This Academic Highlights section of The Journal of Clinical Psychiatry presents the highlights from a series of teleconferences held in the spring of 2014. The series was chaired by Alan F. Schatzberg, MD, Professor and Chairman, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Stanford, California. The faculty were Pierre Blier, MD, PhD, Endowed Chair of Mood Disorders Research, Institute of Mental Health Research, University of Ottawa, Ottawa, Ontario, Canada; Michael E. Thase, MD, Professor, Department of Psychiatry, University of Pennsylvania School of Medicine, Philadelphia; George I. Papakostas, MD, Scientific Director, MGH Clinical Trials Network and Institute, Director, Treatment-Resistant Depression Studies and Associate Professor of Psychiatry, Massachusetts General Hospital, Harvard Medical School, Boston; Larry Culpepper, MD, MPH, Professor and Chair, Department of Family Medicine, Boston University Medical Center, Boston, Massachusetts; and Rakesh Jain, MD, MPH, Director, Psychiatric Drug Research, R/D Clinical Research Center, Lake Jackson, Texas.
Financial disclosure: Dr Schatzberg received grant funding and/or honoraria for lectures and/or participation in advisory boards for McKinsey, Bay City Capital, Naurex, Eli Lilly, EnVivo, Boston Consulting, Gilead, Sire, Xhale, Cervel, Lundbeck/Takeda, National Institute of Mental Health, Pritzker Foundation, Merck, Genetech, Sunovion, MSI, American Psychiatric Association, and Stanford University. Dr Blier received grant funding and/or honoraria for lectures and/or participation in advisory boards for Astra Zeneca, Bristol Myers Squibb, Eli Lilly, Forest, Euthymics, Janssen, Lundbeck, Merck, Otsuka, Pfizer, Pierre Fabre, Servier, Shire, Takeda, and Valeant. Dr Culpepper received grant funding and/or honoraria for lectures and/or participation in advisory boards for Astra Zeneca, Forest Laboratories, H. Lundbeck A/S, Merck & Co, Sunovion, and Takeda. Dr Jain received grant funding and/or honoraria for lectures and/or participation in advisory boards for Addrenex, Forest, Lilly, Lundbeck, Merck, Otsuka, Pamlab, Pfizer, Shionogi, Shire, Sunovion, and Takeda. Dr Papakostas received grand funding and/or honoraria for lectures and/or participation in advisory boards for Avanir, Astra Zeneca, Bristol-Myers Squibb, Brainsway, Eli Lilly, GlaxoSmithKline, Lundbeck, National Institute of Mental Health, Novartis, Pamlab, Pfizer, Ridge Diagnostics, Shire, Sunovion, and Takeda. Dr Thase received grant funding and/or honoraria for lectures and/or participation in advisory boards for AstraZeneca, Bristol Myers Squibb, Cerecor, Eli Lilly, Dey Pharma, Forest Laboratories, Gershon Lehman Group, Guidepoint Global, H. Lundbeck, A/S, MedAvante, Merck, Neuronetics, Otsuka, Ortho-McNeil (Johnson & Johnson, Janssen), Pamlab LLC, Pfizer, Shire US, Sunovion, Supernus Pharmaceuticals, Takeda, Transcept Pharmaceuticals, Agency for Healthcare Research and Quality, Forest Pharmaceuticals, and National Institute of Mental Health.
This evidence-based peer-reviewed Academic Highlights was prepared and independently developed by Healthcare Global Village, Inc., with support from Physicians Postgraduate Press, Inc. The opinions expressed herein are those of the faculty and do not necessarily reflect the opinions of Healthcare Global Village, Inc., or the publisher.
ABSTRACT
Six clinicians provide an overview of the serotonergic antidepressant vortioxetine, which was recently approved for the treatment of major depressive disorder in adults. They discuss the pharmacologic profile and receptor-mediated effects of vortioxetine in relation to potential outcomes. Additionally, they summarize the clinical trials, which demonstrate vortioxetine’s efficacy, and discuss findings related to safety and tolerability that have high relevance to patient compliance.
J Clin Psychiatry 2014;75(12):1411-1418 (doi:10.4088/JCP.14027ah1)
© Copyright 2014 Physicians Postgraduate Press, Inc.
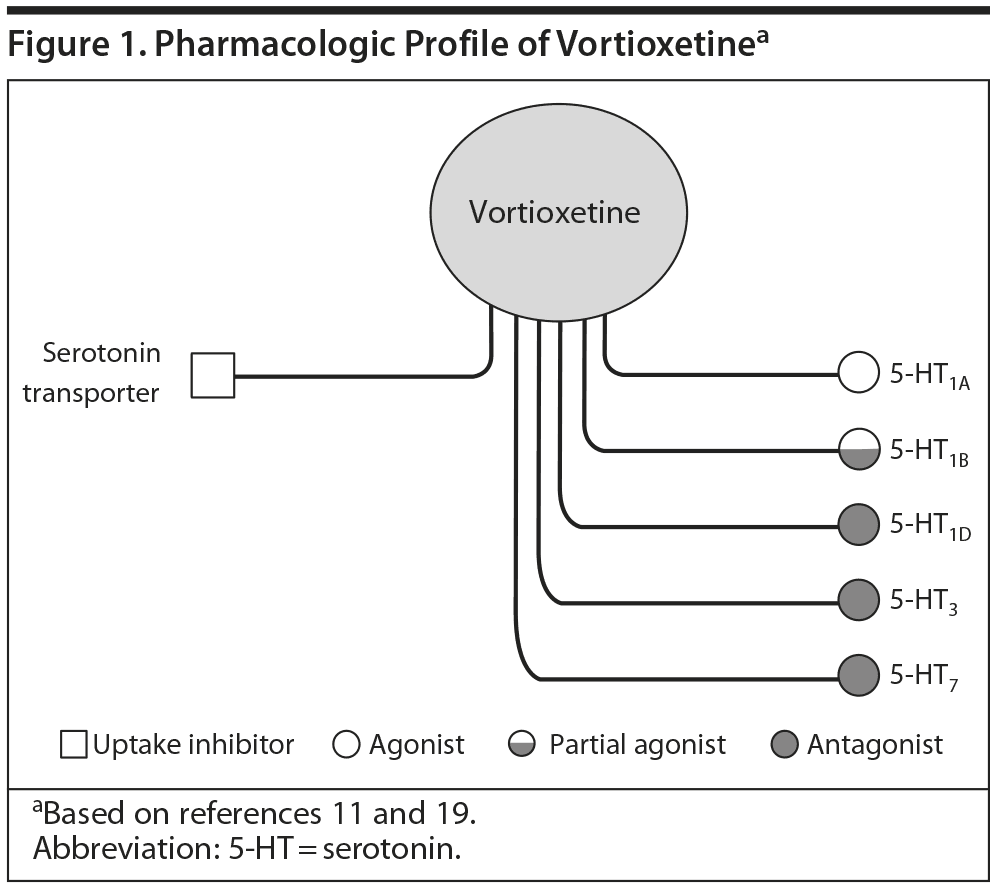
Vortioxetine is a serotonergic (5-HT) antidepressant recently released into the US market. It has a number of pharmacologic effects that extend beyond traditional 5-HT reuptake blockade. For example, the agent is a full agonist at the 5-HT1A and partial agonist at the 5-HT1B receptor. It also antagonizes 5-HT1D, 5-HT3, and 5-HT7 receptors. These various effects may connote potentially unique, beneficial outcomes in patients treated with the agent. A series of teleconferences were held to consider the agent from a broader perspective of preclinical and clinical pharmacology as well as in the context of management of depression in primary care and psychiatry.
In this report of those discussions, the relative receptor and transporter site affinities in preclinical studies are reviewed, and these suggest potential benefit in a number of areas, including side effects observed and improvement in cognition. Clinical trial data from the acute antidepressant trials are reviewed, as are data on the agent in recurrence prevention.
Release of a new agent provides potential benefits to patients as well as their treaters, whether they are in primary care or a psychiatric setting. The importance of managing the behavioral and affective components of medical disorders is also emphasized.
NEUROPHARMACOLOGY, NEUROTRANSMITTERS, AND MECHANISM OF ACTION
Serotonin Receptor Modulation and the Effects of Antidepressants
Vortioxetine is a novel antidepressant with multiple pharmacologic activity, approved by the US Food and Drug Administration (FDA) in 2013 for the treatment of major depressive disorder (MDD) in adults. Like many antidepressants, vortioxetine is believed to work through direct modulation of receptor activity and inhibition of the 5-HT transporter.1 It also has accessory properties that may have therapeutic benefits, particularly in patients with treatment-resistant depression.2
Many researchers believe that an imbalance in serotonin levels plays a role in depression, and it is worth examining in detail the possible links between serotonin receptor modulation and the effects of antidepressants. Serotonin has 7 major families of receptors, each with several subtypes, and influences a range of psychological and body functions.
5-HT1A receptor agonism has an anxiolytic and antidepressant effect, but may cause nausea and light-headedness,3 while 5-HT1B/D and 5-HT2C agonism is associated with low weight gain.4-6 5-HT2A antagonism may be beneficial for sleep maintenance,7 while evidence also indicates that 5-HT7 antagonism plays a role in circadian rhythms and sleep.3,8 The tolerability profile of 5-HT3 antagonism also appears to be favorable, having been associated with a low rate of gastrointestinal effects and nausea.4,5,9
Accessory Properties of Atypical Antipsychotics
Many atypical antipsychotics share certain receptor affinities with some antidepressants, such as 5-HT2A/C antagonism and 5-HT1A agonism, and have been used successfully as adjuncts in treatment-resistant depression. While not fully understood, these characteristics and actions may also contribute to the efficacy of vortioxetine, which shares some of the affinities common in the atypicals (Figure 1). For instance, both aripiprazole and vortioxetine are potent 5-HT1A agonists as well as 5-HT7 antagonists.10
Multimodality of Vortioxetine
Vortioxetine is one of several compounds developed from halogenated benzenes as part of a program to identify a new generation of antidepressants combining at least 2 separate pharmacologic modes of action that would, in theory, have superior clinical efficacy to currently available agents. In vivo nonclinical studies by Bang-Andersen et al11 demonstrated that vortioxetine enhances levels of serotonin, norepinephrine, dopamine, acetylcholine, and histamine in specific areas of the brain.
In addition, it has been shown that vortioxetine enhances memory in rats and increases extracellular acetylcholine and histamine in rat medial prefrontal cortex.12 Substantial evidence suggests that the 5-HT system has an important role in cognitive function, such as learning and memory, through the activation or blockade of 5-HT receptor subtypes, particularly 5-HT3 and 5-HT7. Thus, vortioxetine’s multimodal profile may have contributed to the improvement in cognitive function in patients with MDD observed in a clinical study by Katona et al.13 In this study in patients aged 65 and over, those treated with vortioxetine performed better than the placebo group in cognitive tests measuring processing speed, verbal learning, and memory.
This finding was replicated in a large adult population and reported to be a direct effect of vortioxetine on cognition, that is, being independent of the antidepressant response.14
The modulation of several different neurotransmitters may also have an anxiolytic effect.15 However, both positive and negative findings have been reported in studies evaluating vortioxetine in patients with generalized anxiety disorder.16-18
CLINICAL TRIALS OF VORTIOXETINE
Vortioxetine Trials
The efficacy of vortioxetine was demonstrated in 6 positive 6- to 8-week randomized, double-blind, placebo-controlled studies, including one study conducted in elderly patients and one maintenance study. These studies demonstrated statistically significant improvements in overall symptoms of depression in adults with MDD based on Montgomery-Asberg Depression Rating Scale (MADRS) or Hamilton Depression Rating Scale (HDRS) scores.19

- Vortioxetine, a new serotonergic agent, has shown superiority over placebo in treating major depressive disorder in both adult and geriatric patients.
- The multimodal pharmacologic activity of vortioxetine may convey benefit in cognitive function.
- Vortioxetine’s safety profile is similar to that of other selective serotonin reuptake inhibitors.
- Vortioxetine’s favorable tolerability profile may have meaningful advantages with regard to weight gain and low sexual dysfunction that may benefit patients.
In one of these studies, the change from baseline in MADRS total score was a decrease of 13 in patients treated with 10 mg/d and 14 in those treated with 20 mg/d; in the placebo group, the score decreased by 11 points (full analysis set [FAS], mixed model for repeated measurements [MMRM].19,20 Alvarez et al21 reported a decrease from baseline in MADRS total score at the week 6 primary end point of 20 for patients receiving vortioxetine 10 mg/d versus 15 for placebo (FAS, last observation carried forward [LOCF]). Results from a third study showed the change from baseline to the 8-week end point was a decrease in MADRS total score of 19 for patients randomized to 20 mg/d versus 12 for placebo (FAS, MMRM).22
In the study of elderly patients, the change from baseline in the HDRS total score (FAS, LOCF) was a decrease of 14 for patients treated with 5 mg/d of vortioxetine versus 10 for those in the placebo group.13
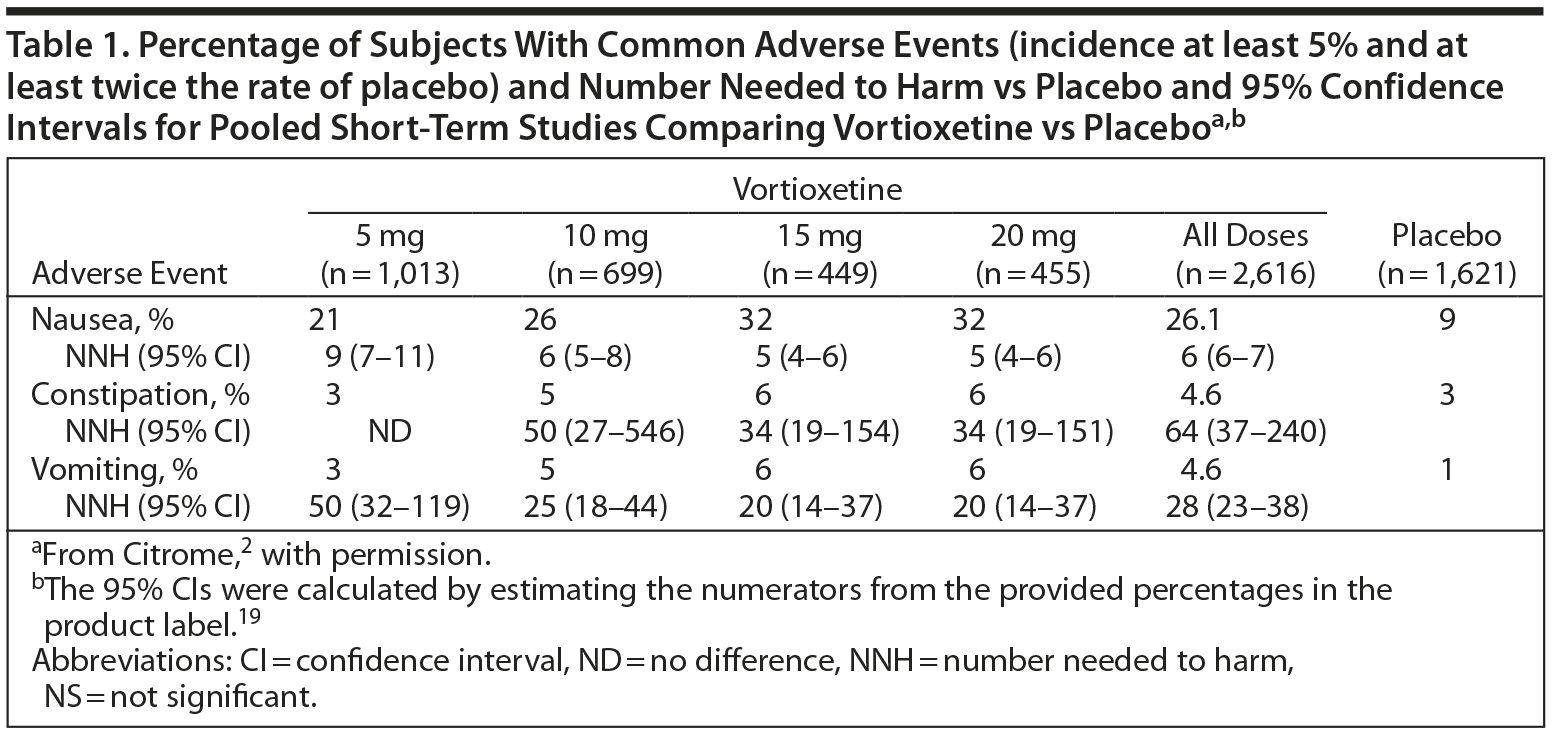
Number Needed to Harm and Number Needed to Treat
The efficacy of antidepressant therapy can be evaluated in several ways; one is by calculating the number needed to harm (NNH) and number needed to treat (NNT). NNT is the number of people who need to be treated over a specific period to promote one additional good outcome or prevent one additional bad outcome, while NNH is the number of people who need to be treated over a specific period before one negative outcome of the treatment will occur.23
The NNH can vary considerably depending on which adverse effect is selected, and a relevant categorical outcome must be chosen to make a fair and credible comparison. Ideally, the NNH should be greater than the NNT. (See Table 1 for data on vortioxetine.2)
The Debate Over Antidepressant Effectiveness
Antidepressants have been widely used since the 1960s. It may seem surprising that, some 50 years later, debate persists over topics such as efficacy in randomized clinical trials versus the far less controlled setting of the psychiatric or primary care practice, the extent and significance of the placebo effect, and which, if any, patient subgroups are most likely to benefit from antidepressants.
One meta-analytic study24 concluded that antidepressants have only modest benefits over placebo treatment, and the authors judged that the magnitude of benefit fell below the accepted criteria for clinical significance. Kirsch et al24 conducted a meta-analysis that comprised 35 clinical trials of 4 newer-generation antidepressants. The weighted mean improvement was 9.6 points on the HDRS in the drug groups and 7.8 in the placebo groups. The difference was statistically significant but did not meet a 3-point drug-placebo criterion for clinical significance that had previously been suggested by the UK’s National Institute of Clinical Excellence.24
The study’s other key finding was that efficacy reached clinical significance only in trials including the most severely depressed patients. The authors stated that was due to a decrease in the response to placebo rather than an increase in response to the antidepressant.24
The narrow mean drug-placebo difference prompted critics to observe that improvements based on rating scales may reflect nonspecific effects associated with many antidepressants rather than a genuine change in mood. They also suggest that amplified placebo effects experienced by patients and observed by raters influence rating scale scores.25
The validity of the findings from the Kirsch meta-analysis was challenged by many; for example, the 2013 study by Fountoulakis et al26 concluded that antidepressants are “clearly superior” to placebo and their efficacy is “unrelated to severity.” But the Kirsch findings were confirmed in a subsequent meta-analytic study27 using the same data from individual patients but also data from trials in which patients had a broader range of baseline symptom severity. The authors concluded that medication offered an advantage over placebo only in patients with very severe symptoms, and benefits were “nonexistent to negligible” among patients with milder depression.27
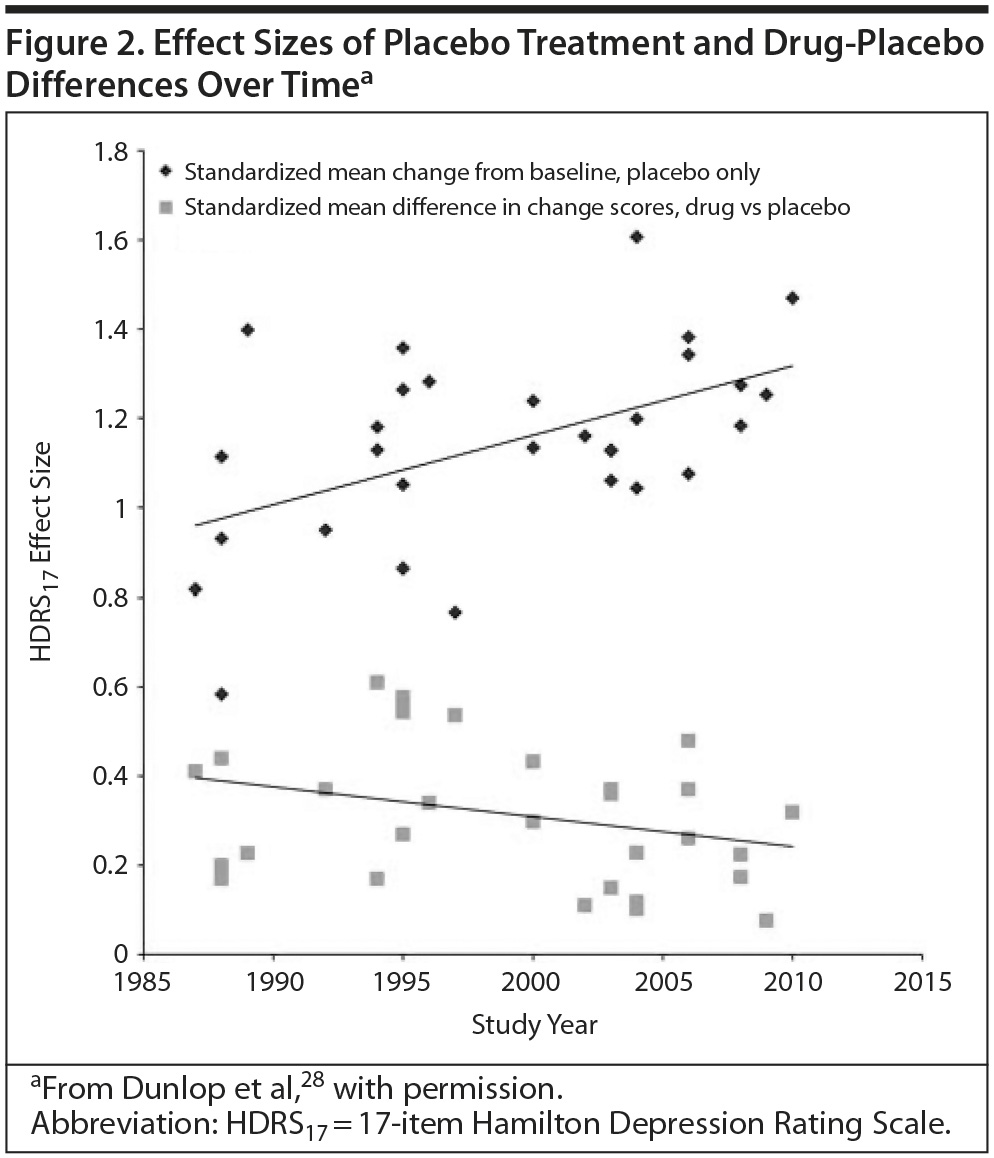
The Diminishing Drug-Placebo Difference
Antidepressant-placebo differences in published trials, also used to measure effect size, have shrunk over the last several decades as the placebo response rate has increased (Figure 2).28 A placebo response rate as high as 50% has been reported.29
The research environment is one possible component of this trend. A meta-analysis of short-term trials of 2 antidepressants over a 20-year period found that a higher percentage of patients enrolled from academic sites predicted a lower placebo response rate, greater drug-placebo separation, and a greater likelihood of positive outcomes.28 However, the majority of patients are now enrolled in industry-sponsored trials.30
Another way to assess the effect of antidepressant therapy versus placebo is through the analytic model. Thase et al31 used a mixture model to identify groups of patients who benefitted from treatment and those who did not. They concluded that “a relatively small mean difference in grouped data can obscure a large difference in benefit in a clinically meaningful proportion of patients.”31(p506)
TOLERABILITY ACROSS THE FDA-APPROVED DOSE RANGE
Adverse Events During Antidepressant Treatment
Adverse or unwanted events that occur during treatment can pose a significant obstacle to successful therapy. While side effects are mild or transient for many patients, for others they can be severe or chronic and may contribute to noncompliance.32
In a study to determine predictors of adherence to antidepressant therapy, Lin et al33 found that approximately 62% of patients who stopped taking medication within 30 days of starting treatment cited problematic side effects as the reason when questioned retrospectively, as did 66% of patients who stopped treatment between 30 and 60 days.
Further, it appears that physicians often underestimate both the incidence of adverse events and the extent to which patients consider these bothersome. Physicians also may expect that certain side effects will be transient, while patients report that they are still bothered by them several months after initiating therapy. In one study of side effects associated with SSRIs, physicians significantly underestimated the frequency of 9 of 17 side effects.34
These findings suggest that physician-patient communication concerning adverse events is inadequate and may be a factor in lack of compliance. The development of antidepressants that have a low incidence of side effects or the use of treatment strategies that alleviate them could improve the standard of care.32
Tolerability Findings From Vortioxetine Studies
Clinical studies suggest that vortioxetine has a good safety and tolerability profile and may have meaningful advantages in terms of tolerability versus the serotonin reuptake inhibitors, especially at doses lower than 20 mg daily.35 Pooled discontinuation rates due to adverse events were low in the short-term, nonelderly clinical trials: 4% in the placebo group, 5% in the 5-mg/d group, 6% in the 10-mg/d group, and 8% in both the 15-mg/d and the 20-mg/d groups.19
Nausea was the most commonly reported adverse event, and its frequency appeared to be dose-related. The incidence of mild nausea ranged from 14% to 20% in patients taking vortioxetine in doses of 5, 10, 15, or 20 mg/d compared to 6% in the placebo group; rates of moderate nausea were from 7% to 13% in the active treatment groups compared to 3% in the placebo group. The incidence of severe nausea was below 2% in all groups.
Nausea was also the most frequent adverse event in the study in elderly patients, occurring in 22% of those taking vortioxetine.13 Similarly, nausea was a common adverse event in the relapse-prevention study. It was the most common adverse event in the open-label period of the study (26%) and the third most common (9%) in the double-blind period.36 The low incidence over an extended period suggests that nausea is unlikely to interfere with compliance during acute treatment and maintenance therapy.
Sexual Dysfunction Associated With Antidepressant Drugs
Sexual dysfunction is common in the general population and even more common in people with depression. Estimates of treatment-emergent sexual dysfunction vary based on study methodology and other factors such as the class of antidepressants.37 However, a 2002 study by Clayton et al38 found that the rate of sexual dysfunction in patients taking SSRIs ranged from 25% to 43%. This study led to proactive exploration of sexual function using questionnaires in evaluation of antidepressants.
In studies of vortioxetine, the voluntarily reported incidence of adverse events related to sexual dysfunction was low and similar to that for placebo in some studies,13,21,39 while slightly higher in others.40-42 For example, Alvarez et al21 reported an incidence of 2% for the 5-mg/d dose of vortioxetine and 1% for the 10-mg/d dose versus 2% for placebo. The incidence of sexual dysfunction was ≤ 5% during both periods of the relapse prevention study.36
In a study using duloxetine as an active control,40 the incidence of treatment-emergent sexual dysfunction reported by men was 0 in the placebo group, 2% in the vortioxetine 2.5-mg group, and 4% in both the vortioxetine 5- and 10-mg groups. The rate was 14% in the duloxetine group.
However, patients may be uncomfortable discussing this sensitive subject, and the incidence may be underestimated if based solely on spontaneous reports.43 The Arizona Sexual Experience Scale (ASEX) is a 5-item scale used to measure sexual dysfunction. Because it is brief and nonintrusive, patients may be more forthcoming.44 The ASEX was used in several studies of vortioxetine, and the data across the studies for patients without sexual dysfunction at baseline (about one-third of the population across all treatment groups) have been summarized. In women, the incidence was 22%, 23%, 33%, and 34% for vortioxetine 5, 10, 15, and 20 mg/d versus 20% for placebo.19 In men, the incidence was 16%, 20%, 19%, and 29% for vortioxetine 5, 10, 15, and 20 mg/d versus 14% for placebo.19
Weight Gain and Other Adverse Effects
Weight gain has been mentioned as one of the most bothersome side effects of antidepressant treatment.34 Vortioxetine had no significant effect on body weight as measured by the mean change from baseline in the placebo-controlled studies.19 The incidence of several other adverse events commonly associated with antidepressant treatment, such as insomnia, fatigue, sedation, and somnolence, was also low.2
PREVENTION OF RECURRENCE
Long-Term Efficacy, Safety, and Tolerability Evaluated
In a series of 6- or 8-week studies, vortioxetine relieved multiple symptoms in adults with MDD. Following these trials, a recurrence prevention study was conducted to evaluate the long-term efficacy of vortioxetine versus placebo as well as to assess the drug’s long-term safety and tolerability.36
This double-blind, randomized, placebo-controlled study36 recruited 639 inpatients and outpatients. The study design consisted of 2 consecutive periods: a 12-week, open-label, flexible-dose treatment period with vortioxetine and a double-blind, fixed-dose, placebo-controlled treatment period of 24-64 weeks. Initially, patients received 5 mg/d of vortioxetine; however, an investigator could increase the dose to 10 mg/d or subsequently decrease it to 5 mg/d from weeks 2 to 8 in the open-label period. The dose remained fixed from week 8 to the end of the period. Patients who were in remission at both week 10 and week 12 were then randomized to placebo or vortioxetine for the double-blind, fixed-dose phase.
Exposure and Efficacy Results
From an initial enrollment of 639 patients in the open-label period, 400 (63%) were randomized to double-blind treatment (206 to vortioxetine and 194 to placebo). The full analysis set was 396 patients.36
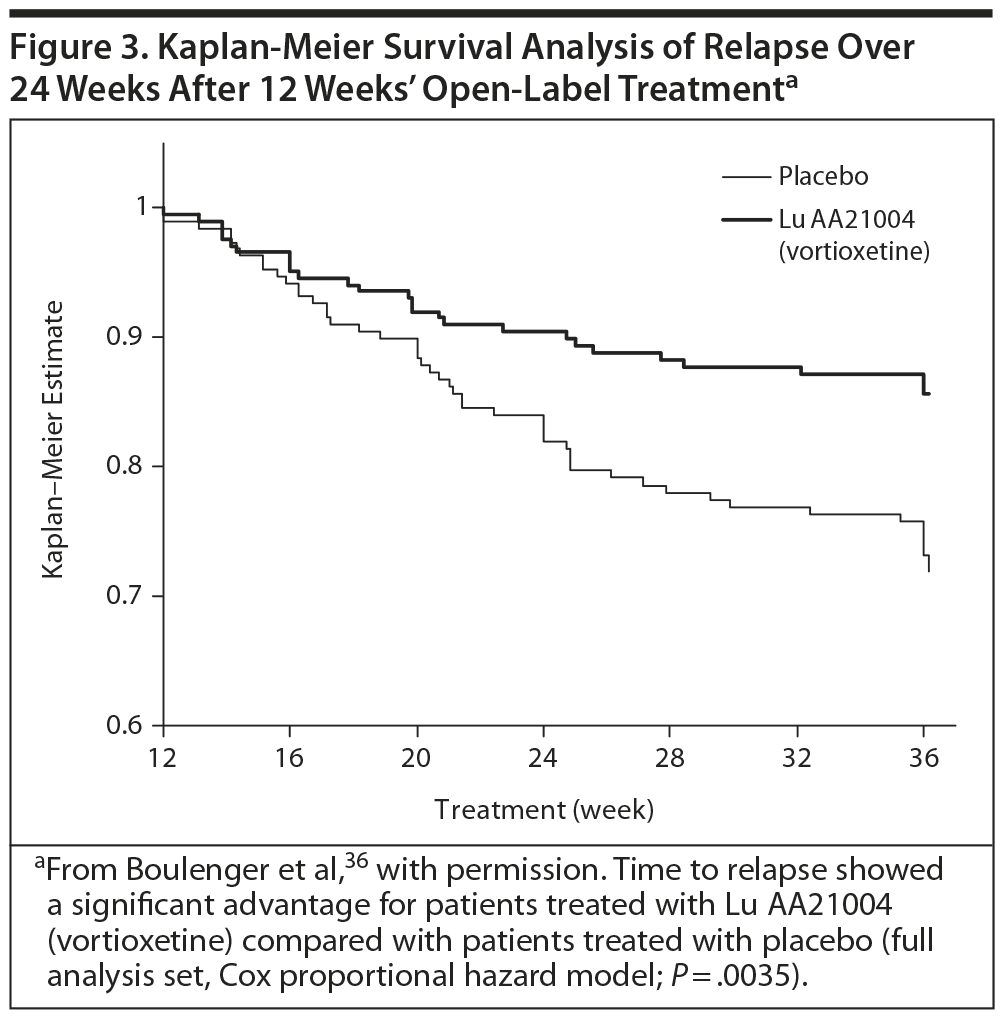
The primary efficacy outcome was the time to relapse of MDD within the first 24 weeks of the double-blind period. In the open-label period, the mean MADRS total score decreased from 32 ± 4 at baseline to 7 ± 6 (all patients treated set [APTS], observed cases [OC]) at week 12. The Clinical Global Impressions-Severity scale (CGI-S) score also decreased, from 4.8 ± 0.7 to 1.8 ± 1.0 (APTS, OC). Overall, 76% (n = 476) of patients had responded to treatment, based on a ≥ 50% decrease in MADRS total score from baseline, and 69% (n = 432) had achieved remission, based on a MADRS total score ≤ 10 (APTS, LOCF).36
In the double-blind phase, 229 of 396 patients in the full analysis set completed the study. The results showed a statistically significant difference in the time to relapse, favoring vortioxetine versus placebo in the first 24 weeks (Figure 3). The hazard ratio was 2.0 (95% confidence interval, CI, 1.3-3.2; P = .003). Another statistically significant finding was that a lower proportion of patients relapsed in the vortioxetine group (13%, n = 27) than in the placebo group (26%, n = 50; P = .001, χ2 test).36
Tolerability Findings
The withdrawal rates due to adverse events were low in both phases of the study, and the investigators classified 87% of the adverse events in the first phase and approximately 90% in the second phase as mild to moderate. In the open-label period, only nausea and headache had an incidence ≥ 10%.36
Also, during this first open-label phase of the trial, 49 patients (8%) withdrew because of adverse events. The events most commonly leading to withdrawal were nausea (n = 17), vomiting (n = 8), headache (n = 5), and fatigue (n = 4). One patient was withdrawn from the study because of suicidal thoughts, another had suicidal thoughts but remained in the study, and one patient attempted suicide and was subsequently withdrawn.36
In the double-blind period, the overall withdrawal rate, excluding relapses, was 16% for the placebo group (n = 30) and 23% for the vortioxetine group (n = 48) (P = .048, χ2 test). Among those who withdrew, the proportion who did so because of adverse events was 8% in the vortioxetine group and 3% in the placebo group.36
The side effects reported in this phase with an incidence ≥ 10% were headache (12% in the vortioxetine group, 13% in the placebo group) and nasopharyngitis (11% in the vortioxetine group, 14% in the placebo group).36
Adverse events related to sexual dysfunction were reported infrequently: 16 patients (3%) during the open-label period and, during the double-blind period, 2 patients in the placebo group and 3 in the vortioxetine group.36
Throughout both periods of the study, no clinically relevant mean changes in clinical safety laboratory values, vital signs, weight, or ECG values were observed.36
Summary of Findings
This study demonstrated that the short-term effects found in previous clinical trials persisted during the full length of an episode of MDD. The risk of relapse for patients randomized to placebo was twice as high as that of patients randomized to vortioxetine, and the relapse rate at 24 weeks in patients receiving placebo was also double that of those taking vortioxetine (26% vs 13%, P < −0.001). Vortioxetine was also well tolerated.36
THE PRIMARY CARE PERSPECTIVE
Affordable Care Act Expands Mental Health Benefits
The Affordable Care Act (ACA) will have an impact on primary care practices in multiple ways, including an uptick in the number of patients with depressive symptoms. More than 32 million Americans are expected to gain access to insurance coverage that includes mental health and/or substance abuse benefits under the ACA, and an additional 30 million who already have such coverage will acquire additional benefits under the Mental Health Parity and Addiction Equity Act.45
Among these millions of newly insured patients, many will be from low income or low socioeconomic status groups, which are at much higher risk of having psychiatric concerns than the typical patient base in many primary care practices. Compared to high status, low socioeconomic status is associated with a 3-fold increased prevalence of MDD (15% vs 5%).46 This trend could affect practices for years to come, since intervals of poverty increase the risk of subsequent major depression well into the future.47 Furthermore, low socioeconomic status is more strongly associated with maintenance of depression than its onset.48
Clinicians also may find that the care of these newly insured patients with mental health concerns is complex due to a legacy of prior conditions and chronic comorbidities that have been inadequately addressed. Depression in combination with one or more chronic diseases worsens health,49 and depressed patients are more than 3 times as likely to be nonadherent.50 Greater depressive symptom severity has been associated with nonadherence not only to medication regimens but to other aspects of treatment, such as dietary, exercise, and rehabilitative recommendations.51 Findings such as these underscore the urgency of diagnosing and treating depressive symptoms as a means of enhancing not only patients’ mental well-being but their physical health.
Value and Sustainability of Behavioral Health Care
The patient-centered medical home (PCMH) is one model primary care practices have adopted to improve health care through strategies such as coordinated care across all elements of the health care system and collaborative care between primary and specialty care providers.52 Reform efforts such as the PCMH model provide access to increased resources, which are coupled with the expectation of improving patient care. Multiple arrangements to align reimbursement with the increased value potentially derived from such models are being tested, including pay for performance contracts, quality contracts, and risk sharing arrangements.53
In addition to coordinating care across health care venues, another strategy being adopted in PCMHs is to integrate care and care management across chronic conditions, replacing older single-disease management approaches. Results from trials of integrated care of patients with multiple chronic diseases, including depression, indicate that this approach is effective.
Antidepressant Selection in Primary Care
Robinson et al54 in a 2005 study estimated that primary care providers are the sole contacts for more than half of patients with mental illness and that depressive symptoms are present in nearly 70% of patients visiting primary care providers. A patient in a primary care setting, particularly one who has recently acquired mental health coverage through the ACA or Mental Health Parity and Addiction Equity Act, may have one or more coexisting acute or chronic conditions, and to the extent possible, an antidepressant that will not aggravate and might be helpful for these conditions is preferable. A favorable tolerability profile also may improve patient adherence to treatment, leading in turn to not only a reduction in depressive symptoms but a reduced risk of behavioral or social actions that could adversely affect the patient’s overall health and well-being.
Drug names: aripiprazole (Abilify), vortioxetine (Brintellix).
REFERENCES
1. Sanchez C, Asin KE, Artigas F. Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data [published online ahead of print July 9, 2014]. Pharmacol Ther. PubMed doi:10.1016/j.pharmthera.2014.07.001
2. Citrome L. Vortioxetine for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant—what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2014;68(1):60-82. PubMed doi:10.1111/ijcp.12350
3. Blier P, Ward NM. Is there a role for 5-HT1A agonists in the treatment of depression? Biol Psychiatry. 2003;53(3):193-203. PubMed doi:10.1016/S0006-3223(02)01643-8
4. Millan MJ. Multi-target strategies for the improved treatment of depressive states: conceptual foundations and neuronal substrates, drug discovery and therapeutic application. Pharmacol Ther. 2006;110(2):135-370. PubMed doi:10.1016/j.pharmthera.2005.11.006
5. Lam DD, Garfield AS, Marston OJ, et al. Brain serotonin system in the coordination of food intake and body weight. Pharmacol Biochem Behav. 2010;97(1):84-91. PubMed doi:10.1016/j.pbb.2010.09.003
6. Halford JC, Harrold JA. 5-HT(2C) receptor agonists and the control of appetite. Handbook Exp Pharmacol. 2012;209(209):349-356. PubMed doi:10.1007/978-3-642-24716-3_16
7. Vanover KE, Davis RE. Role of 5-HT2A receptor antagonists in the treatment of insomnia. Nat Sci Sleep. 2010;2:139-150. PubMed doi:10.2147/NSS.S6849
8. Bonaventure P, Kelly L, Aluisio L, et al. Selective blockade of 5-hydroxytryptamine (5-HT)7 receptors enhances 5-HT transmission, antidepressant-like behavior, and rapid eye movement sleep suppression induced by citalopram in rodents. J Pharmacol Exp Ther. 2007;321(2):690-698. PubMed doi:10.1124/jpet.107.119404
9. Millan MJ. Dual- and triple-acting agents for treating core and co-morbid symptoms of major depression: novel concepts, new drugs. Neurotherapeutics. 2009;6(1):53-77. PubMed doi:10.1016/j.nurt.2008.10.039
10. Mnie-Filali O, Faure C, Lambás-Se×±as L, et al. Pharmacological blockade of 5-HT7 receptors as a putative fast acting antidepressant strategy. Neuropsychopharmacology. 2011;36(6):1275-1288. PubMed doi:10.1038/npp.2011.13
11. Bang-Andersen B, Ruhland T, J׸rgensen M, et al. Discovery of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): a novel multimodal compound for the treatment of major depressive disorder. J Med Chem. 2011;54(9):3206-3221. PubMed doi:10.1021/jm101459g
12. M׸rk A, Montezinho LP, Miller S, et al. Vortioxetine (Lu AA21004), a novel multimodal antidepressant, enhances memory in rats. Pharmacol Biochem Behav. 2013;105:41-50. PubMed doi:10.1016/j.pbb.2013.01.019
13. Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol. 2012;27(4):215-223. PubMed doi:10.1097/YIC.0b013e3283542457
14. McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol. 2014;17(10):1557-1567. PubMed doi:10.1017/S1461145714000546
15. Adell A. Lu-AA21004, a multimodal serotonergic agent, for the potential treatment of depression and anxiety. IDrugs. 2010;13(12):900-910. PubMed
16. Bidzan L, Mahableshwarkar AR, Jacobsen P, et al. Vortioxetine (Lu AA21004) in generalized anxiety disorder: results of an 8-week, multinational, randomized, double-blind, placebo-controlled clinical trial. Eur Neuropsychopharmacol. 2012;22(12):847-857. PubMed doi:10.1016/j.euroneuro.2012.07.012
17. Baldwin DS, Loft H, Florea I. Lu AA21004, a multimodal psychotropic agent, in the prevention of relapse in adult patients with generalized anxiety disorder. Int Clin Psychopharmacol. 2012;27(4):197-207. PubMed doi:10.1097/YIC.0b013e3283530ad7
18. Mahableshwarkar AR, Jacobsen PL, Chen Y, et al. A randomised, double-blind, placebo-controlled, duloxetine-referenced study of the efficacy and tolerability of vortioxetine in the acute treatment of adults with generalised anxiety disorder. Int J Clin Pract. 2014;68(1):49-59. PubMed doi:10.1111/ijcp.12328
19. BRINTELLIX (vortioxetine) tablets, for oral use [package insert]. Deerfield, IL; Takeda Pharmaceuticals America, Inc. 2013.
20. Center for Drug Evaluation and Research. Summary Review. Application number: 204447Orig1s000. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204447Orig1s000SumR.pdf.
21. Alvarez E, Perez V, Dragheim M, et al. A double-blind, randomized, placebo-controlled, active reference study of Lu AA21004 in patients with major depressive disorder. Int J Neuropsychopharmacol. 2012;15(5):589-600. PubMed doi:10.1017/S1461145711001027
22. Boulenger J-P, Loft H, Olsen CK. Efficacy and safety of vortioxetine (Lu AA21004), 15 and 20 mg/day: a randomized, double-blind, placebo-controlled, duloxetine-referenced study in the acute treatment of adult patients with major depressive disorder. Int Clin Psychopharmacol. 2014;29(3):138-149. PubMed doi:10.1097/YIC.0000000000000018
23. US Department of Health and Human Services, Agency for Healthcare Research and Quality, Effective Health Care Program. Glossary of terms. http://effectivehealthcare.ahrq.gov/index.cfm/glossary-of-terms/. Accessed July 14, 2014.
24. Kirsch I, Deacon BJ, Huedo-Medina TB, et al. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5(2):e45. PubMed doi:10.1371/journal.pmed.0050045
25. Moncrieff J. Are antidepressants as effective as claimed? no, they are not effective at all. Can J Psychiatry. 2007;52(2):96-97, discussion 102. PubMed
26. Fountoulakis KN, Veroniki AA, Siamouli M, et al. No role for initial severity on the efficacy of antidepressants: results of a multi-meta-analysis. Ann Gen Psychiatry. 2013;12(1):26. PubMed doi:10.1186/1744-859X-12-26
27. Fournier JC, DeRubeis RJ, Hollon SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303(1):47-53. PubMed doi:10.1001/jama.2009.1943
28. Dunlop BW, Thase ME, Wun CC, et al. A meta-analysis of factors impacting detection of antidepressant efficacy in clinical trials: the importance of academic sites. Neuropsychopharmacology. 2012;37(13):2830-2836. PubMed doi:10.1038/npp.2012.153
29. Brody B, Leon AC, Kocsis JH. Antidepressant clinical trials and subject recruitment: just who are symptomatic volunteers? Am J Psychiatry. 2011;168(12):1245-1247. PubMed doi:10.1176/appi.ajp.2011.11060864
30. Califf RM, Filerman GL, Murray RK, et al. The clinical trials enterprise in the United States: a call for disruptive innovation. National Academy of Sciences, Institute of Medicine Discussion Paper. http://www.iom.edu/~/media/Files/Perspectives-Files/2012/Discussion-Papers/Drug%20Forum-Call%20for%20CTE.pdf. Updated April 13, 2012. Accessed July 22, 2014.
31. Thase ME, Larsen KG, Kennedy SH. Assessing the ‘ true’ effect of active antidepressant therapy v placebo in major depressive disorder: use of a mixture model. Br J Psychiatry. 2011;199(6):501-507. PubMed doi:10.1192/bjp.bp.111.093336
32. Papakostas GI. Limitations of contemporary antidepressants: tolerability. J Clin Psychiatry. 2007;68(suppl 10):11-17. PubMed
33. Lin EH, Von Korff M, Katon W, et al. The role of the primary care physician in patients’ adherence to antidepressant therapy. Med Care. 1995;33(1):67-74. PubMed doi:10.1097/00005650-199501000-00006
34. Hu XH, Bull SA, Hunkeler EM, et al. Incidence and duration of side effects and those rated as bothersome with selective serotonin reuptake inhibitor treatment for depression: patient report versus physician estimate. J Clin Psychiatry. 2004;65(7):959-965. PubMed doi:10.4088/JCP.v65n0712
35. Katona CL, Katona CP. New generation multi-modal antidepressants: focus on vortioxetine for major depressive disorder. Neuropsychiatr Dis Treat. 2014;10:349-354. PubMed doi:10.2147/NDT.S39544
36. Boulenger J-P, Loft H, Florea I. A randomized clinical study of Lu AA21004 in the prevention of relapse in patients with major depressive disorder. J Psychopharmacol. 2012;26(11):1408-1416. PubMed doi:10.1177/0269881112441866
37. Baldwin DS. Sexual dysfunction associated with antidepressant drugs. Expert Opin Drug Saf. 2004;3(5):457-470. PubMed doi:10.1517/14740338.3.5.457
38. Clayton AH, Pradko JF, Croft HA, et al. Prevalence of sexual dysfunction among newer antidepressants. J Clin Psychiatry. 2002;63(4):357-366. PubMed doi:10.4088/JCP.v63n0414
39. Henigsberg N, Mahableshwarkar AR, Jacobsen P, et al. A randomized, double-blind, placebo-controlled 8-week trial of the efficacy and tolerability of multiple doses of Lu AA21004 in adults with major depressive disorder. J Clin Psychiatry. 2012;73(7):953-959. PubMed doi:10.4088/JCP.11m07470
40. Baldwin DS, Loft H, Dragheim M. A randomised, double-blind, placebo controlled, duloxetine-referenced, fixed-dose study of three dosages of Lu AA21004 in acute treatment of major depressive disorder (MDD). Eur Neuropsychopharmacol. 2012;22(7):482-491. PubMed doi:10.1016/j.euroneuro.2011.11.008
41. Mahableshwarkar AR, Jacobsen PL, Chen Y. A randomized, double-blind trial of 2.5 mg and 5 mg vortioxetine (Lu AA21004) versus placebo for 8 weeks in adults with major depressive disorder. Curr Med Res Opin. 2013;29(3):217-226. PubMed doi:10.1185/03007995.2012.761600
42. Jain R, Mahableshwarkar AR, Jacobsen PL, et al. A randomized, double-blind, placebo-controlled 6-wk trial of the efficacy and tolerability of 5 mg vortioxetine in adults with major depressive disorder. Int J Neuropsychopharmacol. 2013;16(2):313-321. PubMed doi:10.1017/S1461145712000727
43. Montejo-González AL, Llorca G, Izquierdo JA, et al. SSRI-induced sexual dysfunction: fluoxetine, paroxetine, sertraline, and fluvoxamine in a prospective, multicenter, and descriptive clinical study of 344 patients. J Sex Marital Ther. 1997;23(3):176-194. PubMed doi:10.1080/00926239708403923
44. McGahuey CA, Gelenberg AJ, Laukes CA, et al. The Arizona Sexual Experience Scale (ASEX): reliability and validity. J Sex Marital Ther. 2000;26(1):25-40. PubMed doi:10.1080/009262300278623
45. Beronio K, Po R, Skopec L, et al. Affordable Care Act Will Expand Mental Health and Substance Use Disorder Benefits and Parity Protections for 62 Million Americans. Washington, DC: ASPE Research Brief, Office of the Assistant Secretary for Planning and Evaluation, US Dept of Health and Human Services; 2013.
46. Sturm R, Gresenz CR. Relations of income inequality and family income to chronic medical conditions and mental health disorders: national survey. BMJ. 2002;324(7328):20-23. PubMed doi:10.1136/bmj.324.7328.20
47. Lynch JW, Kaplan GA, Shema SJ. Cumulative impact of sustained economic hardship on physical, cognitive, psychological, and social functioning. N Engl J Med. 1997;337(26):1889-1895. PubMed doi:10.1056/NEJM199712253372606
48. Lorant V, Deliרge D, Eaton W, et al. Socioeconomic inequalities in depression: a meta-analysis. Am J Epidemiol. 2003;157(2):98-112. PubMed doi:10.1093/aje/kwf182
49. Moussavi S, Chatterji S, Verdes E, et al. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370(9590):851-858. PubMed doi:10.1016/S0140-6736(07)61415-9
50. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107. PubMed doi:10.1001/archinte.160.14.2101
51. Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160(21):3278-3285. PubMed doi:10.1001/archinte.160.21.3278
52. Agency for Healthcare Research and Quality. Patient-centered medical home resource center: defining the PCMH. http://www.pcmh.ahrq.gov/page/defining-pcmh. Accessed July 17, 2014.
53. Wexler R, Hefner J, Welker MJ, et al. Health care reform: possibilities & opportunities for primary care. J Fam Pract. 2014;63(6):298-304. PubMed
54. Robinson WD, Geske JA, Prest LA, et al. Depression treatment in primary care. J Am Board Fam Pract. 2005;18(2):79-86. PubMed doi:10.3122/jabfm.18.2.79