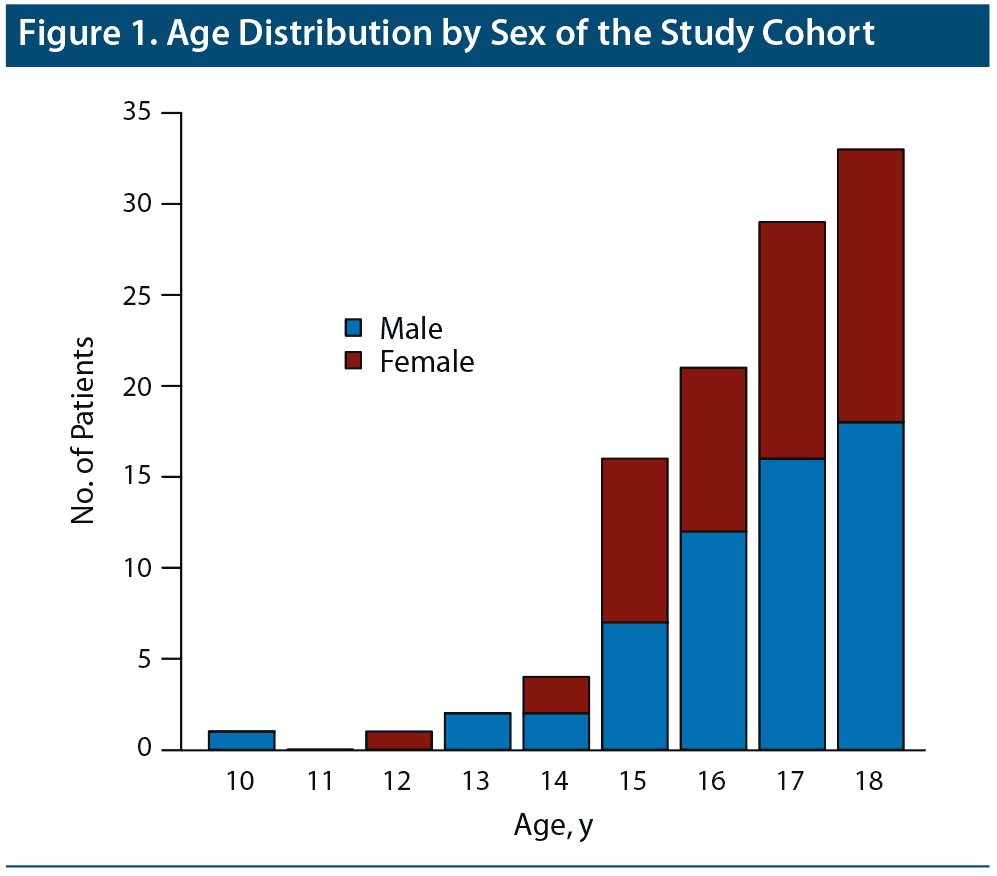
Analyses were performed using R statistical software, version 3.2.4 (R Foundation, 2016), and the RStudio environment, version 0.99.892.27 Baseline variables were tested as predictors of 3 types of clinical outcome: response on the CGI-I (dichotomous), number of ECT treatments administered (continuous), and presence of reported side effects (dichotomous). Side effects were further distinguished by type: headache, nausea/vomiting, muscle soreness, and memory problems. Logistic regression (eg, glm function, logit model) was used to test baseline predictors of ECT response. To evaluate associations between dichotomous and continuous variables, the Welch unequal-variances 2-sample t test was used, and the standardized mean difference (Hedges g) was estimated as a measure of effect size. To examine associations between 2 dichotomous variables, the Fisher exact test was used, and the odds ratio was estimated as a measure of effect size. To evaluate associations between 2 continuous variables, the Pearson correlation was computed. Because the distribution of age was strongly skewed in our sample (Figure 1), age was also examined with nonparametric tests (eg, Spearman correlation, Wilcoxon rank sum test).
RESULTS
Baseline Characteristics
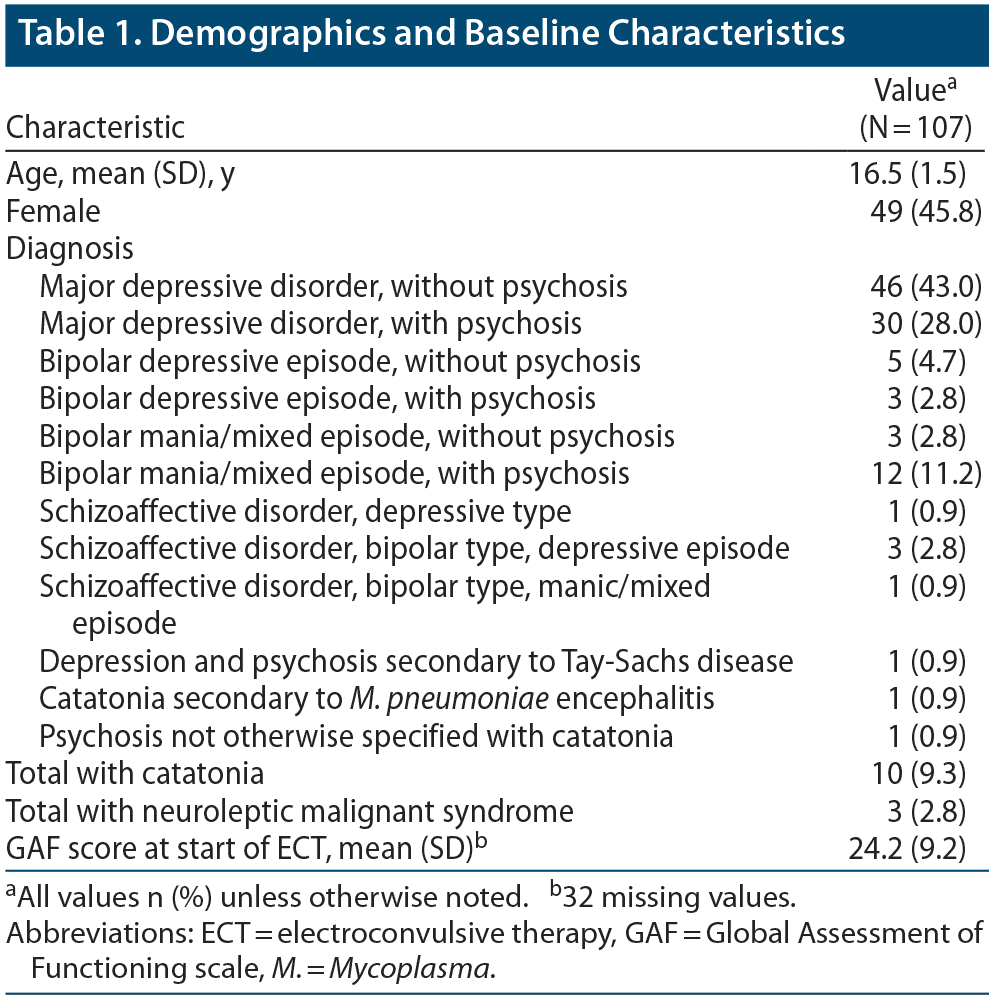
One hundred ten children and adolescents received ECT for a psychiatric disorder at the University Neuropsychiatric Institute during the period 1985–2016. Three patients with autism received ECT for self-injurious behavior and, because they did not have concomitant affective, psychotic, or catatonic diagnoses, were not included in the analysis. We therefore report on 107 patients. Forty-nine patients (46%) were female, and the mean age at the start of the treatment series was 16.5 years. The age range was 10–18 years (Figure 1). The most common diagnoses were major depressive disorder (MDD) and bipolar disorder (BD) (Table 1). Twelve patients had a secondary diagnosis of autism spectrum disorder, and 2 had intellectual disability.
GAF scores at the time of consultation for ECT were available for 75 patients. The mean score was 24.2, with a range from 6 to 45, indicating that the patients were typically seriously impaired and unable to function in almost all social domains.
Younger patients (aged 10–14 years) had more severe symptoms and functional impairment. There was a significant association between younger age and the presence of psychosis (P = .038, Wilcoxon rank sum test) and catatonia (P = .015, Wilcoxon rank sum test). Younger age also correlated with lower GAF score (ρ = 0.247, P = .032, Spearman correlation). All of the patients in our sample under 15 years of age (n = 8) were psychotic and/or catatonic. There was no statistically significant association between sex and other baseline variables (P > .05).
Stimulus dosing and electrode placement reflected the changing practice at the institution over the years of the study. Earlier patients were typically administered a brief pulse waveform, and later patients primarily received ultrabrief stimulation (< 0.5 ms). Bifrontal lead placement became standard at our institution in 2001. Overall, 1 patient received right unilateral lead placement, 2 received mixed lead placement (started right unilateral and converted to bitemporal), 13 received bitemporal lead placement, and 91 received bifrontal lead placement.
Clinical Outcomes
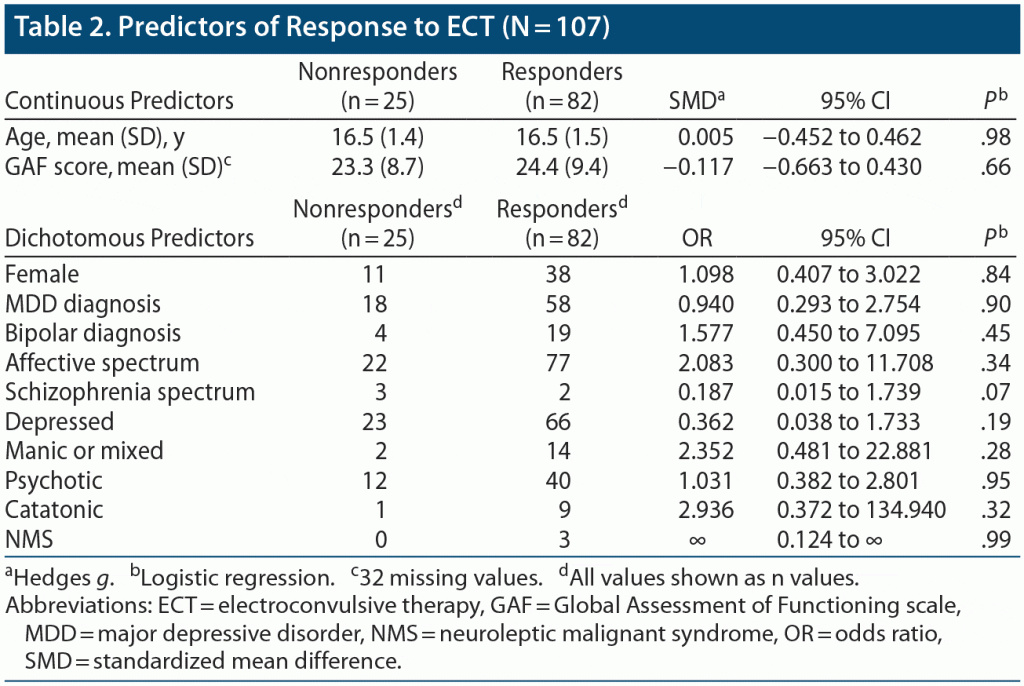
Response. The overall response rate for the sample was 77%. The response rate was lower in patients with a schizophrenia-spectrum diagnosis than in other patients (40% vs 78%, odds ratio [OR] = 0.19), but this difference did not reach statistical significance (P = .07, Fisher exact test). Psychotic features did not predict response (OR = 1.0). Manic/mixed episodes, catatonia, and neuroleptic malignant syndrome (NMS) tended to respond better than other predictors, but none of these reached statistical significance (Table 2). In our sample, patients with depression (MDD or BD) had a response rate of 74%, while patients in a manic or mixed episode had a response rate of 88%. Patients who had a diagnosis of catatonia in addition to their principal mood or psychotic disorder had a response rate of 90%, and all 3 patients with NMS responded. In further analyses restricted to the subgroup of patients with affective disorders, there was similarly no significant association between baseline variables and response rate.
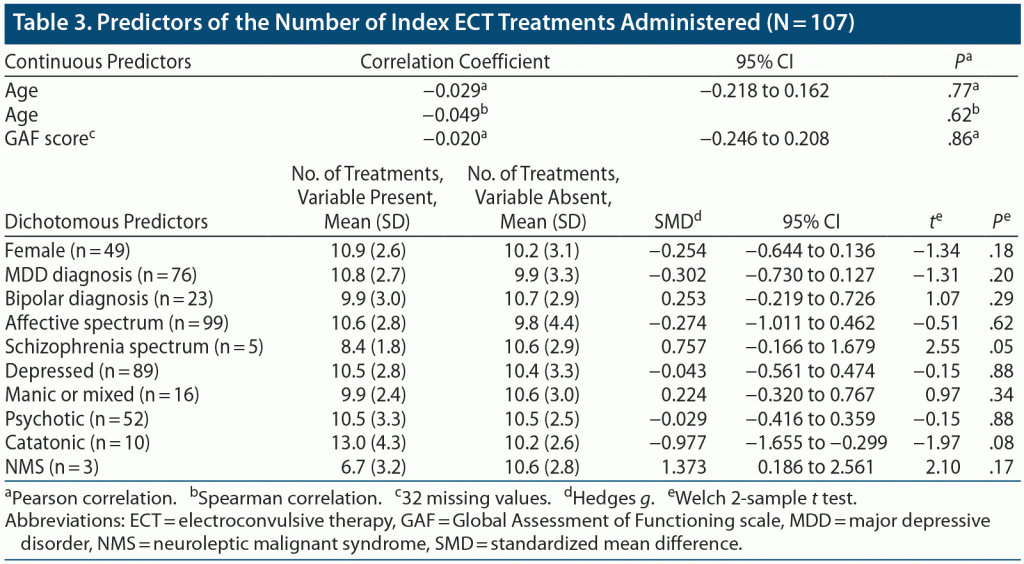
Number of treatments. The mean number of treatments in the acute series was 10.5. There was no statistically significant association between any baseline demographic or clinical variable and the number of treatments (Table 3), although patients with schizophrenia-spectrum illness tended to receive fewer treatments on average than other patients (mean of 8.4 vs 10.6, P = .051). This association was independent of the association between a schizophrenia-spectrum illness and ECT response: in a logistic regression that included both ECT response and the number of treatments as predictors, each remained associated with schizophrenia-spectrum illness (P = .035 and P = .051, respectively). Patients with catatonia received a greater number of treatments on average than other patients (mean of 13.0 vs 10.2, P = .078).
Side effects. Ninety percent of the sample reported at least 1 side effect during the treatment course. The most commonly reported side effect of ECT was headache (75%), followed by memory problems (65%), nausea/vomiting (50%), and muscle soreness (29%). One patient experienced tardive seizures on 2 occasions during her continuation course (not during the index series). These occurred within 30 minutes of her treatment and were resolved with propofol and midazolam. There were no other serious side effects.
In tests for associations of baseline variables with side effects, we found that muscle soreness was reported less commonly by patients with psychosis than by those without psychosis (14% vs 43%; OR = 0.22; 95% CI, 0.07–0.61; P = .014 after Bonferroni correction for 12 tests). No statistically significant predictors were found for memory problems, headache, nausea/vomiting, or muscle soreness (all P > .05 after Bonferroni correction).
Associations Between Clinical Outcomes
We performed exploratory analyses to evaluate for associations among 6 clinical outcomes: CGI-I response, number of treatments, headache, memory problems, muscle soreness, and nausea/vomiting. A positive association was found between nausea/vomiting and headache (P = .001, Fisher exact test, Bonferroni-corrected for 15 tests). There were no other significant associations (all P > .05 after Bonferroni correction). There was also no association between lead placement and clinical outcomes (P > .05).
DISCUSSION
This study describes, to our knowledge, the largest cohort of children and adolescents treated with ECT in the United States. The large sample size improved the estimates of short-term outcomes and allowed for an analysis of predictors of outcomes, which has not been previously well described in the literature. The highest response rates were observed in mania, catatonia, and NMS. Age and sex did not predict ECT outcomes in youth, which is notable, given that significant neurobiological changes occur between childhood and late adolescence. It also provides evidence against the notion that ECT is safer or more effective in adults than in adolescents.16
Although patients in this sample were typically seriously impaired in functioning, the majority experienced clinically significant improvement after a course of ECT. The response rate of this sample, 77%, is very close to those reported in other recent retrospective studies. A sample of 51 youth from the Mayo Clinic, which was similar to ours in terms of diagnoses treated, had a 77% response rate.23 Two cohorts from tertiary care centers in India were reported to have response rates of 76%20 and 77%,21 although those samples had a greater proportion of patients with psychotic and catatonic symptoms than the US sample. All 3 studies defined response identically to this study (CGI-I rating of much improved or very much improved). A sample of 13 adolescents from Canada, in which “reliable improvement” was defined as a decrease in the Beck Depression Inventory-II score of 10 points or more, demonstrated an improvement rate of 77%.22 In older studies, the response rates were more variable.28–31 Thus, our findings are consistent with those of more recent studies that show response rates of 76%–77% among seriously ill youth.
Previous reports of side effects are highly variable in the adolescent literature. This variability may be due to differences in how this information is collected as well as variability in the patient population and in administration of treatments. Reports of the per-person rates of memory complaints range from 0% to 85%; headaches, from 8% to 80%; nausea/vomiting, from 8% to 64%; and muscle soreness, from 0.5% to 69%.20–23,28–35 The rates of the side effects in our study fit within these ranges.
Tardive seizures have occurred in 0%–9% of youth described in retrospective studies.23,28,30,34,35 The 1 patient in our study who experienced tardive seizures during her continuation course represents 0.9% of our sample. Because of the large sample size, this rate most likely represents an improved estimate of the risk of tardive seizures in youth receiving ECT.
Previous studies have suggested that youth with a schizophrenia spectrum diagnosis have a lower ECT response rate than those with an affective disorder.30,36 We observed a similar pattern in our sample, although this pattern did not reach statistical significance. Patients with a schizophrenia spectrum diagnosis received fewer treatments than other patients, independent of ECT response; this finding has not previously been reported in the literature to our knowledge. Together, these findings suggest that youth with schizophrenia spectrum disorders may have a lower chance of response relative to youth with affective disorders, but those who do respond may respond more rapidly. However, while the number of treatments may indicate speed of response, for the nonresponders, it may instead indicate when the psychiatrist determines to stop trying. It is possible that, in schizophrenia spectrum disorders, response happens quickly, or if it does not, the psychiatrist stops treating, perhaps based on knowledge from the adult literature of the lower response rate in these patients. Similarly, in catatonia and affective disorders, even in the face of nonresponse, clinicians may be more likely to continue treating based on the evidence that these disorders are highly responsive to ECT. These findings require confirmation in a larger sample.
Studies20,36 on youth with catatonia have suggested that this disorder is highly responsive to ECT. A recent study23 demonstrated similar changes in CGI score between patients with and without catatonia. Data on ECT response among youth with NMS are limited, with a recent case series37 describing significant improvement in 5 youth with this condition. In our sample, patients with catatonia or NMS appeared to respond more frequently to ECT than did patients without these disorders (90% and 100%, respectively), although these differences did not reach statistical significance due to the small number of patients. Previous studies have not reported on how catatonia or NMS predict other clinical outcomes. In our study, subjects with NMS received fewer treatments on average compared with other subjects (6.7 vs 10.6), whereas patients with catatonia received a greater number of treatments (13.0 vs 10.2), although these findings did not reach statistical significance due to the small number of patients with these conditions.
In our sample, the syndrome of psychosis did not predict treatment response or number of treatments. In a meta-analysis36 of studies from 1993 to 2003, the ECT response rate of youth with depression with psychotic features was 85%, while the response rate for youth without psychotic features was 69%. More recently, a study22 of adolescents with depressive disorders demonstrated a 50% response rate in subjects with psychosis, compared with 67% in subjects without. Thus, psychotic features appear to be an inconsistent predictor of ECT response in youth, as similarly found in the adult literature.3
Our sample included 8 patients younger than 15 years old, and all of them were either psychotic or catatonic. Because of the limited evidence base for the safety and efficacy of ECT in children and the belief of many mental health professionals that ECT is safer in adolescents than in children,16 it is possible that only those with the most severe illness are referred at a young age for ECT. In addition, many patients receive ECT only after a long depressive illness course, which is more likely to be present in older adolescents.
This sample included 16 youth in a manic or mixed episode. Few studies have assessed ECT outcomes of youth with mania, but the suggestion of the literature29,30 is that this condition is highly responsive to ECT. A systematic review38 done in 1997 showed an 80% response rate in youth with mania (20 subjects), compared with a 63% response rate for depression (40 subjects). Our study showed an 88% response rate in mania and a 74% response rate in depression.
This retrospective study has a number of limitations. First, without a comparison group that did not receive ECT, this study cannot determine the true incremental impact of ECT in youth. In addition, only patients who were referred for an ECT consultation by the primary psychiatrist were eligible to receive ECT, thus introducing the possibility of sampling bias. This study did not assess for many factors that may have influenced outcomes, for example, comorbid psychiatric disorders, long duration of illness, history of abuse, and family discord.39–41 Self-injurious behavior in autism was not studied and remains an area in need of further research. As with any retrospective study, a potential for confirmation bias exists (ratings biased toward treatment response). Raters were not blinded to other variables such as diagnosis or number of treatments. Nonetheless, to improve reliability of ratings, 2 experienced independent raters reviewed each chart and came to a consensus on each case. Underreporting of side effects, particularly common or mild ones, may underestimate their frequency. Furthermore, cognitive side effects were measured subjectively. This study reports on short-term outcomes only. Information about long-term benefits or side effects from ECT cannot be determined from these data. Finally, that these data derive from a single-site study limits the study’s generalizability.
Overall, this study provides retrospective evidence suggesting ECT is a safe and effective treatment for youth with certain severe psychiatric illnesses. The data presented in this study show that, particularly for patients aged 15–18 years, for whom there are the most data, those with severe affective disorders and catatonia respond to ECT similarly to adults and that psychiatrists should consider ECT in their clinical decision making for these patients. Whether younger children, or youth with schizophrenia spectrum disorders, respond similarly to adults remains unclear.
Submitted: November 12, 2019; accepted August 5, 2020.
Published online: February 23, 2021.
Potential conflicts of interest: Dr Mickey has received research support from Novartis and LivaNova for unrelated work. Drs Pierson, Gilley, and Weeks have no potential conflicts of interest.
Funding/support: None.
Acknowledgments: The authors thank Kelly Smith, MD, and Jeremy Kendrick, MD, both from the University of Utah, Salt Lake City, Utah, for providing some of the data used in the analysis. Drs Smith and Kendrick have no conflicts of interest to declare. We thank all the physicians, nurses, technicians, and administrators of the Treatment Resistant Mood Disorders clinic for providing care for our patients and families.
Clinical Points
- A large body of research informs the use of electroconvulsive therapy (ECT) in adults, but there are limited data on its use in youth.
- These data suggest that youth aged 15–18 years receiving ECT for a severe affective disorder or catatonia respond similarly to adults. Clinicians should consider its use in these patients.
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Childhood and Adolescent Mental Health section. Please contact Karen D. Wagner, MD, PhD, at [email protected].
References (41)

- Prudic J, Sackeim HA, Devanand DP. Medication resistance and clinical response to electroconvulsive therapy. Psychiatry Res. 1990;31(3):287–296. PubMed CrossRef NLM
- Prudic J, Haskett RF, Mulsant B, et al. Resistance to antidepressant medications and short-term clinical response to ECT. Am J Psychiatry. 1996;153(8):985–992. PubMed CrossRef NLM
- Haq AU, Sitzmann AF, Goldman ML, et al. Response of depression to electroconvulsive therapy: a meta-analysis of clinical predictors. J Clin Psychiatry. 2015;76(10):1374–1384. PubMed CrossRef NLM
- Luchini F, Medda P, Mariani MG, et al. Electroconvulsive therapy in catatonic patients: efficacy and predictors of response. World J Psychiatry. 2015;5(2):182–192. PubMed CrossRef NLM
- Crow TJ, Johnstone EC. Controlled trials of electroconvulsive therapy. Ann N Y Acad Sci. 1986;462:12–29. PubMed CrossRef NLM
- Pande AC, Grunhaus LJ, Haskett RF, et al. Electroconvulsive therapy in delusional and non-delusional depressive disorder. J Affect Disord. 1990;19(3):215–219. PubMed CrossRef NLM
- Petrides G, Fink M, Husain MM, et al. ECT remission rates in psychotic versus nonpsychotic depressed patients: a report from CORE. J ECT. 2001;17(4):244–253. PubMed CrossRef NLM
- O’Connor MK, Knapp R, Husain M, et al. The influence of age on the response of major depression to electroconvulsive therapy: a CORE Report. Am J Geriatr Psychiatry. 2001;9(4):382–390. PubMed CrossRef NLM
- Tew JD Jr, Mulsant BH, Haskett RF, et al. Acute efficacy of ECT in the treatment of major depression in the old-old. Am J Psychiatry. 1999;156(12):1865–1870. PubMed NLM
- Abraham KR, Kulhara P. The efficacy of electroconvulsive therapy in the treatment of schizophrenia: a comparative study. Br J Psychiatry. 1987;151(2):152–155. PubMed CrossRef NLM
- Kho KH, Blansjaar BA, de Vries S, et al. Electroconvulsive therapy for the treatment of clozapine nonresponders suffering from schizophrenia—an open label study. Eur Arch Psychiatry Clin Neurosci. 2004;254(6):372–379. PubMed CrossRef NLM
- Pompili M, Lester D, Dominici G, et al. Indications for electroconvulsive treatment in schizophrenia: a systematic review. Schizophr Res. 2013;146(1–3):1–9. PubMed CrossRef NLM
- Dodwell D, Goldberg D. A study of factors associated with response to electroconvulsive therapy in patients with schizophrenic symptoms. Br J Psychiatry. 1989;154(5):635–639. PubMed CrossRef NLM
- Datto CJ. Side effects of electroconvulsive therapy. Depress Anxiety. 2000;12(3):130–134. PubMed CrossRef NLM
- Parmar R. Attitudes of child psychiatrists to electroconvulsive therapy. Psychiatr Bull. 1993;17(1):12–13. CrossRef
- Ghaziuddin N, Kaza M, Ghazi N, et al. Electroconvulsive therapy for minors: experiences and attitudes of child psychiatrists and psychologists. J ECT. 2001;17(2):109–117. PubMed CrossRef NLM
- Ghaziuddin N, Walter G. Electroconvulsive Therapy in Children and Adolescents. New York, NY: Oxford University Press; 2013.
- Thompson JW, Blaine JD. Use of ECT in the United States in 1975 and 1980. Am J Psychiatry. 1987;144(5):557–562. PubMed CrossRef NLM
- Walter G, Rey JM. An epidemiological study of the use of ECT in adolescents. J Am Acad Child Adolesc Psychiatry. 1997;36(6):809–815. PubMed CrossRef NLM
- Grover S, Malhotra S, Varma S, et al. Electroconvulsive therapy in adolescents: a retrospective study from north India. J ECT. 2013;29(2):122–126. PubMed CrossRef NLM
- Jacob P, Gogi PK, Srinath S, et al. Review of electroconvulsive therapy practice from a tertiary child and adolescent psychiatry centre. Asian J Psychiatr. 2014;12:95–99. PubMed CrossRef NLM
- Zhand N, Courtney DB, Flament MF. Use of electroconvulsive therapy in adolescents with treatment-resistant depressive disorders: a case series. J ECT. 2015;31(4):238–245. PubMed CrossRef NLM
- Puffer CC, Wall CA, Huxsahl JE, et al. A 20 year practice review of electroconvulsive therapy for adolescents. J Child Adolesc Psychopharmacol. 2016;26(7):632–636. PubMed CrossRef NLM
- American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
- American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Revised Edition. Washington, DC: US Department of Health, Education, and Welfare; 1976.
- R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2016.
- Ghaziuddin N, King CA, Naylor MW, et al. Electroconvulsive treatment in adolescents with pharmacotherapy-refractory depression. J Child Adolesc Psychopharmacol. 1996;6(4):259–271. PubMed CrossRef NLM
- Cohen D, Paillère-Martinot ML, Basquin M. Use of electroconvulsive therapy in adolescents. Convuls Ther. 1997;13(1):25–31. PubMed CrossRef NLM
- Walter G, Rey JM. Has the practice and outcome of ECT in adolescents changed? findings from a whole-population study. J ECT. 2003;19(2):84–87. PubMed CrossRef NLM
- Stein D, Kurtsman L, Stier S, et al. Electroconvulsive therapy in adolescent and adult psychiatric inpatients—a retrospective chart design. J Affect Disord. 2004;82(3):335–342. PubMed NLM
- Strober M, Rao U, DeAntonio M, et al. Effects of electroconvulsive therapy in adolescents with severe endogenous depression resistant to pharmacotherapy. Biol Psychiatry. 1998;43(5):335–338. PubMed CrossRef NLM
- Baeza I, Flamarique I, Garrido JM, et al. Clinical experience using electroconvulsive therapy in adolescents with schizophrenia spectrum disorders. J Child Adolesc Psychopharmacol. 2010;20(3):205–209. PubMed CrossRef NLM
- Zhang ZJ, Chen YC, Wang HN, et al. Electroconvulsive therapy improves antipsychotic and somnographic responses in adolescents with first-episode psychosis—a case-control study. Schizophr Res. 2012;137(1–3):97–103. PubMed CrossRef NLM
- Maoz H, Nitzan U, Goldwyn Y, et al. When can we predict the outcome of an electroconvulsive therapy course in adolescents? a retrospective study. J ECT. 2018;34(2):104–107. PubMed CrossRef NLM
- Consoli A, Benmiloud M, Wachtel L, et al. Electroconvulsive therapy in adolescents with the catatonia syndrome: efficacy and ethics. J ECT. 2010;26(4):259–265. PubMed CrossRef NLM
- Ghaziuddin N, Hendriks M, Patel P, et al. Neuroleptic malignant syndrome/malignant catatonia in child psychiatry: literature review and a case series. J Child Adolesc Psychopharmacol. 2017;27(4):359–365. PubMed CrossRef NLM
- Rey JM, Walter G. Half a century of ECT use in young people. Am J Psychiatry. 1997;154(5):595–602. PubMed CrossRef NLM
- Emslie GJ, Rush AJ, Weinberg WA, et al. Fluoxetine in child and adolescent depression: acute and maintenance treatment. Depress Anxiety. 1998;7(1):32–39. PubMed CrossRef NLM
- Curry J, Rohde P, Simons A, et al; TADS Team. Predictors and moderators of acute outcome in the Treatment for Adolescents with Depression Study (TADS). J Am Acad Child Adolesc Psychiatry. 2006;45(12):1427–1439. PubMed CrossRef NLM
- Asarnow JR, Emslie G, Clarke G, et al. Treatment of selective serotonin reuptake inhibitor-resistant depression in adolescents: predictors and moderators of treatment response. J Am Acad Child Adolesc Psychiatry. 2009;48(3):330–339. PubMed NLM
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top