This CME activity is expired. For more CME activities, visit cme.psychiatrist.com.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation.
This Academic Highlights section of The Journal of Clinical Psychiatry presents the highlights of the series “A Multidisciplinary Approach for Addressing Challenges in Alzheimer’s Disease,” which consisted of teleconferences and meetings held between September 2018 and April 2019. In conjunction with the activity Chair and faculty, this report was prepared and independently developed by the CME Institute of Physicians Postgraduate Press, Inc., and was supported by educational grants from ACADIA Pharmaceuticals Inc.; Allergan; Avanir Pharmaceuticals, Inc.; and Biogen MA, Inc.
The program was chaired by Alireza Atri, MD, PhD, from Banner Sun Health Research Institute, Sun City, Arizona; and Brigham and Women’s Hospital, and Harvard Medical School, Boston, Massachusetts. The faculty were Danielle Goldfarb, MD, from Banner Alzheimer’s Institute, Banner Sun Health Research Institute, and University of Arizona College of Medicine, Phoenix, Arizona; Simon Sheard, DO, from Banner Health Center, Maricopa, Arizona; and Lynn Shaughnessy, PsyD, from Beth Israel-Deaconess Hospital and Harvard Medical School, Boston, Massachusetts.
CME Objective
After studying this article, you should be able to:
- Provide pharmacologic and nonpharmacologic treatment options to diminish cognitive, behavioral, and psychological symptoms of Alzheimer’s disease in conjunction with other health care providers
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Release, Expiration, and Review Dates
This educational activity was published in December 2019 and is eligible for AMA PRA Category 1 Credit™ through December 31, 2021. The latest review of this material was November 2019.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Marlene P. Freeman, MD, Editor in Chief, has received research funding from JayMac and Sage; has been a member of the advisory boards for Otsuka, Alkermes, and Sunovion; has been a member of the Independent Data Safety and Monitoring Committee for Janssen; and, as a Massachusetts General Hospital (MGH) employee, works with the MGH National Pregnancy Registry, which is sponsored by Teva, Alkermes, Otsuka, Actavis, and Sunovion, and works with the MGH Clinical Trials Network and Institute, which receives research funding from multiple pharmaceutical companies and the National Institute of Mental Health. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears on the next page.
J Clin Psychiatry 2019;80(6):MS18002AH3C
To cite: Atri A, Goldfarb D, Sheard S, et al. Current and emerging solutions to challenges in the management of Alzheimer’s disease. J Clin Psychiatry. 2019; 80(6):MS18002AH3C
To share: https://doi.org/10.4088/JCP.MS18002AH3C
© Copyright 2019 Physicians Postgraduate Press, Inc.
Alzheimer’s disease (AD) is a clinically heterogeneous, progressive, and ultimately fatal condition in which neurodegenerative processes cause a vicious cycle of inflammatory, microvascular, and neurochemical dysfunction and damage that leads to progressive cognitive, behavioral, and functional changes. These changes manifest insidiously over years through a wide range of symptoms that can include forgetfulness; word-finding or speaking difficulties; poor decision-making, judgment, planning, organization, and task completion; trouble with calculations or navigation; sleep problems; apathy, withdrawal, irritability, anxiety, and low mood; and changes in personality, and comportment.
Clinicians must first appropriately detect impairments, evaluate the nature and cause(s) and contributing factors, and disclose the diagnosis in a timely and compassionate fashion that involves both the person with the illness and a care partner (often a spouse or family member). Then, they will use a combination of nonpharmacologic, behavioral, and pharmacologic treatments to ease the burden of this disorder on patients and care partners. In this Academic Highlights, Drs Atri, Goldfarb, Sheard, and Shaughnessy discuss best practices for addressing the symptoms and impairments experienced by patients with AD, as well as the importance of working with and assessing the needs of the care partner.
BUILDING A THERAPEUTIC ALLIANCE AND DEVELOPING A CARE PLAN
According to Dr Atri, after an appropriate diagnosis and disclosure process, effective management of AD should include 3 essential patient-centered and personalized elements: (1) nonpharmacologic and behavioral psychoeducation and interventions; (2) pharmacologic management; and (3) establishment and maintenance of a triadic therapeutic alliance with the patient-care partner dyad for care planning, implementation, and monitoring.1 The extent to which a clinician is successful in achieving the third element will largely determine the success of the other two elements.
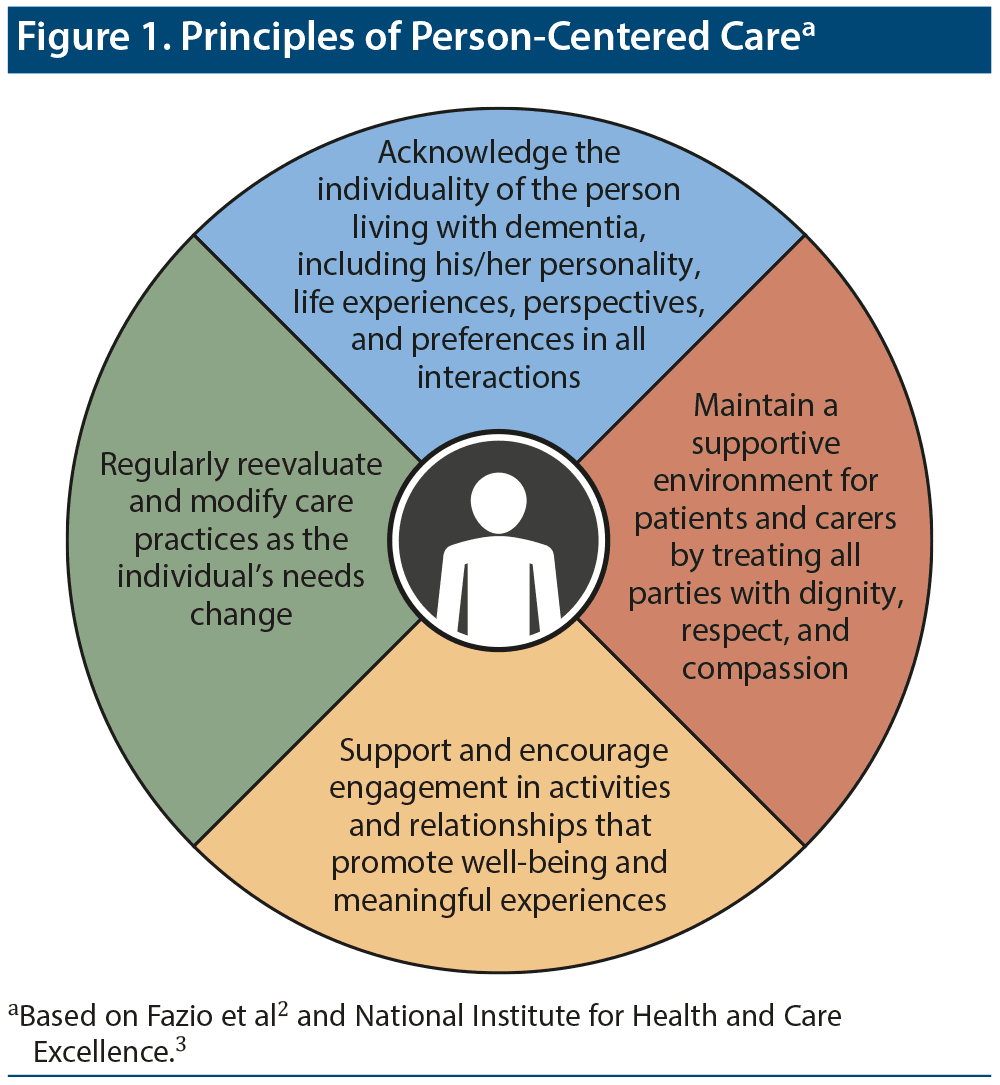
Current guidelines2,3 for the management of patients with AD and related dementias (ADRD) emphasize that care must be person-centered. Person-centered care is based on several principles that revolve around the idea that people living with dementia are individuals with unique experiences and personalities, and their perspectives, goals, and lives have value (Figure 1).
As part of practicing person-centered care, Dr Atri communicates to all of his patients and their care partners that he is their “advisor”; they are “the boss”; and that he will always be their advocate and honestly share his assessment and recommendations with them truthfully and compassionately. Dr Sheard added that clinicians must be clear about uncertainties regarding a patient’s prognosis because dementia manifests, progresses, and responds to treatment differently in every person. There should also be a focus on making the best of the present, rather than becoming trapped by fear regarding what the future may hold. Dr Goldfarb stated that clinicians must be mindful of the new reality confronting the patient and care partner because their identities and sense of self are changing, as is their relationship with each other.4 Dr Shaughnessy emphasized that it is important to consider cultural, social, educational, and resilience factors; environmental supports; and vulnerabilities when characterizing the person with dementia’s profile with regard to cognitive and functional strengths and weaknesses. She stated the critical importance of these considerations both during the assessment and when providing personalized recommendations that leverage strengths and shore up support systems to pragmatically implement interventions and care plans that benefit both patients and care partners.
An individual living with AD described his concerns:
Patient Perspectives
“I’m scared of the unknown. I don’ t know if I have 6 months to communicate or 6 years. I worry about going to bed at night and whether I’ m going to be as bad tomorrow. This disease is with you 24/7. It’s in my brain and I can’ t get away from it—it is a scary thing.”5
Clinicians should begin and interweave conversations about long-term life and care planning during the diagnostic disclosure process.6 Drs Atri, Goldfarb, Sheard, and Shaughnessy underscored the importance of addressing key topics, such as the patient’s preferences for care, advance directives, potential safety issues, and financial planning, as soon as possible to maximize the potential that the patient is able to participate in decision-making and to minimize the potential for harm to the patient, care partner(s), and others. As Dr Goldfarb explained, the goals of life and care planning are to keep the patient safe and autonomous as long as possible, to help and care for her/his care partner(s), and to minimize the risk of crisis and potentially avoidable poor life and health outcomes (eg, falls, accidents, financial ruin).
Dr Shaughnessy identified critical topics to address, including medication management, driving, and finances, because these are areas in which difficulties are likely to arise as the patient’s disease progresses. Therefore, it is important to initiate and have continuing conversations, often over several visits and with the aid of assessment, psychoeducation, and counseling by colleagues and community resources (eg, neuropsychology, social work, occupational therapy, Alzheimer’s Association care consultation) to agree on and implement concrete plans for ongoing assessment, monitoring, and appropriate support at home and in the community.
Dr Atri stated that having plans in place as early as possible, which are periodically evaluated, can identify when the patient is no longer able to safely complete these tasks and other activities of daily living independently, increase patient autonomy in decision-making, identify when the patient no longer has decision-making capacity (triggering predetermined proxy decision-making), minimize catastrophic outcomes, and avoid crisis situations. It can also facilitate triggering action regarding the need for additional support at home or transition in living and care environment, such as moving into continuing residential care, assisted living, or a memory care/dementia unit.
PSYCHOEDUCATION AND NONPHARMACOLOGIC INTERVENTIONS
Psychoeducation and nonpharmacologic or behavioral interventions are the first foundational element of care employed in the management of AD; they involve both the patient and care partner and should continue throughout the disease course.2 Patients and care partners should receive education about AD in general, possible symptoms, prognosis, treatment options, safety issues, and support and care resources.7 This information should be conveyed in a way that is understandable to patients and care partners and relevant to the patient’s unique circumstances.3
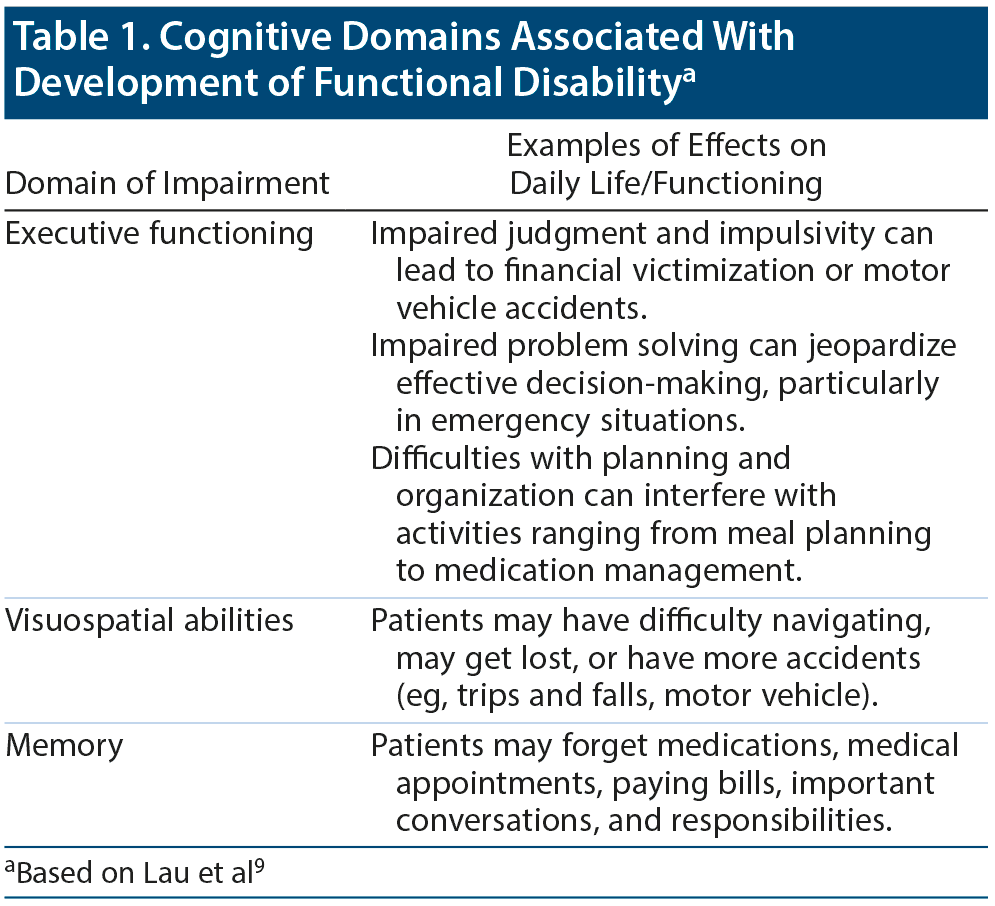
Dr Goldfarb pointed out that the results of neuropsychological testing done for diagnostic purposes can also reveal important areas of deficit that can be addressed with adaptive or compensatory strategies.8 Dr Shaughnessy explained that, even in individuals with very mild changes or cognitive impairment, lower performance in particular cognitive domains is highly predictive of progressive decline and later functional disability.9 Changes in these domains are important for planning considerations and as targets for psychoeducation, compensatory strategies, and nonpharmacologic interventions. Older adults with normal cognition or with mild cognitive impairment at baseline who reported more severe baseline cognitively based functional limitations on cognitive assessments were 4 times more likely to later develop functional disability in instrumental activities of daily living.9 The cognitively based functional domains associated with the greatest risk of later functional disability were everyday planning (associated with the cognitive domain of executive function), everyday memory, and everyday visuospatial abilities (Table 1).
Lifestyle Interventions
Clinicians should take a holistic approach when treating patients with AD.2 Lifestyle interventions can be used to promote the individual’s physical, emotional, and cognitive functioning. Health-related issues such as hypertension, obesity, diabetes, depression, hearing loss, smoking, and physical inactivity are all risk factors for dementia,10 and interventions that promote a brain-healthy lifestyle may help support the patient and mitigate clinical decline.10,11 Following a brain-healthy diet and engaging in frequent physical and cognitive activity have been found to be associated with a reduced incidence of AD.12 Dr Sheard recommended that patients with AD follow the same type of diet as patients with cardiovascular conditions and engage in regular physical and social activities. Physical activity does not have to be strenuous—especially if the patient has been fairly inactive—but it does have to be routine. Activities need to be targeted to the patient’s needs, preferences, abilities, and level of functioning.
An individual with AD shared how exercise has helped in maintaining a positive attitude toward his diagnosis:
Patient Perspectives
“When I was diagnosed, I took a positive approach and said, ‘ Okay, this is who I am; it’s part of my life.’ I decided to maximize what I’ ve got rather than be depressed about it. I also chose to be physically active because early in life I learned that physical activity raises my spirits. I got back on my bike, and I even ride in the winter—it’s exhilarating! I can honestly say that giving up my driver’s license enriched my life.”13
Drs Atri and Goldfarb discussed the importance of sleep for patients with AD. Sleep problems, including short sleep duration, sleep fragmentation, altered circadian rhythms, and sleep disordered breathing and sleep apnea, occur in up to 45% of patients with AD.14 The pathogenesis of AD involves accumulation and aggregation of toxic β-amyloid42 protein (Aβ42) species, and the sleep-wake cycle is involved in regulating Aβ42 clearance.15 Therefore, disruptions to the sleep-wake cycle may lead to increasing AD-related toxicity and pathology.
Dr Atri pointed out that, in persons with AD, poor sleep and reversal of wake-sleep cycle often contribute to irritability, anxiety, low mood, agitation, or even aggression and are a large source of stress and burden for caregivers. He stated that clinicians must be proactive to assess for sleep problems and other potential triggers and contributors to behavioral issues, such as anxiety, fear, loneliness, depression, dehydration, hunger, constipation, chronic pain (eg, arthritis), metabolic or electrolyte derangements, and infections/medical illness (eg, urinary tract infection). Promoting a consistent and supporting routine; “just right” activities, exercise, and social engagement; hydration; a healthy diet; and good sleep hygiene is important for overall health and could have a therapeutic impact in reducing stress and anxiety, mitigating AD symptoms, and preventing crisis.1
Behavioral/Environmental Interventions
Integrating nonpharmacologic strategies for managing behavioral symptoms of patients with AD is key (Table 2).16 Dr Sheard emphasized the importance of tailoring these types of interventions to each patient.6 For instance, if a patient has gait imbalance and is at risk for falls, environmental modifications such as removing rugs and adding a bar in the shower, and also making a referral for evaluation of balance and gait, are helpful. He suggested that referral to physical therapy and occupational therapy home safety evaluations or a visit from a home health nurse can assist care partners in finding and addressing these issues.
Dr Atri recommended that care partners try to remain calm, positive, and reassuring in interactions and try to simplify the environment and make it calm, predictable, and comforting. To avoid or diffuse agitation or aggression in individuals with AD, he recommended that care partners use a soothing tone of voice or gentle touch; avoid confrontation, correction, or convincing; and not say “no” and allow the moment to pass unless there is a dire need to address an immediate issue of safety. Because individuals with AD often have difficulty with processing speed, working memory, and multistep commands, care partners should talk slowly and deliberately and should simplify and condense instructions.
Dr Atri emphasized that as dementia progresses, care partners will need to learn “a new way and language” to communicate. This involves being mindful and selective about what, how, and when to provide information, to sometimes compassionately withhold information that may upset the person with dementia, and to use distraction or redirection to avoid topics that the patient may find disturbing. For example, when a person with dementia forgets that her/his spouse, sibling, or other loved one has died and repeatedly asks about them, it may be better not to continue to remind them that the loved one died; the person with dementia would continue to forget, yet each time may experience intense grief, and the feelings of sadness and anxiety often remain with them for hours or days, long after they forget the information. He emphasized that care partners need ongoing psychoeducation and emotional and psychological support to be able to implement and maintain these strategies, since they are under chronic stress and what they do is challenging mentally, emotionally, and physically.
A care partner shared her thoughts on withholding distressing information from her mother:
Care Partner Perspectives
“There are times when honesty is painful for everyone when a loved one has dementia. When my mom wondered when Dad was coming home from the hospital, I initially walked her through his death and how we were all surrounding him. I still puddle at the memory of these conversations and it’s been more than 5 years since I had them with Mom. She relived the pain as did I. Why didn’ t I just say that he would be home in a few days? I had a fixed belief that honesty was the best policy . . . but there were many times when it didn’ t serve my mom.”17
Care Partner-Directed Interventions
Dr Sheard stated that he considers the care partners of his patients with AD to be his second patients, and he screens them for issues such as fatigue, stressors, anxiety, depression, and need for respite. Dr Goldfarb added that, ideally, care partners should use the time they get from respite care to do something they find enjoyable, rather than always running errands. Dr Goldfarb also stated that care partners feel validated and comforted when health care providers simply acknowledge their importance and show concern for their needs.
Current AD practice guidelines2,3,18 provide recommendations for assessing and supporting the needs of care partners throughout the stages of AD. Interventions for care partners can include psychoeducation and skills training designed to instruct them in the best methods for dealing with dementia-related difficulties and practical aspects for providing care.3,18 Care partners may find this challenging if they have been in a long-term relationship with the patient because, as Drs Atri and Goldfarb explained, they have established ways of interacting with each other.6 When the patient says something that is incorrect or does something disagreeable, the care partner’s impulse may be to correct or argue with the patient. Dr Goldfarb pointed out that care partners may need to be reminded that they are not going to win an argument with a person with AD. Dr Sheard agreed and stated that when conflicts arise, rather than arguing with or contradicting the person with AD, care partners should try to diffuse the situation by distracting or redirecting the individual. Dr Atri provided some strategies that care partners can use in situations in which their loved one with AD may become agitated or aggressive, such as redirecting them to engage in an activity that is calming and enjoyable, which could be anything from folding towels to retelling a favorite story. Dr Atri reiterated that he often tells his care partners that, unless safety is at risk, they should avoid reactively saying “No” to the patient. Instead, they should calmly redirect, remove the trigger for potential confrontation or agitation, and use forgetfulness as an ally “to allow the moment to pass.” Dr Atri recommended the DICE (Describe, Investigate, Create, Evaluate) approach to assessing and managing the behavioral and psychological symptoms of AD and dementia.19
Case Practice Question
Discussion of the best response can be found at the end of the activity.
Case 1. Carlos is a 78-year-old man with moderate to severe AD dementia. His wife was his primary care partner until her death a year ago. Carlos moved in with his daughter, Maria, and her husband, who have 3 children—one married, one away at college, and one in high school. Maria put Carlos in her oldest child’s room, which is still full of her belongings. At first, Carlos enjoyed walking the dog daily with his grandson, playing his violin, and working jigsaw puzzles with Maria. However, when the school year ended, Maria’s 2 sons were both at home, often watching television or playing video games, and Carlos’ condition deteriorated. He has stopped activities he previously enjoyed, is no longer going on his daily walk, now frequently asks for his wife, and often becomes agitated when he can’ t find his keys because he thinks he needs to leave for work, although he is retired and has stopped driving. He is increasingly hostile in his interactions with Maria and his grandsons. Which of the following strategies is the least likely to aid Carlos, Maria, and her family?
a. To avoid conflict, encourage Maria to restrict Carlos more to his room so as not to get overstimulated by his grandsons; provide a rocking chair to replace walks outside; and provide his own listening music to drown out the noise from the television and video games.
b. Instruct the family to remain calm in their interactions, help Carlos by not reminding him that his wife died or that he retired, and redirect his attention when he is agitated.
c. Advise Maria to declutter Carlos’ room to provide a calming environment, and suggest her sons wear headphones for video games and use subtitles on the television so that the volume can be lowered.
d. Suggest that Maria or her son resume daily walks with Carlos, and also consider sending him to a day-care facility for people with AD to help him participate in suitable activities and to provide a break for Maria and her family.
PHARMACOLOGIC TREATMENT
The goals of pharmacologic treatment of AD are to manage symptoms and slow decline.13 However, Drs Sheard and Atri emphasized that before clinicians consider adding pharmacotherapy, they should review the patient’s current treatment regimen to determine if any medications can be eliminated. Dr Atri said that patients diagnosed with dementia often exhibit “medication drift,” which is a phenomenon in which, over time, patients are prescribed more and more medications to treat various symptoms or side effects. Many of the medications act in opposition, are relatively contraindicated, and create additional symptoms that lead to additional medication prescriptions. Dr Goldfarb added that polypharmacy is frequently an issue with psychiatric medications and pointed out that any changes in a patient’s medication regimen should involve the primary care doctor and any specialists.
According to Dr Atri, the American Geriatrics Society’s Beers Criteria for Potentially Inappropriate Medication Use in Older Adults20 is a useful resource for determining which medications should be removed or avoided in patients with AD (Table 3).
Stepwise Approach to the Pharmacologic Treatment of Cognitive Symptoms of AD
Currently, no treatment can modify the Alzheimer’s disease process, but a number of pharmacotherapies have a beneficial effect on cognitive and neuropsychiatric symptoms of dementia and help to mitigate clinical decline in cognition, daily function, and behavior.21 Medications approved by the US Food and Drug Administration include the cholinesterase inhibitors (ChEIs) donepezil, rivastigmine, and galantamine and the N-methyl-d-aspartate (NMDA) antagonist memantine. The selection of pharmacotherapy should be dictated by the stage, severity, and particular circumstances of the patient. After appropriate evaluation and counseling, in patients for whom adequate supervision and monitoring are available, treatment with AD medications is recommended: starting a ChEI in mild stages, adjusting dose (gradually up to intermediate range),7 and then implementing add-on combination therapy (memantine added to background ChEI) as dementia stages progress.18,22
Dr Atri stated that he uses donepezil as first-line treatment for AD. Donepezil is a generic oral medication approved for treating all stages of AD. There is a central cholinergic deficit from the loss of cholinergic brain neurons in individuals with AD. Like the other ChEIs, donepezil derives its therapeutic effect from inhibiting acetylcholinesterase, increasing the availability of acetylcholine in the synaptic cleft, and thus facilitating cholinergic signal transduction.23,24
The benefits of treatment by all 3 ChEIs, as well as memantine, in AD have been reviewed in systematic reviews and meta-analyses that include 30-142 studies and tens of thousands of participants.24-27 Randomized placebo-controlled trials lasting 24 to 52 weeks have demonstrated small to medium effect size treatment benefits of ChEIs at the group level for improving, stabilizing, or delaying decline in cognition, activities of daily living, and global status and for ameliorating behavioral and psychiatric symptoms and caregiver burden.1,7 For example, a Cochrane Database review24 of 30 studies found that donepezil treatment was associated with benefits in cognitive function, activities of daily living, and clinician-rated global clinical state in patients in all stages of AD.
Similar reviews of rivastigmine28 and galantamine29 have also been conducted. All show modest but significant treatment benefits.26,27
Dr Atri uses memantine as second-line add-on combination treatment for AD as the clinical stage progresses. Memantine, which is approved for the treatment of moderate-to-severe AD, either alone or in combination with donepezil, is a low-to-moderate-affinity antagonist of NMDA glutamate receptors.7,30 Early pathological changes in AD affect brain regions with high concentrations of NMDA glutamate receptors such as the hippocampus, a critical area for binding of new information, learning, and encoding of episodic memory. Memantine is a low-to-moderate affinity NMDA glutamate receptor channel blocker with rapid kinetics. It is postulated to exert its effects in the AD brain by improving neurochemical signal transduction signal-to-noise characteristics and mitigating potential calcium-induced glutamate/NMDA-receptor-mediated neurotoxicity, working in a complementary fashion with ChEIs.30-32
In randomized controlled trials, systematic reviews, and meta-analyses of trials in persons in the moderate or severe stages of AD dementia, memantine monotherapy and memantine add-on combination therapy to background ChEI have been found to produce significant benefits on cognition, behavioral disturbances, activities of daily living, and global functioning and be well-tolerated.25-27,33
Current international guidelines support use of ChEIs and memantine in the pharmacologic treatment of AD dementia18,22,34 and recommend using a combination of ChEIs and memantine in patients with moderate-to-severe AD.22
Short-term responses to AD medications vary between individuals. Aggregate data support that during the initial 6-12 months of treatment, cognition, activities of daily living, behavioral symptoms, or global clinical impression of change may improve in a minority (10%-20%), plateau in nearly half (30%-50%), and continue to deteriorate in about a third (20%- 40%) of treated patients.1,7 Discontinuation of ChEI treatment is, on aggregate, harmful.1,7 On average, patients taken off, or those inconsistently taking, AD medications progress more rapidly than those who continue treatments, particularly ChEIs. Discontinuation trials of ChEIs to see if there is worsening should be avoided, as even temporary discontinuation is associated with irreversible decline and greater risk of nursing home placement.35-38
Sustained treatments provide a modest expectation of short-term stabilization or improvement and longer-term slowing of clinical decline. As the disease progresses over several months to years, patients who may initially show improvement or stability will eventually decline. Dr Atri stressed that clinicians should communicate about practical issues associated with pharmacologic treatment, including rationale, need for monitoring, and expectations.
Dr Atri noted that AD medications can be continued in late stages of dementia to support basic psychomotor processes and behavioral responses that assist caregivers to deliver basic care by maintaining praxis, functional communication, and the elementary processes of movement and eating. The benefits of maintaining treatment may also extend to reducing emergence or increasing severity of behavioral issues and need for antipsychotic use.
He said that in the long run, current pharmacologic treatments for AD mitigate decline but do not prevent it, so as the disease progresses to very severe or end-stage dementia, clinicians must, on a case-by-case basis and in a patient-centered collaboration with family and care partners, weigh and discuss the calculus regarding discontinuation of AD medications and turning the focus to palliation, hospice, and comfort care.1,7,39
Case Practice Question
Discussion of the best responses can be found at the end of the activity.
Case 2. Glenda is a 62-year-old woman who just moved to the area to live with her daughter, and this is her first visit at your primary care clinic. Glenda has been treated for depression on and off since her early forties. About 1.5 years ago, shortly after the death of her husband, she began to experience insomnia, irritability, and forgetfulness, which she ascribed to depression. She was not taking an antidepressant at the time; therefore, her previous primary care doctor started her on 10 mg of escitalopram each morning. Her mood symptoms improved, but her sleep issues and forgetfulness worsened. She was diagnosed with early-stage AD and started on a ChEI taken in the evening. Her cognitive status stabilized, but her sleep difficulties worsened, and 4 months ago she started using over-the-counter (OTC) diphenhydramine for her insomnia. Her daughter reports that she has been much more confused in the last few months. Considering Glenda’s diagnosis of AD, which of the following is the best next step?
a.Discontinue escitalopram and her ChEI.
b.Advise her to discontinue OTC sleep aids, move dosing of her ChEI to after breakfast, and move her escitalopram dose to bedtime.
c.Advise her to switch from OTC sleep aids to a prescription sleep aid such as eszopiclone or zolpidem.
d.Refer Glenda to a specialist.
PHARMACOTHERAPIES FOR BEHAVIORAL AND PSYCHOLOGICAL SYMPTOMS
Behavioral and psychiatric symptoms (BPSs) in persons with AD can be very distressing and challenging to treat.16 These symptoms include mood disturbance, anxiety, sleep disturbance, wandering, repetitive questioning, agitation, aggression, and psychosis. Nearly all individuals with AD will experience at least one BPS, with depression, apathy, and anxiety being the most common.19 These symptoms are thought to stem from neurodegeneration in brain systems that regulate mood and behavior. Interpersonal and environmental triggers may also cause or contribute to BPSs. These symptoms increase care partner burden and the likelihood of nursing home placement, reduce quality of life, and accelerate disease progression and functional decline.16 A care partner described his experience with his mother wandering out of the house at night:
Care Partner Perspectives
“Right before the tipping point, she started staying awake really odd hours. She would not sleep for 48-72 hours straight, then sleep all day. When she was awake and I [was] asleep, she would knock on my bedroom door in the middle of the night to make sure I was still here. As hard as that was, it was nothing compared to the call from our local police when they found her a good distance from our home, not aware of where she was or even who she was. Luckily for me, the police were aware of the situation and knew who she was and where she lived. But it made me realize that if she had wandered into the street and a car had hit her, I would have to live with the knowledge that I would have indirectly caused the situation by not taking action. I could not imagine how a person would feel had they accidentally hit my mother, and I never wanted to feel that way. So for me, that was the moment when I realized I could no longer take care of her at home and that I needed to find a different solution, as hard as that ‘ different solution’ was (and believe me, it was HARD).”40
Although nonpharmacologic interventions should be the preferred treatment for behavioral symptoms, practice guidelines acknowledge that some patients may warrant pharmacologic treatment if they are a safety risk to themselves or others or if their psychological symptoms are causing severe distress.2,3 Dr Atri stated that some of the medications used to treat the cognitive symptoms of AD may also have a therapeutic effect on symptoms such as mood, apathy, aggression, and irritability.7 Dr Goldfarb mentioned that antidepressants, namely selective serotonin reuptake inhibitors (SSRIs), can be helpful for mood symptoms. The SSRIs sertraline, citalopram, and escitalopram have been found to have efficacy for reducing agitation and irritability.41
Antipsychotics have been investigated for treating BPSs, but these agents have been found to confer limited benefits while carrying considerable morbidity risks, including increasing mortality.19 Antipsychotics should be considered a last resort and only used for patients with severe behavioral disturbances that have not responded to other treatments or for patients who pose an immediate safety risk to themselves or others.7,19,42 After discussing the risks with the patient’s family and care partners, if the decision is made to start antipsychotic treatment, the lowest possible dose should be used, the patient must be closely monitored, and the medication should be discontinued as soon as possible. Dr Sheard stated that he has had some success using quetiapine in patients with severe hallucinations. Risperidone, which is approved for the treatment of BPSs in Europe but not in the United States, may also be considered.19
Case Practice Question
Discussion of the best response can be found at the end of the activity.
Case 3. Russell, a retired law enforcement officer, and Jeannette, a retired school teacher, have been married for over 50 years. Jeanette has been diagnosed with AD, which is now of moderate severity. Russell has been caring for her at home with help from their grown children and home health nurses. A safety assessment of their home determined that Russell owns several firearms, but because these are in a locked safe, the care team determined that they did not pose a risk. As Jeanette’s AD progressed, she would get up and wander at night and often mistake Russell for an intruder. Recently, Jeannette confronted Russell with one of his guns because she thought he was a burglar. The gun was not loaded, and Russell was able to calm Jeanette and obtain the gun. Russell is unsure if Jeanette found the key to open the safe or if he had accidentally left it unlocked. Would an antipsychotic be an appropriate treatment for Jeanette’s symptoms?
a.Yes
b.No
Clinical Points
- Practice person-centered communication and care when evaluating and managing persons with Alzheimer’s disease.
- Strive to develop and maintain a strong triadic therapeutic relationship that involves both the patient and care partner(s) and to provide ongoing psychoeducation and support regarding the condition, care considerations, and resources.
- Begin discussions about safety and care planning early in the process to maximize the potential for the patient to be involved in current and future decision-making.
- Provide counseling regarding expectations of treatment, which are to mitigate long-term clinical, functional, and behavioral decline as the disease progresses.
- Advise patients and care partners how to take and monitor medications (to ensure proper adherence).
- Implement, monitor, and slowly adjust/titrate stage-dependent cholinesterase inhibitors and/or memantine monotherapy or combination therapy.
- Always provide psychoeducation and implement nonpharmacologic interventions before considering pharmacotherapy for behavioral and psychiatric symptoms (BPSs).
- Use antipsychotics sparingly and only under specific conditions in some individuals with severe refractory BPSs, which should always be done with thoughtful risk-benefit calculus and with extreme caution as a last resort for severe and refractory BPSs or when risk to safety of self or others is involved.
- Support patients, families, and care partners by advocating pragmatic care plans and activities that promote general health, safety, and well-being for the patient and the care partner(s).
Discussion of Case Practice Questions
Case 1: Preferred response is a. To avoid conflict, encourage Maria to restrict Carlos more to his room so as not to get overstimulated by his grandsons; provide a rocking chair to replace walks outside; and provide his own listening music to drown out the noise from the television and video games.
Stopping physical activity, increasing isolation, and allowing noisy environments are not recommended for patients with AD. Carlos’ problems seemed to arise when his routine and environment were disrupted and became too noisy, while his physical activity (daily walks) and engagement diminished. Adjusting his environment, having him resume his daily walks, and providing his family some relief in the form of day care would be advisable.
Case 2: Preferred response is b. Advise her to discontinue OTC sleep aids, move dosing of her ChEI to after breakfast, and move her escitalopram dose to bedtime.
First-generation antihistamine sleep aids and prescription sleep aids are included in the Beers Criteria of medications to avoid in patients with dementia or cognitive impairment—they are anticholinergic, counteracting the cholinergic benefits of ChEI and suppressing cognitive functions. ChEIs can increase alertness and cause vivid dreams and disrupt sleep if given at night. Moving the ChEI dose to the morning, to be given after food (if taken orally) to mitigate potential cholinergic side effects on an empty stomach (eg, nausea, flatulence, loose stools), can be beneficial to mitigate sleep problems. Because Glenda did experience some improvement in mood symptoms following initiation of escitalopram, this treatment should be continued, and because sedation is a common side effect of this agent, moving her dose to bedtime may ease her insomnia.
Case 3: Preferred response is b. No.
Because of the risks of antipsychotic treatment, they should be used only if the patient is an imminent threat to herself or others. Although Jeanette confronted her husband with a gun, she was amenable to Russell’s calming techniques. The best option would be to eliminate the firearms from the household. Furthermore, Jeanette should have a comprehensive evaluation (eg, the DICE approach19) to assess triggers and any physical, medical, and other causes of her psychological symptoms.
Published online: December 3, 2019.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, sertraline, citalopram, escitalopram, quetiapine, and risperidone are not approved by the US Food and Drug Administration for the treatment of Alzheimer’s disease, but their potential use in selective clinical situations for mood, anxiety, irritability, agitation, aggression, and psychosis symptoms in patients with Alzheimer’s disease was discussed.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Academy of Physician Assistants (AAPA) accepts certificates of participation for educational activities certified for AMA PRA Category 1 Credit™ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 1 hour of Category I credit for completing this program.
Financial Disclosure
Dr Atri has no equity, shares, or salary from any pharmaceutical or biotechnology company and is not a member of any pharmaceutical company’s speakers’ bureau. In the last 12 months, Dr Atri has received, or may receive, honoraria for consulting, educational lectures/programs/materials, or medical/scientific advisory/data safety monitoring boards from AbbVie, Alzheimer’s Association, Biogen, Eisai, Grifols, Harvard Medical School Graduate Continuing Education, Novo Nordisk, Roche/Genentech, Suven, and Synexus. Dr Atri’s institution (Banner Health) has received, related to Dr Atri’s activities, observational study/clinical trial study-, infrastructure development- and/or outreach and awareness-related funding from Arizona Alzheimer’s Research Consortium, National Institutes of Health/NIA, Novartis, Alzheimer’s Clinical Trial Consortium (ACTC), Alzheimer’s Therapeutic Research Institute (ATRI), University of Indiana School of Medicine, and Global Alzheimer’s Platform (GAP). Dr Atri has received book royalties from Oxford University Press. Drs Sheard, Goldfarb, and Shaughnessy have no personal affiliations or financial relationships with any commercial interest to disclose relative to the activity.
Review Process
The faculty member(s) agreed to provide a balanced and evidence-based presentation and discussed the topic(s) and CME objective(s) during the planning sessions. The faculty’s submitted content was validated by CME Institute staff, and the activity was evaluated for accuracy, use of evidence, and fair balance by the Chair and a peer reviewer who is without conflict of interest.
The opinions expressed herein are those of the faculty and do not necessarily reflect the opinions of the CME provider and publisher or the commercial supporters.
REFERENCES
1. Atri A. The Alzheimer’s disease clinical spectrum: diagnosis and management. Med Clin North Am. 2019;103(2):263-293. PubMed CrossRef
2. Fazio S, Pace D, Maslow K, et al. Alzheimer’s Association Dementia Care Practice Recommendations. Gerontologist. 2018;58(suppl_1):S1-S9. PubMed CrossRef
3. National Institute for Health and Care Excellence (UK). Dementia: Assessment, Management and Support for People Living with Dementia and Their Carers. London, UK: National Institute for Health and Care Excellence; 2018.
4. Goldfarb D, Sheard S, Shaughnessy L, et al. Disclosure of Alzheimer’s disease and dementia: patient- and care partner-centric decision-making and communication. J Clin Psychiatry. 2019;80(2):MS18002BR1C. PubMed CrossRef
5. Botek A-M. Another World: People With Alzheimer’s Share Their Perspectives. Aging Care website. https://www.agingcare.com/Articles/alzheimers-patients-share-their-experiences-153702.htm. 2019.
6. Atri A, Sheard S, Goldfarb D. A multidisciplinary approach for addressing challenges in Alzheimer’s disease [webcast]. J Clin Psychiatry. 2019;80(5):MS18002WC4C. PubMed CrossRef
7. Atri A. Current and future treatments in Alzheimer’s disease. Semin Neurol. 2019;39(2):227-240. PubMed CrossRef
8. Shaughnessy L, Sheard S, Goldfarb D, et al. Cognitive assessment of Alzheimer’s disease and dementias in clinical practice: pragmatics of brief instruments and neuropsychological evaluation. J Clin Psychiatry. 2019;80(4):MS18002BR2C. PubMed CrossRef
9. Lau KM, Parikh M, Harvey DJ, et al. Early cognitively based functional limitations predict loss of independence in instrumental activities of daily living in older adults. J Int Neuropsychol Soc. 2015;21(9):688-698. PubMed CrossRef
10.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673-2734. PubMed CrossRef
11.Small GW. Detection and prevention of cognitive decline. Am J Geriatr Psychiatry. 2016;24(12):1142-1150. PubMed CrossRef
12.Rolandi E, Frisoni GB, Cavedo E. Efficacy of lifestyle interventions on clinical and neuroimaging outcomes in elderly. Ageing Res Rev. 2016;25:1-12. PubMed CrossRef
13.#StillHere: Five Personal Stories. Alzheimer Society Manitoba website. https://alzheimer.mb.ca/still-here-five-personal-stories/. 2019.
14.Bubu OM, Brannick M, Mortimer J, et al. Sleep, cognitive impairment, and Alzheimer’s disease: a systematic review and meta-analysis. Sleep. 2017;40(1). PubMed CrossRef
15.Musiek ES, Xiong DD, Holtzman DM. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp Mol Med. 2015;47(3):e148. PubMed CrossRef
16.Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308(19):2020-2029. PubMed CrossRef
17.Bransford KH. Lying to the Ones We Love. Dealing with Dementia website. https://dealingwithdementia.wordpress.com/2019/05/14/lying-to-the-ones-we-love/. May 2019.
18.World Health Organization. mhGAP Intervention Guide: Version 2.0 for Menta,. Neurological and Substance Use Disorders in Non-Specialized Health Settings. Geneva, Switzerland: World Health Organization; 2016.
19.Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350(7):h369. PubMed CrossRef
20.American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. PubMed CrossRef
21.Tisher A, Salardini A. A comprehensive update on treatment of dementia. Semin Neurol. 2019;39(2):167-178. PubMed CrossRef
22.Schmidt R, Hofer E, Bouwman FH, et al. EFNS-ENS/EAN Guideline on concomitant use of cholinesterase inhibitors and memantine in moderate to severe Alzheimer’s disease. Eur J Neurol. 2015;22(6):889-898. PubMed CrossRef
23.Kumar A, Sharma S. Donepezil. StatPearls. Treasure Island, FL: StatPearls Publishing; 2019.
24.Birks JS, Harvey RJ. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst Rev. 2018;6(1):CD001190. PubMed
25.Kishi T, Matsunaga S, Oya K, et al. Memantine for Alzheimer’s disease: an updated systematic review and meta-analysis. J Alzheimers Dis. 2017;60(2):401-425. PubMed CrossRef
26.Knight R, Khondoker M, Magill N, et al. A systematic review and meta-analysis of the effectiveness of acetylcholinesterase inhibitors and memantine in treating the cognitive symptoms of dementia. Dement Geriatr Cogn Disord. 2018;45(3-4):131-151. PubMed CrossRef
27.Tricco AC, Ashoor HM, Soobiah C, et al. Comparative effectiveness and safety of cognitive enhancers for treating Alzheimer’s disease: systematic review and network metaanalysis. J Am Geriatr Soc. 2018;66(1):170-178. PubMed CrossRef
28.Birks JS, Chong LY, Grimley Evans J. Rivastigmine for Alzheimer’s disease. Cochrane Database Syst Rev. 2015;22(9):CD001191. PubMed CrossRef
29.Loy C, Schneider L. Galantamine for Alzheimer’s disease and mild cognitive impairment. Cochrane Database Syst Rev. 2006;(1):CD001747. PubMed CrossRef
30.McShane R, Westby MJ, Roberts E, et al. Memantine for dementia. Cochrane Database Syst Rev. 2019;3:CD003154. PubMed CrossRef
31.Danysz W, Parsons CG. The NMDA receptor antagonist memantine as a symptomatological and neuroprotective treatment for Alzheimer’s disease: preclinical evidence. Int J Geriatr Psychiatry. 2003;18(suppl 1):S23-S32. PubMed CrossRef
32.Parsons CG, Danysz W, Dekundy A, et al. Memantine and cholinesterase inhibitors: complementary mechanisms in the treatment of Alzheimer’s disease. Neurotox Res. 2013;24(3):358-369. PubMed CrossRef
33.Matsunaga S, Kishi T, Iwata N. Memantine monotherapy for Alzheimer’s disease: a systematic review and meta-analysis. PLoS One. 2015;10(4):e0123289. PubMed CrossRef
34.Ihl R, Bunevicius R, Frölich L, et al; WFSBP Task Force on Mental Disorders in Primary Care; WFSBP Task Force on Dementia. World Federation of Societies of Biological Psychiatry guidelines for the pharmacological treatment of dementias in primary care. Int J Psychiatry Clin Pract. 2015;19(1):2-7. PubMed CrossRef
35.Howard R, McShane R, Lindesay J, et al. Nursing home placement in the Donepezil and Memantine in Moderate to Severe Alzheimer’s Disease (DOMINO-AD) trial: secondary and post-hoc analyses. Lancet Neurol. 2015;14(12):1171-1181. PubMed CrossRef
36.Deardorff WJ, Feen E, Grossberg GT. The use of cholinesterase inhibitors across all stages of Alzheimer’s disease. Drugs Aging. 2015;32(7):537-547. PubMed CrossRef
37.Raskind MA, Peskind ER, Truyen L, et al. The cognitive benefits of galantamine are sustained for at least 36 months: a long-term extension trial. Arch Neurol. 2004;61(2):252-256. PubMed CrossRef
38.Farlow M, Anand R, Messina J Jr, et al. A 52-week study of the efficacy of rivastigmine in patients with mild to moderately severe Alzheimer’s disease. Eur Neurol. 2000;44(4):236-241. PubMed CrossRef
39.Renn BN, Asghar-Ali AA, Thielke S, et al. A systematic review of practice guidelines and recommendations for discontinuation of cholinesterase inhibitors in dementia. Am J Geriatr Psychiatry. 2018;26(2):134-147. PubMed CrossRef
40.StillHere. Wandering. Alz Connected website. https://www.alzconnected.org/discussion.aspx?g=posts&t=2147497794. May 2013.
41.Mathys M. Pharmacologic management of behavioral and psychological symptoms of major neurocognitive disorder. Ment Health Clin. 2018;8(6):284-293. PubMed CrossRef
42.National Institute for Health and Care Excellence. Dementia: assessment, management and support for people living with dementia and their carers [NICE guideline NG97]. NICE website. https://www.nice.org.uk/guidance/ng97. June 2018.
This PDF is free for all visitors!
Save
Cite