ABSTRACT
Background: Positive and Negative Syndrome Scale (PANSS) data from a pivotal phase 3 study in participants with schizophrenia of RBP-7000, a recently marketed long-acting subcutaneous injectable risperidone formulation, were examined to determine if dose-response relationships existed for different items of the PANSS.
Methods: Changes in the 30 PANSS items were analyzed individually and using the 5 factor-analysis–derived dimensions defined by Marder and colleagues. Subgroups of patients who could benefit from the RBP-7000 120 mg dose were investigated.
Results: 337 participants were randomized and received study medication (RBP-7000 90 mg n = 111, RBP-7000 120 mg n = 114, placebo n = 112). Dose-dependent responses were observed in items from the study-specified PANSS positive and general psychopathology exploratory subscales. Dose-dependent responses were observed across all 5 Marder dimensions, with the largest effect sizes observed with the 120 mg dose in the uncontrolled hostility/excitement (UHE) and anxiety/depression dimensions. Participants with baseline UHE dimension scores ≥ 9 demonstrated greater improvement in PANSS total score at the 120 mg dose compared to the 90 mg dose.
Conclusions: RBP-7000 demonstrated efficacy across both the primary and exploratory PANSS study endpoints and the post hoc Marder dimensions. Schizophrenia patients with higher baseline Marder UHE scores may benefit from initiation of treatment at the 120 mg dose.
Trial Registration: ClinicalTrials.gov identifier: NCT02109562
J Clin Psychiatry 2021;82(5):21m13906
To cite: Le Moigne A, Csernansky J, Leadbetter RA, et al. PANSS individual item and Marder dimension analyses from a pivotal trial of RBP-7000 (monthly extended-release risperidone) in schizophrenia patients. J Clin Psychiatry. 2021;82(5):21m13906.
To share: https://doi.org/10.4088/JCP.21m13906
© Copyright 2021 Physicians Postgraduate Press, Inc.
aGlobal Research & Development, Indivior Inc., North Chesterfield, Virginia
bDepartment of Psychiatry and Behavioral Sciences, Feinberg School of Medicine, Northwestern University, Chicago, Illinois
cCollaborative Neuroscience Network LLC, Garden Grove, California
dWashington University School of Medicine, St Louis, Missouri, and Thriving Mind South Florida, Miami, Florida
eSemel Institute for Neuroscience and Human Behavior, University of California at Los Angeles, Los Angeles, California
*Corresponding author: Anne Le Moigne, MD, Clinical/Data Sciences and Operations, Indivior Inc., 10710 Midlothian Turnpike, Ste 125, North Chesterfield, VA 23235 ([email protected]).
A novel once-monthly extended-release subcutaneous injectable formulation of risperidone (RBP-7000) that provides rapid and sustained achievement of clinically relevant exposure in a single dose without supplemental dosing was approved by regulatory authorities for the treatment of schizophrenia in adults. A post hoc analysis of the data was undertaken to determine whether further analyses of the collected Positive and Negative Syndrome Scale (PANSS) data, including the Marder dimensions,5 might indicate a dose-response relationship to guide clinical dosage choices.
The efficacy of this subcutaneous injectable monthly risperidone (SMR) was established in a double-blind, placebo-controlled, 8-week phase 3 study in which patients hospitalized with moderate to severe exacerbations of schizophrenia were randomized to receive SMR 90 mg or 120 mg or matching placebo. After 8 weeks of treatment (2 injections of SMR or placebo), statistically significant improvements were observed versus placebo on the PANSS total score (the primary endpoint), as well as the exploratory endpoints PANSS positive scale score and PANSS general psychopathology scale score.1,2
The PANSS total score is the sum of a clinician-administered 30-item assessment that evaluates each symptom-based item on a 7-point scale (1 = absent; 7 = extreme). Positive symptoms and negative symptoms subscales are the sum of 7 items each, and general psychopathology subscale is the sum of 16 items.3
Marder and colleagues4 used factor analysis to propose a reorganization of PANSS items into 5 dimensions. An analysis based on this reorganization found substantial improvement for oral risperidone 6–16 mg daily versus placebo in total score effect size (ES) of 0.29 and these 5 dimensions, positive symptoms (ES: 0.26), disorganized thoughts (ES: 0.26), uncontrolled hostility/excitement (UHE; ES: 0.30), anxiety/depression (ES: 0.18), and negative symptoms (ES: 0.15).4 The Marder dimensions have been previously used in analyses of various antipsychotic agents.5–19
To further characterize the responses at SMR 90 mg and 120 mg, we conducted an analysis of PANSS item-level data from the SMR pivotal study and subsequently investigated the 5-dimension restructuring proposed by Marder.
METHODS
The current analysis uses data collected during the SMR phase 3 inpatient study (ClinicalTrials.gov identifier: NCT02109562) that enrolled adult participants 18–55 years old with acute exacerbation of DSM-IV-TR–defined schizophrenia, excluding those with documented failure of adequate treatment with 2 or more antipsychotics or treatment-resistant disease and those taking ≥ 6 mg oral risperidone.1,2 Participants were hospitalized, washed out of previous oral medication for up to 8 days, and required to have a PANSS total score of 80–120 and a score of ≥ 4 on ≥ 2 of 4 positive symptoms subscale items at screening: hallucinatory behavior, delusions, conceptual disorganization, or suspiciousness/persecution. Enrolled participants were randomized 1:1:1 to receive SMR 90 mg, SMR 120 mg, or matching placebo on days 1 and 29.1
The PANSS was administered at screening, at the baseline visit, prior to study drug administration, and on days 15, 29, 43, and 57. The primary efficacy endpoint was change from baseline to day 57 in PANSS total score in the intent-to-treat (ITT) population, which included all randomized participants who received ≥ 1 dose of study medication and had ≥ 1 postbaseline PANSS total score assessment. Exploratory outcome measures included PANSS subscale scores (ie, positive symptoms, negative symptoms, general psychopathology).1
Statistical Analyses
For the current analyses, change from baseline through day 57 was evaluated using the same analysis methodology as for the original analyses, a mixed-effects model for repeated measures (MMRM).2,20 The MMRM model included fixed effects for each scheduled efficacy visit as a categorical variable, baseline score (last measurement on or before the randomization date), treatment, and treatment-by-visit interaction, assuming an unstructured covariance matrix. Estimates for least-squares (LS) means, their associated 95% confidence intervals (CIs), and P values were calculated.
These analyses comprised the 30 individual PANSS items and the 5 PANSS item dimensions described by Marder. The analyses were expanded to include the change from baseline to each interim assessment following the first injection (ie, days 15, 29, and 43).
Consistent with US Food and Drug Administration recommendations and information presented in the current US package insert for SMR, PANSS scores from unscheduled early termination visits were excluded and early termination scores were not carried forward to day 57.20
The ES (standardized difference) for each Marder dimension for SMR 90 mg or 120 mg compared with placebo was calculated using adjusted mean changes from baseline (difference in the LS mean for active vs placebo treatment) divided by the pooled standard deviation (SD).
Additionally, exploratory analyses were undertaken to determine whether a set of individual baseline PANSS items could identify subgroups of participants who may benefit from the higher SMR 120 mg dose. Based on eligibility criteria, and the largest between-dose differences for the Marder dimensions, the following 3 sets of PANSS items were chosen for defining the subgroups: the 4 entry criteria items, the 3 avoidance and withdrawal items (emotional withdrawal, passive/apathetic social withdrawal, and active social avoidance), and the 4 Marder UHE items (excitement, hostility, uncooperativeness, and poor impulse control). For each set of items, 2 subgroups were defined using a cut-point of baseline scores that produced approximately equally sized subgroups. Differences in change from baseline to day 57 in PANSS total score between 120 mg and 90 mg were assessed for these subgroups using MMRM as previously described.
Because of the exploratory nature of these analyses and for consistency with the original Marder analysis of oral risperidone used as reference, P values are assessed for significance using a 0.05 2-sided α level.
Safety/Tolerability
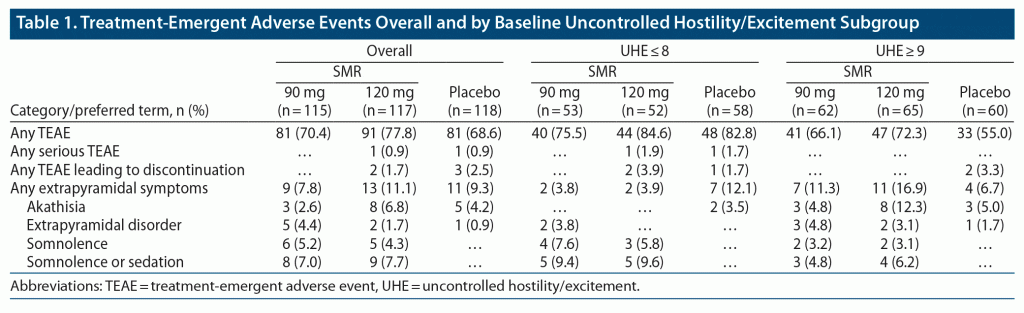
Standard safety and tolerability assessments were based on the safety population, which included participants who received ≥ 1 dose of study medication. Published results are not repeated here.1,21 The percentage of participants who experienced at least 1 treatment-emergent adverse event (TEAE), treatment-emergent serious AEs, TEAE leading to discontinuation, somnolence, and extrapyramidal symptoms (EPS) were summarized overall and for the subgroups selected by the efficacy analyses. To provide context to the EPS TEAEs, changes from baseline to day 57 were summarized for the objective measures of Abnormal Involuntary Movement Scale (AIMS), Simpson-Angus Scale (SAS), and Barnes Akathisia Rating Scale (BARS), overall and for the same subgroups.
RESULTS
Study Population
A total of 354 participants were randomized to treatment, including 350 participants who received ≥ 1 dose of study medication (SMR 90 mg n = 115; SMR 120 mg n = 117; placebo n = 118) and constituted the safety population. A total of 337 participants constituted the ITT population (SMR 90 mg n = 111; SMR 120 mg n = 114; placebo n = 112). A total of 259 randomized participants (73.2%) completed the study. The most common reasons for discontinuation were withdrawal of consent (18.6%), withdrawal by investigator (3.4%), insufficient clinical response (1.7%), and adverse event (1.4%).
Treatment groups were generally well matched as to demographics and disease status. Participants were primarily black/African American (72.1%) and male (76.6%), with an overall mean age of 41.2 years. Overall, participants had been diagnosed with schizophrenia for an average of 17 years (SD = 9.7) before entering the study. Mean (SD) PANSS total scores at baseline were 95.5 (9.2), 94.9 (8.1), and 94.1 (8.9) for participants receiving SMR 90 mg, 120 mg, and placebo, respectively.
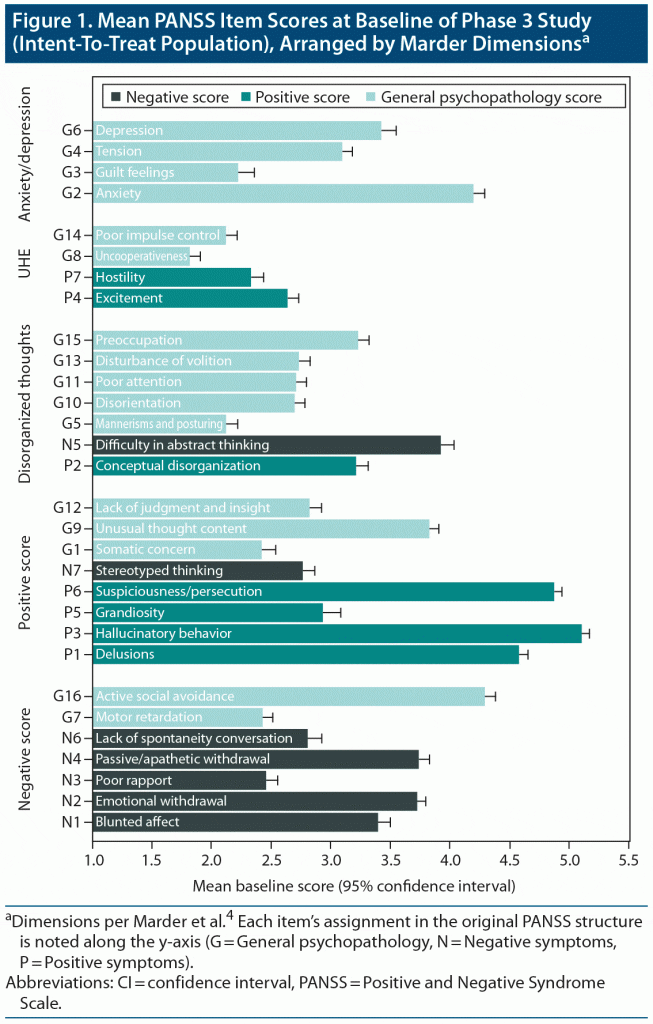
PANSS Item Scores at Baseline
Mean (SD) baseline PANSS scores for the ITT population are displayed by item in Figure 1, grouped according to the Marder dimensions.
Individual Item Changes From Baseline to Day 57
The largest significant improvements for both SMR doses compared with placebo were observed in the 4 positive symptoms (hallucinatory behavior, conceptual disorganization, suspiciousness/persecution, delusions), each required by the inclusion criteria, and in 6 general psychopathology items (active social avoidance, depression, uncooperativeness, poor attention, tension, anxiety). Additionally, in the 120 mg group only, significant improvements were observed in the following items: hostility, excitement, emotional withdrawal, passive/apathetic social withdrawal, poor impulse control, and somatic concern. ESs for the items that statistically differed from placebo ranged from 0.27 to 0.63.
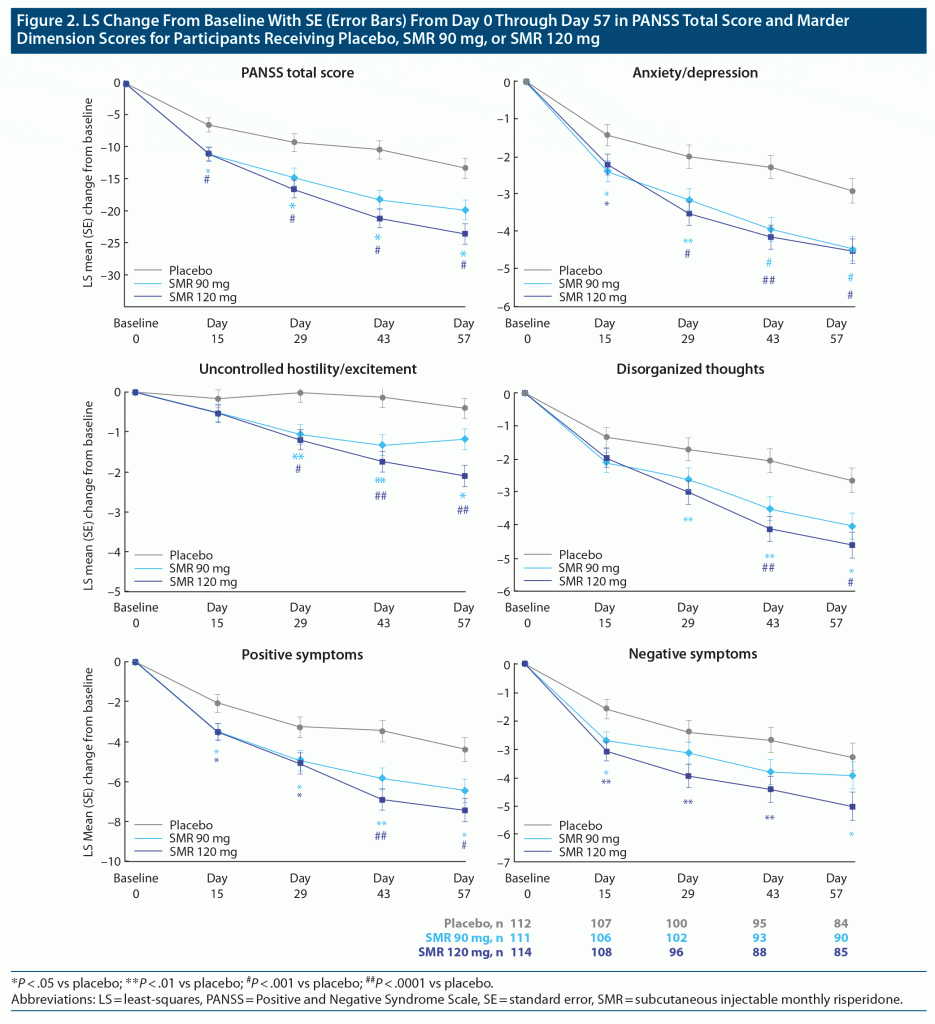
PANSS Marder Dimension Scores Over Time
The time courses for mean changes (95% CIs) in the Marder dimensions from baseline to day 57 and also, as a reference, in PANSS total score are presented in Figure 2. Both SMR doses produced improvements versus placebo across the Marder dimensions that, in general, increased over time. Significant improvements were observed by day 15 for both SMR doses in the anxiety/depression, positive symptoms, and negative symptoms dimensions. Except for the anxiety/depression dimension, SMR 120 mg generally produced more pronounced improvements than SMR 90 mg. In the SMR 90 mg group, UHE dimension scores plateaued after day 29, while continuous improvement of this dimension score was noted in the 120 mg group.
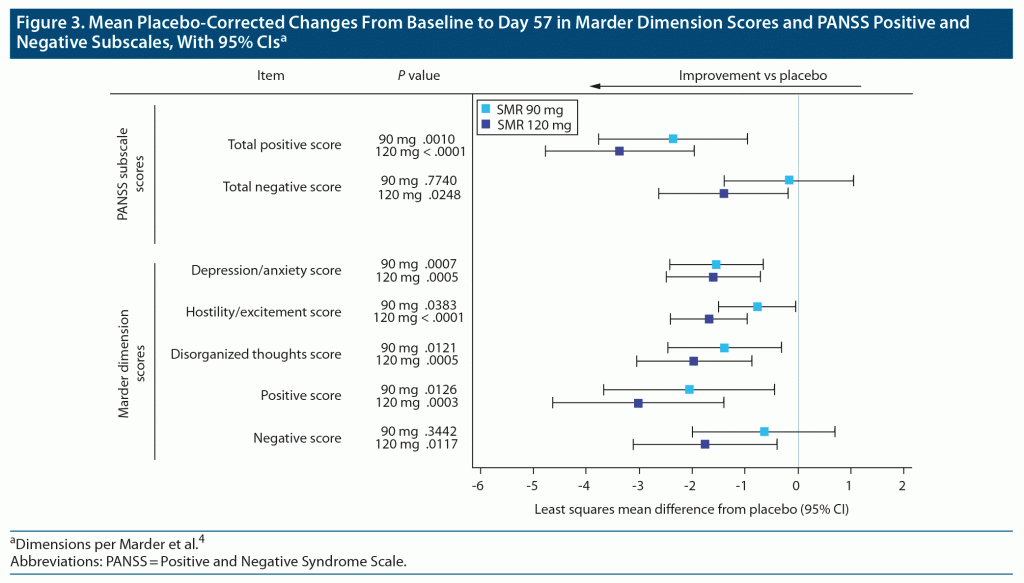
The placebo-corrected item-level mean changes from baseline to day 57 and 95% CIs were compiled for each of the 5 Marder dimensions and, as a reference, the PANSS positive and negative subscales (Figure 3). At day 57, significant improvements versus placebo were observed for both SMR groups in the Marder UHE, anxiety/depression, disorganized thoughts, and positive symptoms dimensions. Significant improvement versus placebo in the Marder negative symptoms dimension was observed only with SMR 120 mg.
ESs from the original Marder PANSS factor analysis of oral risperidone 6–16 mg doses are used for reference and generally were small in magnitude, ranging from 0.15 to 0.30.4 The ESs for PANSS total score for SMR 90 mg and 120 mg were 0.40 and 0.63, respectively, suggesting a greater overall effect with the higher dose. Moreover, an examination of ESs for both SMR doses demonstrates that the long-acting injectable formulation delivers efficacy across multiple symptom domains (ESs ranging from 0.12 to 0.63, small to medium in magnitude) as was shown for the 6–16 mg daily doses of oral risperidone used in the Marder analysis.
In this study, the largest between-dose differences in ES suggested a greater effect for SMR 120 mg compared to 90 mg with respect to the negative symptom and UHE dimensions; dose-related increases in ES were more modest for PANSS total score and the other Marder dimensions. Because negative symptom improvement may be confounded by improvement in positive symptoms during acute antipsychotic treatment, we chose not to pursue further analyses in the negative symptoms. However, we examined the effects of baseline UHE dimension score on treatment response with SMR 90 mg and 120 mg.
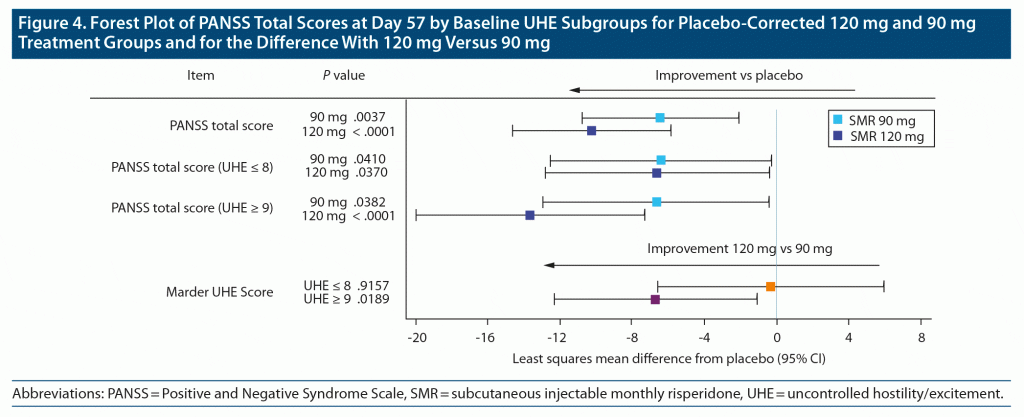
To compare the impact of the SMR doses, differences in PANSS total scores were assessed using the subgroups identified (the 4 PANSS entry criteria items, the PANSS avoidance and withdrawal items, and the Marder UHE items) and cut-points that produced similar sizes for each of the SMR doses. The Marder UHE subgroups demonstrated the largest differences between doses, and therefore other cut-points were explored, but the original cut-point of 9 yielded the most substantial between-group dose-dependent differences in response.
The difference in changes from baseline in PANSS total score between each dose group and placebo as well as the difference between SMR 120 mg and 90 mg are summarized by baseline UHE dimension scores in Figure 4. Improvement in PANSS total score (mean placebo-corrected) for the 120 mg dose in the high baseline UHE dimension score group was nearly twice that in the low group (LS mean −13.67 vs −6.63, respectively), whereas similar improvements were observed for both baseline UHE groups with the 90 mg dose. Confirming this observation, there was no additional improvement at the 120 mg dose over the 90 mg dose in the low baseline UHE group (LS means [95% CI]: −0.34 [−6.62 to 5.95]); however, there was a statistically significant benefit of 120 mg versus 90 mg in the high baseline UHE group (LS means [95% CI]: −6.75 [−12.36 to −1.14]).
Safety and Tolerability
The previously published safety observations are not repeated here1,21; however, these data were evaluated in the baseline UHE subgroups identified in the efficacy analysis (Table 1). The proportion of participants with any TEAE was slightly higher in the low versus the high baseline UHE score group. The incidence of somnolence or sedation was also slightly higher in the low UHE score group. Incidence of somnolence was similar for both SMR doses in the high UHE score groups. The incidence of a TEAE of akathisia was greater in the high versus the low UHE score group. Within the high UHE score group, a TEAE of akathisia was greater with SMR 120 mg versus 90 mg. It is important to note that no clinically meaningful changes from baseline occurred in the BARS total score between groups. Taken together, these findings indicate that different UHE baseline score groups may reflect different levels of “activation.” Thus, participants with a low baseline UHE may be associated with increased risk of somnolence, and a high UHE baseline may be associated with akathisia. There was no evidence of change from baseline, regardless of treatment group or baseline UHE score, on other measures of EPS, including AIMS total score and SAS total score.
DISCUSSION
The equivalence of 3 mg oral risperidone/90 mg and 4 mg oral risperidone/120 mg is based on the results from a Multiple Ascending Dose Study.22 In the phase 3 study of SMR 90 mg and 120 mg, improvements in PANSS item-level responses across multiple symptom domains were observed in comparison to placebo. These improvements were noted despite higher placebo responses reported in the literature since the Marder analysis of oral risperidone was published.4
An extension study including rollover participants from this study and new stable outpatients further demonstrated both safety and long-term effectiveness of SMR.21
Original analyses of the PANSS subscales suggested a dose-dependent response. With respect to individual PANSS items, SMR 90 mg and 120 mg demonstrated significant improvements versus placebo (P < .05), respectively, in 4/7 and 6/7 positive items; 0/7 and 2/7 negative items; and 6/16 and 8/16 general psychopathology items.2,20
Significant improvements for both SMR doses across all dimensions except for negative symptoms with SMR 90 mg were also demonstrated in the analysis of the Marder dimensions. Confirming our original observations,1,23 SMR 120 mg was consistently associated with a more substantial decrease in negative symptoms than SMR 90 mg, suggesting that a higher risperidone exposure is needed to ameliorate these symptoms. Analysis of the Marder factor-analysis–derived dimensions also strongly suggested a dose-dependent effect for SMR. Adjusted mean changes from baseline to day 57 were greater with the 120 mg dose than the 90 mg dose for all Marder dimensions, and responses for the UHE and the negative symptoms dimensions were more than 2-fold greater for the higher dose than for the lower dose of SMR.
PANSS total score was evaluated in baseline UHE dimension subgroups to identify participants who may benefit from treatment with the higher dose, given the overall larger ES and difference between doses in this dimension. Participants with higher baseline UHE dimension scores (≥ 9) had more than twice the reduction in PANSS total score with 120 mg versus 90 mg. The similar between-dose incidence of somnolence as a TEAE suggests that the occurrence of this TEAE does not contribute to the observed pattern of reductions in PANSS total score. Therefore, it appears that patients with higher baseline UHE scores may benefit preferentially across symptoms by initiating SMR at the higher dose.
While it was not a main objective of this research, comparison of the current results with those from Marder suggests a more robust placebo response in the current study, as well as somewhat higher ES for both SMR doses compared with the oral risperidone doses (6–16 mg). However, the range of doses evaluated in the Marder study complicates making rigorous comparisons with the 2 SMR doses utilized in this study, which correspond to approximately 3 and 4 mg of oral risperidone. Moreover, the original Marder analysis4 was based on the North American oral risperidone studies conducted in the early 1990s. At that time, most of the participants enrolled had been previously exposed only to first-generation antipsychotic drugs. Direct comparison of the ES between Marder and our study is further limited by differences in modeling techniques employed (analysis of covariance versus MMRM) and different methods for managing missing data (last observation carried forward versus missing at random, utilizing all observed data). Both studies were post hoc analyses and did not adjust for multiple comparisons. An additional limitation is that the pivotal study used here enrolled participants who may have had lower UHE scores at baseline in order to conform with current guidelines for informed consent, as compared with studies done 30 years earlier. In contrast, our inclusion criteria may have skewed to participants who have predominantly positive symptoms and therefore may have led to an underestimation of the effect on the other 4 Marder dimensions.
Notwithstanding these caveats, the pattern of treatment response described by Marder and colleagues4 across their redefined PANSS dimensions is similar to the findings in this study of SMR. Based on observed ES, responses in this study were similar or greater in magnitude across multiple dimensions. The most substantial differences between the original analysis by Marder and the current study were greater responses in the anxiety/depression dimension with both SMR doses and a substantially increased response in the UHE dimension with the 120 mg dose. Broadly, these findings are also consistent with other post hoc analyses examining treatment response based on the Marder dimensions (eg, Citrome et al 2018,7 Kane et al 2007,13 Marder et al 200716).
CONCLUSIONS
This analysis of item-level results from an SMR pivotal trial confirmed the efficacy of this formulation to reduce the severity of PANSS item symptoms, using both the original PANSS subscales and the dimensions defined during a factor analysis conducted by Marder and colleagues. The improvements in the original PANSS negative symptoms subscale at the SMR 120 mg dose may have been at least partially attributable to improvements in positive symptoms. These data suggest that patients with higher baseline scores (≥ 9) on the Marder UHE dimension, as well as those with high negative symptoms scores, might benefit from initiating treatment with the 120 mg instead of the 90 mg dose.
Submitted: January 30, 2021; accepted July 7, 2021.
Published online: September 21, 2021.
Author contributions: Study design: all authors (for current analysis); study investigator: Dr Walling; enrolled patients: Dr Walling; collection and assembly of data: Mss Heath and Le Moigne; data analysis: Mss Heath and Le Moigne; data interpretation: all authors; manuscript review and revisions: all authors.
Potential conflicts of interest: Mss Le Moigne and Heath are employees of Indivior Inc. Dr Csernansky has served as a Data Safety Monitoring Board member for Eli Lilly and Sanofi-Aventis. Drs Andorn, Leadbetter, and Graham were employees of Indivior Inc. at the time of the study. Dr Walling has received grants from Alkermes, Janssen, Otsuka, Forum, Lundbeck, Sunovion, Acadia, Allergan, IntraCellular, Noven, Merck, AbbVie, and Roche. Dr Newcomer has received grant support from the National Institutes of Health, Substance Abuse and Mental Health Services Administration, and has served as a consultant to Indivior, Sunovion, Intra-Cellular Therapies, Otsuka, and Alkermes; has been involved in patent litigation on behalf of Sunovion; and serves on a Data Safety Monitoring Board for Amgen. Dr Marder has been a paid consultant for Biogen, Lundbeck, Roche, Newron, Avanir, Allergan, and Sunovion.
Funding/support: This manuscript was funded by Indivior Inc, North Chesterfield, Virginia.
Role of the sponsor: This research and manuscript were funded by Indivior Inc, North Chesterfield, Virginia.
Acknowledgments: Medical writing assistance and editorial support was provided to the authors by Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, and funded by Indivior, Inc.
Data sharing statement: The authors will not make data collected for the study available to others, including individual participant data and a data dictionary defining each field in the set.
Clinical Points
- A once monthly subcutaneous risperidone injection is efficacious for many of the symptoms of schizophrenia taken in whole (PANSS total score) or in part, via traditional subscales (PANSS negative, PANSS positive, and the Marder subscale).
- In addition to our previous observations that the 120 mg dose was more effective than placebo on negative symptoms, these new post hoc analyses suggest that patients with high uncontrolled hostility/excitement scores on the Marder scale may benefit from a starting dose of 120 mg subcutaneous monthly risperidone rather than the 90 mg dose.
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Psychosis section. Please contact Ann K. Shinn, MD, MPH, at [email protected].
References (23)

- Nasser AF, Henderson DC, Fava M, et al. Efficacy, safety, and tolerability of RBP-7000 once-monthly risperidone for the treatment of acute schizophrenia: an 8-week, randomized, double-blind, placebo-controlled, multicenter phase 3 study. J Clin Psychopharmacol. 2016;36(2):130–140. PubMed CrossRef
- Le Moigne A, Fava M, Csernansky J, et al. Reanalysis of a phase 3 trial of a monthly extended-release risperidone injection for the treatment of acute schizophrenia. J Clin Psychopharmacol. 2021;41(1):76–77. PubMed CrossRef
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. PubMed CrossRef
- Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry. 1997;58(12):538–546. PubMed CrossRef
- Bitter I, Lieberman JA, Gaudoux F, et al. Randomized, double-blind, placebo-controlled study of F17464, a preferential D3 antagonist, in the treatment of acute exacerbation of schizophrenia. Neuropsychopharmacology. 2019;44(11):1917–1924. PubMed CrossRef
- Brannan SK, Sawchak S, Miller AC, et al. Muscarinic cholinergic receptor agonist and peripheral antagonist for schizophrenia. N Engl J Med. 2021;384(8):717–726. PubMed CrossRef
- Citrome L, Risinger R, Cutler AJ, et al. Effect of aripiprazole lauroxil in patients with acute schizophrenia as assessed by the Positive and Negative Syndrome Scale-supportive analyses from a Phase 3 study. CNS Spectr. 2018;23(4):284–290. PubMed CrossRef
- Davis JM, Chen N. The effects of olanzapine on the 5 dimensions of schizophrenia derived by factor analysis: combined results of the North American and international trials. J Clin Psychiatry. 2001;62(10):757–771. PubMed CrossRef
- Deutsch SI, Schwartz BL, Schooler NR, et al. Targeting alpha-7 nicotinic neurotransmission in schizophrenia: a novel agonist strategy. Schizophr Res. 2013;148(1-3):138–144. PubMed CrossRef
- Fleischhacker W, Galderisi S, Laszlovszky I, et al. The efficacy of cariprazine in negative symptoms of schizophrenia: post hoc analyses of PANSS individual items and PANSS-derived factors. Eur Psychiatry. 2019;58:1–9. PubMed CrossRef
- Gastpar M, Masiak M, Latif MA, et al. Sustained improvement of clinical outcome with risperidone long-acting injectable in psychotic patients previously treated with olanzapine. J Psychopharmacol. 2005;19(5 suppl):32–38. PubMed CrossRef
- Ismail Z, Peters-Strickland T, Miguelez M, et al. Aripiprazole once-monthly in the treatment of acute psychotic episodes in schizophrenia: post hoc analysis of positive and negative syndrome scale Marder factor scores. J Clin Psychopharmacol. 2017;37(3):347–350. PubMed CrossRef
- Kane J, Canas F, Kramer M, et al. Treatment of schizophrenia with paliperidone extended-release tablets: a 6-week placebo-controlled trial. Schizophr Res. 2007;90(1-3):147–161. PubMed CrossRef
- Li H, Rui Q, Ning X, et al. A comparative study of paliperidone palmitate and risperidone long-acting injectable therapy in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(4):1002–1008. PubMed CrossRef
- Lloyd K, Latif MA, Simpson S, et al. Switching stable patients with schizophrenia from depot and oral antipsychotics to long-acting injectable risperidone: efficacy, quality of life and functional outcome. Hum Psychopharmacol. 2010;25(3):243–252. PubMed CrossRef
- Marder SR, Kramer M, Ford L, et al. Efficacy and safety of paliperidone extended-release tablets: results of a 6-week, randomized, placebo-controlled study. Biol Psychiatry. 2007;62(12):1363–1370. PubMed CrossRef
- Nasrallah HA, Gopal S, Gassmann-Mayer C, et al. A controlled, evidence-based trial of paliperidone palmitate, a long-acting injectable antipsychotic, in schizophrenia. Neuropsychopharmacology. 2010;35(10):2072–2082. PubMed CrossRef
- Potkin SG, Phiri P, Szegedi A, et al. Long-term effects of asenapine or olanzapine in patients with persistent negative symptoms of schizophrenia: a pooled analysis. Schizophr Res. 2013;150(2–3):442–449. PubMed CrossRef
- Schmidt ME, Kent JM, Daly E, et al. A double-blind, randomized, placebo-controlled study with JNJ-37822681, a novel, highly selective, fast dissociating D2 receptor antagonist in the treatment of acute exacerbation of schizophrenia. Eur Neuropsychopharmacol. 2012;22(10):721–733. PubMed CrossRef
- Andorn AC, Graham J, Fava M, et al. Efficacy of monthly extended-release risperidone injections (RBP-7000) for the treatment of schizophrenia: an analysis of individual PANSS items [poster]. Presented at: Annual Congress of the Schizophrenia International Research Society; April 10–14, 2019; Orlando, Florida.
- Andorn A, Graham J, Csernansky J, et al. Monthly extended-release risperidone (RBP-7000) in the treatment of schizophrenia: results from the phase 3 program. J Clin Psychopharmacol. 2019;39(5):428–433. PubMed CrossRef
- Laffont CM, Gomeni R, Zheng B, et al. Population pharmacokinetics and prediction of dopamine D2 receptor occupancy after multiple doses of RBP-7000, a new sustained-release formulation of risperidone, in schizophrenia patients on stable oral risperidone treatment. Clin Pharmacokinet. 2014;53(6):533–543. PubMed CrossRef
- Perseris [package insert]. North Chesterfield, VA: Indivior Inc; 2019.
This PDF is free for all visitors!
Save
Cite