Buprenorphine is a partial-agonist opioid that is prescribed as a medication-assisted treatment (MAT) for opioid use disorder (OUD). Buprenorphine is also a potent analgesic with high opioid-receptor affinity and binding coefficient; when buprenorphine is administered simultaneously with a μ-opioid receptor full agonist (“full agonist opioid” [FAO]), the combination can yield unexpected outcomes depending on dosing and timing. Buprenorphine is sometimes perceived as a powerful competitive opioid blocker that will hamper pharmacologic management that necessitates the use of FAO. When patients receiving buprenorphine-MAT (BUP-MAT) formulations have presented for operative procedures, there has been clinical variance in approach to their BUP-MAT management. Recognizing the risk management challenge from both analgesia and BUP-MAT perspectives, we convened a multidisciplinary group of clinicians who treat BUP-MAT patients and completed a literature review with the goal of generating a guideline for appropriate management of these patients presenting for a broad spectrum of surgical procedures. Our conclusion is that continuous simultaneous administration of buprenorphine products with FAO is safe when accounting for dose and timing, including surgeries that historically produce moderate to severe pain, and may further provide an analgesic advantage, lessen FAO burden, and reduce relapse risk to this group.

ABSTRACT
Buprenorphine is a partial-agonist opioid that is prescribed as a medication-assisted treatment (MAT) for opioid use disorder (OUD). Buprenorphine is also a potent analgesic with high opioid-receptor affinity and binding coefficient; when buprenorphine is administered simultaneously with a μ-opioid receptor full agonist (“full agonist opioid” [FAO]), the combination can yield unexpected outcomes depending on dosing and timing. Buprenorphine is sometimes perceived as a powerful competitive opioid blocker that will hamper pharmacologic management that necessitates the use of FAO. When patients receiving buprenorphine-MAT (BUP-MAT) formulations have presented for operative procedures, there has been clinical variance in approach to their BUP-MAT management. Recognizing the risk management challenge from both analgesia and BUP-MAT perspectives, we convened a multidisciplinary group of clinicians who treat BUP-MAT patients and completed a literature review with the goal of generating a guideline for appropriate management of these patients presenting for a broad spectrum of surgical procedures. Our conclusion is that continuous simultaneous administration of buprenorphine products with FAO is safe when accounting for dose and timing, including surgeries that historically produce moderate to severe pain, and may further provide an analgesic advantage, lessen FAO burden, and reduce relapse risk to this group.
J Clin Psychiatry 2020;81(1):19com12810
To cite: Acampora GA, Nisavic M, Zhang Y. Perioperative buprenorphine continuous maintenance and administration simultaneous with full opioid agonist: patient priority at the interface between medical disciplines. J Clin Psychiatry. 2020;81(1):19com12810.
To share: https://doi.org/10.4088/JCP.19com12810
© Copyright 2020 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry and Department of Anesthesia Critical Care and Pain Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts
*Corresponding author: Gregory Alexander Acampora, MD, Department of Psychiatry, MGH, 55 Fruit St, M76, Boston, MA 02114 ([email protected]).
Medication-assisted treatment (MAT) is a treatment priority in the US government initiative to address the current opioid crisis per the Substance Abuse and Mental Health Services Administration (SAMHSA; a branch of the US Department of Health and Human Services).1 One of the results of this effort is an increase in the number of patients receiving MAT who present for surgical intervention. Buprenorphine is a μ-opioid receptor partial agonist used clinically to treat opioid use disorder and pain. Buprenorphine is an oripavine (thebaine metabolite) semisynthetic derivative with activity-interactions at the 4 identified opioid peptide (OP) receptors (MOP [mu, μ], DOP [delta, δ], KOP [kappa, κ] and NOP [nociception]) that result in unique pharmacokinetic and pharmacodynamic exposure-response relationships.2 Clinically, the high MOP receptor affinity, partial agonism, and binding coefficient of buprenorphine produces many of the putative therapeutic effects observed; however, when buprenorphine is administered simultaneously with a μ-opioid receptor full agonist (“full agonist opioid” [FAO]), the combination can yield unfamiliar outcomes depending on dosing and timing.3
Buprenorphine products are produced in various strengths alone or in combination with the μ-opioid receptor antagonist naloxone in several formulations. In developing our treatment guideline, most target patients were receiving the sublingual (SL) preparations of Suboxone 4/1 ratio (buprenorphine/naloxone) formulation pre-, peri-, and postoperatively. The term “BUP-MAT” will be used throughout this article for the buprenorphine/naloxone combination, realizing that some patients may be on buprenorphine-only formulations. The buprenorphine parent compound dominates the pharmacologic interactions addressed in this guideline development article. The naloxone component of the SL formulations is considered to have negligible effects with low picogram plasma levels achieved; however, the FDA did acknowledge less negligible effects if doses of 16/4 or more were taken at one time.4
Buprenorphine is commonly perceived by clinicians and patients to be a powerful competitive opioid blocker, lending to the conclusion that it will hinder both intraoperative and postoperative pharmacologic management that uses FAO. Thus, continuation of BUP-MAT may generate concerns in the surgical/procedural setting. This apprehension may result in discord between clinician disciplines that inherently have different outcome priorities. Surgeons, proceduralists, and anesthesiologists tend to focus on acute pain mitigation and may emphasize fully tapering off BUP-MAT to optimize acute pain management. Substance use disorder (SUD) specialists are aware of risks (relapse and overdose) associated with patients stabilized on BUP-MAT and may prioritize strategies that preserve BUP-MAT. Patients can become entangled in this issue as they bring their own concepts of BUP-MAT effectiveness for pain management, often based on street-lore reports, internet information gathering, and rumors of possible antagonistic interference between BUP-MAT and FAO. All of these can negatively influence anticipatory planning for perioperative and postoperative pain control and cause considerable distress and anxiety for the patient and teams alike.

- Opioid use disorder patients on BUP-MAT present a dilemma in the perioperative period with regard to balancing risk of relapse and pain management. Previous clinical practice treated this as a dichotomous choice that our team sought to resolve using preclinical and clinical published papers to develop a new interdisciplinary guideline for this complex patient population.
- The guideline offers clinicians a structured dosing strategy throughout the surgical period to avoid patient withdrawal and relapse risk preoperatively while maximizing opioid pain management postoperatively. This single guideline offers a consistent message that can be shared with patients by the addiction and surgical care clinicians.
- We recommend up to 8 mg twice daily (16 mg) BUP-MAT the day before surgery for relapse prevention. This is followed by 4 mg twice daily the day of surgery and throughout the postoperative period to minimize risk of full and partial opioid agonist competition while providing supplemental pain control with FAO. This also permits restabilization of the patient’s effective dose of BUP-MAT without discharge opioids.
Perioperative pain management in patients on BUP-MAT has been dealt with in diverse ways by institutions.5 University of Michigan guidelines initially recommended discontinuing buprenorphine up to 5 days prior to higher complexity surgery, then using conventional FAO for peri- and postoperative pain control. Boston Medical Center guidelines recommended holding BUP-MAT on the day of the surgery, then using extended release FAO agents for baseline pain control, as well as short-acting FAO for breakthrough pain management. University of Kentucky guidelines recommended continuing BUP-MAT perioperatively and consideration of opioid-sparing alternatives (eg, regional anesthesia) in addition to conventional FAO treatment for postoperative pain control. Traditionally, the Massachusetts General Hospital Department of Anesthesia Critical Care and Pain Medicine (MGH DACCPM) followed the University of Michigan guidelines—patients scheduled for procedures associated with higher postoperative pain would be instructed to taper off their BUP-MAT in anticipation of surgery for fear that coadministration of buprenorphine and FAO could lead to suboptimal pain control. For opioid use disorder (OUD) treaters, this protocol generated considerable concern as patients would be tapered off treatment and often would return home on FAO tapers that could result in reemergence of cravings and potential relapse to use. To address these concerns, a multidisciplinary team of MGH providers (anesthesia, psychiatry, and addiction medicine) who care for patients with a history of OUD formed a task force to examine perioperative BUP-MAT use and propose a new collective guideline for this patient population.
This guideline development report provides an overview of the history of buprenorphine and its applications, including recent prescribing trends and perceived conflicts of coadministration with FAO. We describe the preclinical and clinical data used to propose our rationale for continuous use of buprenorphine with traditional FAO analgesia without the need to stop MAT.
Historical Perspective on Opioid Prescribing
A review of the history of opioid administration indicates early recognition of the association of pain relief benefit with misuse potential. Development of semisynthetic and synthetic opioid variants represents attempts to create potent analgesics with limited side effects and less addiction hazard. Since 2000, the mortality rates associated with opioids have expanded exponentially, particularly with the high potency synthetic opioids.6
In 1928, the New York City Bureau of Social Hygiene convened the Committee on Drug Addiction, which produced an in-depth book, titled The Opium Problem, that identified 2 concerning international trends with opioids: (1) misuse after therapeutic applications and (2) the spread of heroin.7 This initiated efforts to separate opioid analgesic properties from associated risk of addiction. The decades leading to the 1960s resulted in production of more agonists, then the development of antagonists, and finally an attempt to mitigate burgeoning addiction with opioid agonist maintenance replacement (methadone) in a highly regulated environment.8 In 1966, the discovery of buprenorphine in Hull, England, was the result of a trend to create safe over-the-counter opioids with analgesic but not addicting qualities.9 Quickly identified as a very potent analgesic, with its unique μ-opioid receptor partial agonist activity, buprenorphine was studied as a putative “addict treatment” at the Addiction Research Center in Kentucky.10 Thereafter, buprenorphine applications follow a convoluted path through regulatory layers to their eventual approval as an acceptably safe office-based opioid treatment with US Congressional passage of the Drug Addiction Treatment Act (DATA) 2000.11 With emphasis on buprenorphine as a primary MAT, use as a primary analgesic option tended to be limited. Buprenorphine resurfaced as a putative safer opioid analgesic, but use of it is now confusing due to requirements of adhering to combinations of DATA 2000 and Drug Enforcement Administration rules and regulations.12 More recently, clinicians again find themselves at the clinical crossroads of prescribing for pain with the addiction problem in mind.
Development of the MGH Perioperative Buprenorphine Protocol
MGH instituted a novel Strategic Plan in 2004 and in 2014 renewed the plan with 12 key strategies as institutional imperatives. The fifth strategy was to advance care of chronic disease with a specific focus to include SUD as an important contributor to health burden and financial costs.13 Adding to the existing Psychiatry and Internal Medicine addictions foci, the MGH SUD Initiative created a multidisciplinary Addiction Consult Team to provide comprehensive care to patients admitted to the general hospital with co-occurring OUD diagnoses. The anesthesiology acute and chronic pain services became part of the association. These interdisciplinary teams independently realized the risks to patients on BUP-MAT coming for a procedure that may require FAO who, per MGH DACCPM practice, were told to discontinue BUP-MAT perioperatively. Accordingly, in 2018, a collaborative working group of these interdisciplinary teams was convened to address the needs of patients on BUP-MAT scheduled for surgery while integrating the treatment priorities of the treatment stakeholders. The central goal of this process was to prioritize safety and comfort for patients already on BUP-MAT while acknowledging the potential challenges in addressing pain, avoiding withdrawal, and other issues created by coadministering BUP-MAT with FAO. We initiated a comprehensive PubMed National Center for Biotechnology Information database search without publication or language restriction for established methodological guideline peer-reviewed clinical studies using buprenorphine, buprenorphine, AND naloxone linked to chronic pain, acute pain, surgical post op pain, analgesia, OUD, competition, and synergy. This search was later amplified by Google searches. We then restricted the search to articles on the outcomes of periprocedural continuation or discontinuation of BUP-MAT in patients with history of OUD. We excluded articles that covered periprocedural use of buprenorphine as part of pain medication regimens.14 This yielded the evidence-based recommendation for our new institution-wide BUP-MAT guideline that was unanimously accepted by the SUD working group, as well as the entire DACCPM at MGH.
Initial review of the literature revealed disparity in optimal perioperative management strategies for patients on buprenorphine formulations. Our workgroup was aware of risk of relapse in our target BUP-MAT population both pre- and postoperatively when there was a transition off BUP-MAT and a BUP-MAT reinduction postoperatively.15 There were several case reports suggesting possible acute pain control interference from buprenorphine, but we did not find a persuasive pattern. Other groups suggested that it was feasible to continue buprenorphine for certain postoperative pain and that it can be beneficial when continued during the postoperative period.16-18 The data with the highest-quality evidence for combined use of BUP-MAT with FAO for moderate to severe pain were found in the obstetric population.17,19-21 Lacking were data for continuation of BUP-MAT on other surgery types such as large open incision perineal and abdominal procedures, thoracotomy, multilevel spine fusion, and joint replacement. We remained uncertain about the optimal dose and timing of BUP-MAT in these cases. We found the following articles particularly relevant to our guideline solution.
We emphasized a preclinical (rat tail-flick) study by Kögle et al22 that tested the interaction of buprenorphine with FAO (morphine, oxycodone, hydromorphone, and fentanyl) and full antagonist opioids (naloxone, naltrexone, and clocinnamox). Their results verified the antagonistic effects by buprenorphine in high dose (up to 6.81 mg/kg IP) but revealed additive or superadditive (synergistic) effects when buprenorphine was coadministered at analgesic dose range (0.0316 mg/kg IV). Additionally, if the tested FAO was given after the acute buprenorphine effect for pain (approximately 8 h), the FAOs showed full analgesic effect, suggesting that the slow receptor kinetics of buprenorphine do not have a negative influence on the availability of opioid receptors beyond the analgesic duration. This was evidence that an analgesic dose of buprenorphine could be additive or synergistic in preclinical studies at or less than ED50 (effective dose for 50% of the studied population) of buprenorphine notwithstanding the verification of the surmised antagonistic effects of buprenorphine at higher doses.
Next, we sought broader clinical examples of successful human administration of buprenorphine and FAO combinations as we wanted to infer an optimal in vivo human analgesic dose. In a 2009 double-blind study by Oifa et al23 of adults without OUD undergoing abdominal surgery (gastrectomy, large bowel resection, or partial pancreatectomy), 120 lower risk adult patients were given postoperative patient-controlled analgesia (PCA) with buprenorphine (BUP) and/or morphine (MO) in a 4-arm trial involving intravenous (IV) infusion + bolus (BUP/BUP, MO/MO, BUP/MO, MO/BUP). IV PCA with BUP alone or in combination with MO provided equivalent postoperative analgesia to IV PCA with MO alone in patients who had undergone major abdominal surgery. At the analgesic doses and modes of administration used, BUP continued to be effective and was well tolerated when given IV both alone and in combination with morphine. The average BUP infusion rate was 0.4 μg/kg/h ח 70 ח 12 = 336 μg per 24 hours. The average BUP bolus (0.15 μg/kg each) amount per 12 hours was 135-178 μg per 70 kg. With a mean bolus value of 150 μg per 70 kg in 12 hours, the result was 300 μg by bolus BUP daily. The average combined bolus + infusion 24-hour dose of BUP was 972 μg per day. Assuming a 30% BUP oral bioavailability calculation,24 we concluded from this paper that approximately 3 mg (300 μg) SL BUP would be the derived sufficient BUP analgesic dose.
This was evidence that BUP-MAT could be continued throughout the perioperative period. The next clinical challenge was to identify optimal dosing that would provide both additive pain control effect and also reduce risk for relapse in a complex patient population taking a range of BUP-MAT doses and undergoing surgical procedures with variable postoperative pain expectations.
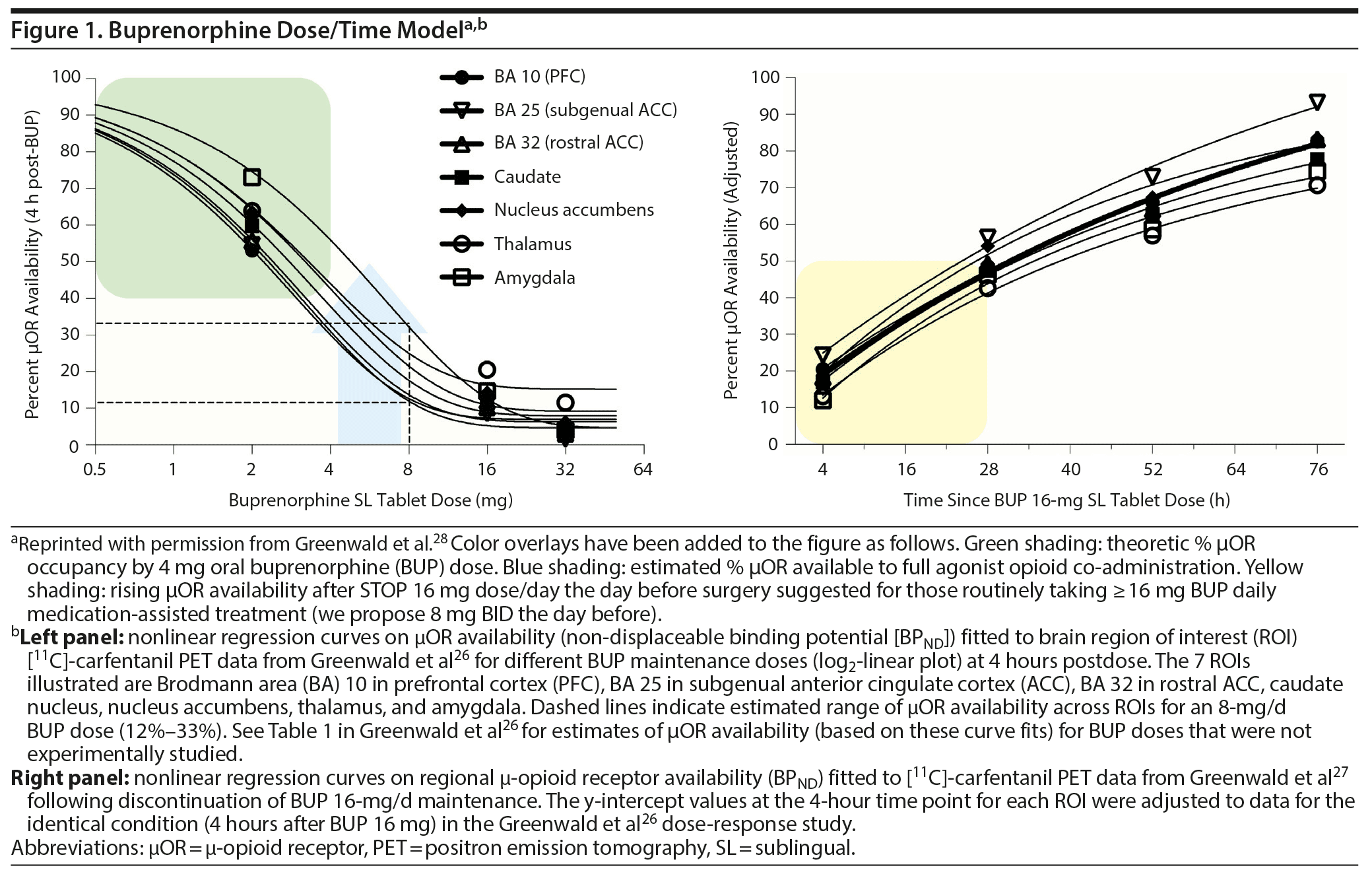
A series of elegant studies conducted over 15 years by Greenwald, Zubieta, and colleagues25-28 provided a platform to guide our recommending BUP-MAT dosing and timing to allow both analgesia and protection against craving. The researchers used carbon 11 tagged carfentanil positron emission tomography imaging (11C-cPET) of μ-opioid receptor (μOR) availability in subcortical brain substructures of subjects with a history of heroin OUD. In the first study, subjects were administered 2 mg and 16 mg of liquid oral buprenorphine followed by (11C-cPET) that revealed μOR low occupancy at the low doses and high occupancy at the high dose.25 The next study found that oral tablets produced nearly identical PET occupancy at 16 mg (85%-92% μ receptor occupancy vs 90% with liquid), and there was no statistical improvement in subjective response to doses up to 32 mg (94%-98% except thalamus).26
The third study made 11C-cPET measurements at 4, 28, 52, and 76 hours after stopping 16 mg/d buprenorphine, revealing recovered μOR availability at 30%, 54%, 67%, and 82%, respectively. Subjects in that study reported adequate withdrawal symptom suppression at 50%-60% buprenorphine occupancy.27 The 2014 paper28 summated their research findings in a mathematical graph (Figure 1). Figure 1, left panel, shows μOR availability in subcortical regions of the brain relative to log-scale increasing doses of BUP. Figure 1, right panel, is the availability of μOR after 16 mg is held. The authors in the last paper determined that there is great variability in clinical settings and restricting to fixed dosing may not be optimal.
In summary, our major conclusions from the above outlined literature search were as follows:
- Buprenorphine shows high affinity, low efficacy, and long duration but clinically reversible action at the MOP receptor.
- Buprenorphine provides significant analgesia at relatively SL low doses.
- Buprenorphine at high doses competes with FAO and, as expected, can reduce efficacy of FAO.
- When analgesic doses of BUP-MAT, ideally 4 mg BID, are used in combination with FAO, it can produce a synergistic analgesic effect.
- Withdrawal symptoms are suppressed with μ availability of 50%-60%.
- 24 hours after buprenorphine 16 mg daily is withheld, 40% of opioid receptors become available again. No significant increase of withdrawal or craving was noted compared to 4 hours post buprenorphine administration.
Following literature review and the multidisciplinary meeting, we identified key goals for the updated recommendations for patients on BUP-MAT as follows:
- Avoid inducing BUP-MAT withdrawal—preferably without resorting to FAO to achieve this.
- Minimize the risk of prescribed opioid use and minimize the reinforcement effect of FAO, while not negatively impacting analgesia and ensuring adequate perioperative pain control.
- Minimize risk of patients’ BUP-MAT being held for prolonged time periods (eg, up to 5 days preoperation), as this may be unnecessary and increases below-outlined risks.
- Having an analgesic dose of BUP-MAT may not only be safe with FAO but also provide additional benefits in pain control and reduce overall FAO utilization.
We also concluded that the historic DACCPM recommendations were inadequate largely given the multiple risks associated with discontinuation of BUP-MAT, as outlined here:
- Precipitation of BUP-MAT withdrawal
- Increased relapse risk as BUP-MAT washes out and withdrawal/cravings begin
- Difficult reinduction with longer washout so as to avoid precipitated withdrawal effects
- Patient anxiety associated with getting off and back on BUP-MAT treatment
- Acute exacerbation of chronic pain with multiple medication changes
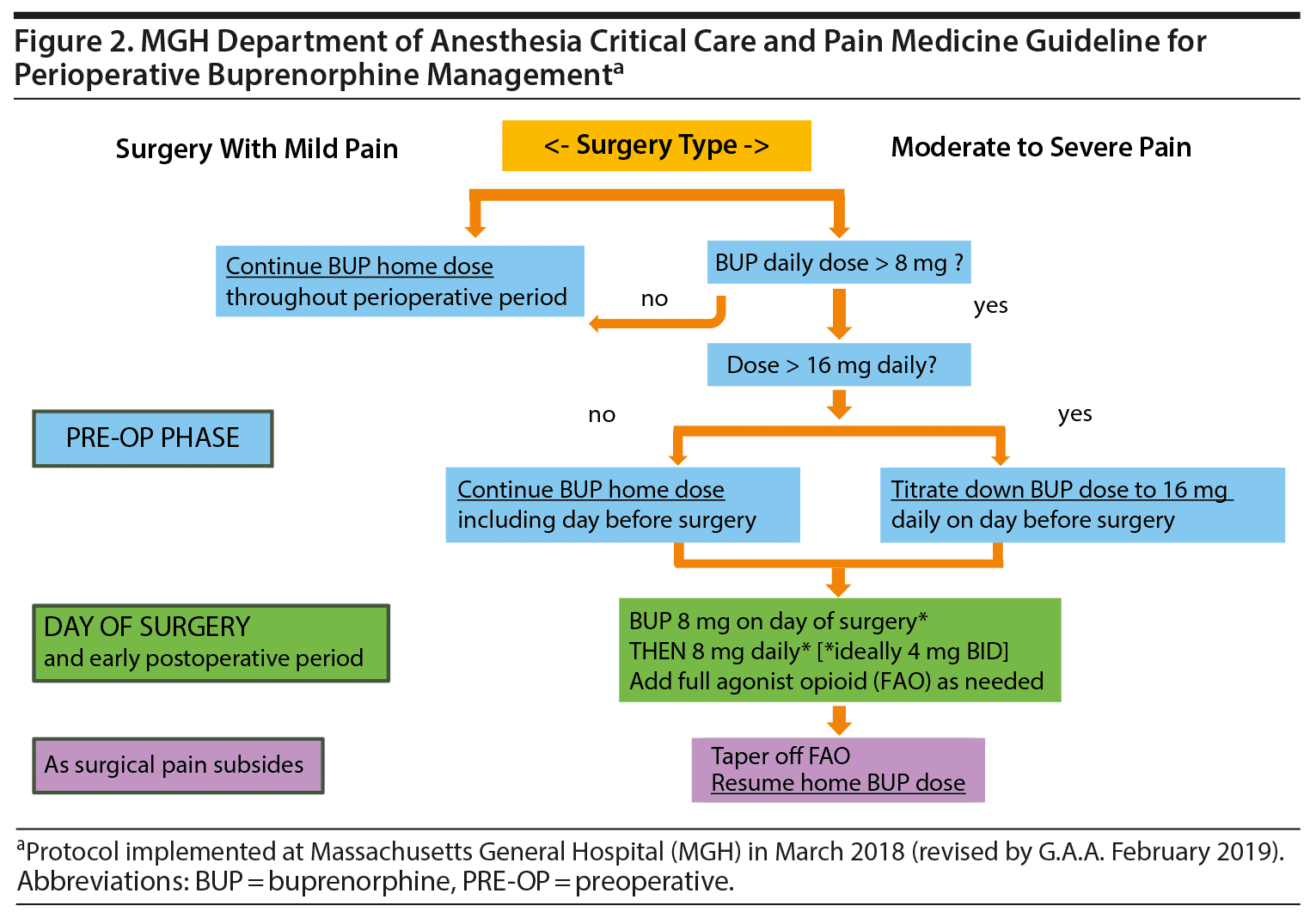
Hence, the new DACCPM perioperative BUP-MAT dose recommendations* are as follows:
- BUP-MAT dose 8 mg per day or less: Continue baseline regimen throughout.
- BUP-MAT dose greater than 8 mg per day:
If mild pain is anticipated postoperatively (ie, procedures in which historically less than 5-day courses of low dose oxycodone or hydrocodone are prescribed, including cesarean section): continue baseline regimen throughout (expectation is no need to add-on opioids).
If moderate to severe pain is anticipated postoperatively:
Day before surgery:
- If BUP-MAT < 16 mg daily, continue regimen but suggest 8 mg bid.
- If BUP-MAT dose > 16 mg daily, reduce dose to 16 mg daily (recommend 8 mg twice a day BID vs single 16 mg dose).
Day of surgery and throughout hospital stay:
Continue BUP-MAT at 8 mg per day (preferably 4 mg BID vs 8 mg QD), use additional opioid agonists as needed.
After discharge:
When surgical pain subsides, taper off opioid agonists and then return to previous BUP dose.
A graphic representation of these recommendations is shown in Figure 2.
We also recommended increased pre- and postoperative communication between the various clinical disciplines caring for the patient, including the following:
- Identify the name and contact information of the physician who provides the patient’s BUP-MAT and contact that physician whenever possible to reach consensus on management plan.
- Ensure addiction consultation service is engaged whenever appropriate to help identify resources and support available for patient postoperatively.
- Upon discharge, pain service consultation teams and/or addiction consultation team will contact patient’s buprenorphine prescriber and provide a handoff of the buprenorphine management course and postdischarge plan.
- All nonopioid adjuvant (multimodal) pain medications (gabapentin, pregabalin, tricyclic antidepressants, muscle relaxers, acetaminophen, etc) should be considered postoperatively and postdischarge.
DISCUSSION
There exist clinically unique dose and timing effects of buprenorphine that can be exploited for both analgesia and relapse prevention during the perioperative period. This is a guideline to permit BUP-MAT continuously during all perioperative phases of any level of surgery while allowing for simultaneous administration of FAO intra- and postoperatively. Specifically, allow a relatively high BUP-MAT dose late in the preoperative period (maintain up to 16 mg/d at 8 mg BID BUP-MAT the day before surgery) to suppress craving risks. For day of and postoperative phases, administer a continuous lower dose (up to 8 mg/d at 4 mg BID) to complement analgesia while leaving receptors available for flexible use of FAO as needed. Finally, convert back to BUP-MAT-only maintenance.
There has been a practice to limit use of BUP-MAT to a dichotomous choice at the intersection of OUD and pain. This clinical approach generated a challenge when considering perioperative analgesia for surgical patients on BUP-MAT. Preclinical data indicate unique properties of buprenorphine including beneficial interactions regarding pain management. The literature is trending toward perioperative BUP-MAT continuation protocols despite some case reports suggesting possible interference. Within the body of preclinical and clinical observations, we found compelling evidence to recommend a continuation strategy.
Other institutions and authors have proposed continuation of buprenorphine in patients with OUD through the perioperative period. A 2019 editorial by Lembke et al29 suggested that buprenorphine should be continued during the perioperative period and provided a diagram of dose, preoperative, day of surgery, postoperative, and discharge strategies using 12 mg buprenorphine as the anchor dose. We chose to emphasize the time course studies by Greenwald et al that support our belief that up to 16 mg BUP-MAT preoperatively provides greater protection against craving risks. Thereafter, 4 mg twice daily dosing on the day of surgery and in the postoperative period reduces the risk of full agonist and partial agonist competition when providing analgesia.
One historic approach to understanding relative potency of μ-opioid full and partial receptor agonists relied on the use of equivalency charts and in vitro binding characteristics such as Ki. These preclinical metrics have been useful in predicting some of the dynamic involved in the pharmacokinetic and pharmacodynamic exposure-response relationships when dosing, mixing, or alternating opioids but are insufficient to explain the distinct clinical effect of each FAO.30 For more detailed pharmacologic descriptions of buprenorphine in acute and chronic pain settings we recommend articles by Lutfy and Cowan,2 Raffa et al,31 and Khanna and Pillarisetti.32
It is beyond the scope of this article to discuss the effect of buprenorphine active metabolites norbuprenorphine, buprenorphine-3-glucuronide, and norbuprenorphine-3-glucuronide and their activity-interactions at the 4 identified opioid peptide receptors. It is likely that these interactions help account for some of the discrepancies in the literature when discussing “ceiling effect,” “synergy,” and “efficacy” of buprenorphine.33
We did not address buprenorphine-only pain management products such as Belbuca buccal films at 75-900 μg = 0.075-0.9 mg per 12 hours, Butrans patch at 5-20 μg = 0.005-0.020 mg per hour for 7 days, Buprenex 300 μg = 0.3 mg IV/IM every 8 hours, or the depot Sublocade IM 100/300 mg monthly. All but the Sublocade formulations represent sub-1 mg daily doses and would fall into the “less than 8 mg/d” part of our guideline. Sublocade FDA-approved manufacturing insert data34 show steady-state plasma levels achieved by the 100 mg/mo IM product approximating 12 mg daily dosing of Subutex (buprenorphine only) sublingual product; notably, the 300 mg/mo IM product daily plasma levels exceed that achieved by the 24 mg daily dosing of Subutex sublingual product that could create undesirable competition if FAO is relied upon perioperatively.
This guideline represents a significant shift at our institution in the specific management of BUP-MAT for OUD patients who present for surgical procedures, particularly procedures that are associated with moderate to severe pain both intra- and postoperatively including large open incision perineal and abdominal procedures, thoracotomy including CABG, multilevel spine fusion, and joint replacement. Our treatment guideline satisfies the perioperative therapeutic goals of differing disciplines while keeping safety and comfort a priority for patients on BUP-MAT and encouraging team communication and cooperation.
Limitations of this paper include the following: (1) this proposal is based on clinical and preclinical studies; (2) this guideline generalizes to a buprenorphine dose with the implication that Suboxone formulations would be most commonly used; (3) while we noted the potential for BUP-MAT to provide adequate analgesia, we did not discuss in detail buprenorphine as a stand-alone analgesic; (4) this guideline does not address buprenorphine-only pain management product because its focus is striking a balance between addressing risk of relapse preoperatively with pain management postoperatively and return to longitudinal BUP-MAT; and (5) the ideal dose of buprenorphine in humans is not yet known but may be determined by new preclinical and clinical research into cellular level, tissue levels, and activity of its metabolites.
Our interdisciplinary team reviewed clinical and preclinical literature to generate an evidence-based guideline for the continuous administration of BUP-MAT throughout planned surgeries of any level of complexity. Patient safety and comfort are maximized by limiting disruption of the preoperative BUP-MAT dose and with ongoing interdisciplinary communication, while also satisfying specialty clinician priorities. We implemented this protocol at MGH in March 2018.
Published online: January 7, 2020.
Potential conflicts of interest: None.
Funding/support: None.
REFERENCES
1.Medication-Assisted Treatment (MAT). SAMHSA website. https://www.samhsa.gov/medication-assisted-treatment.
2.Lutfy K, Cowan A. Buprenorphine: a unique drug with complex pharmacology. Curr Neuropharmacol. 2004;2(4):395-402. PubMed CrossRef
3.Gambús PL, Trocóniz IF. Pharmacokinetic-pharmacodynamic modelling in anaesthesia. Br J Clin Pharmacol. 2015;79(1):72-84. PubMed CrossRef
4.Center for Drug Evaluation and Research Application Number. 022410Orig1s000. Clinical Pharmacology and Biolpharmaceutics Review(s). FDA website. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022410Orig1s000ClinPharmR.pdf. pages 16-20
5.Ward EN, Quaye AN, Wilens TE. Opioid use disorders: perioperative management of a special population. Anesth Analg. 2018;127(2):539-547. PubMed CrossRef
6.Spencer MR, Warner M, Bastian BA, et al. Drug overdose deaths involving fentanyl, 2011-2016. Natl Vital Stat Rep. 2019;68(3):1-19. PubMed
7.Terry CE, Pellens M. The Opium Problem. New York, NY: The Committee on Drug Addictions in collaboration with The Bureau of Social Hygiene; 1928.
8.Campbell ND, Lovell AM. The history of the development of buprenorphine as an addiction therapeutic. Ann N Y Acad Sci. 2012;1248(1):124-139. PubMed CrossRef
9.Lewis J. Nathan B. Eddy Award lecture: In pursuit of the Holy Grail. The College on Problems of Drug Dependence (CPDD) website. https://cpdd.org/Media/Index/AwardSpeeches/LewisJ._Eddy1998.pdf. 1998.
10.Jasinski DR, Pevnick JS, Griffith JD. Human pharmacology and abuse potential of the analgesic buprenorphine: a potential agent for treating narcotic addiction. Arch Gen Psychiatry. 1978;35(4):501-516. PubMed CrossRef
11.Drug Addiction Treatment Act of 2000 Pub. L. 106-310, div. B, title XXXV, Sections 3501 and 3502. Congress.gov website. https://www.congress.gov/106/plaws/publ310/PLAW-106publ310.pdf. Oct. 17, 2000. Page 122.
12.Heit HA, Covington E, Good PM. Dear DEA. Pain Med. 2004;5(3):303-308. PubMed CrossRef
13.Substance Use Disorders Initiative. Massachusetts General Hospital website. https://www.massgeneral.org/substance-use-disorders-initiative.
14.Quaye AN, Zhang Y. Perioperative management of buprenorphine: solving the conundrum [online ahead of print November 30, 2018]. Pain Med. 2018. PubMed
15.Ling W, Hillhouse M, Domier C, et al. Buprenorphine tapering schedule and illicit opioid use. Addiction. 2009;104(2):256-265. PubMed CrossRef
16.Kornfeld H, Manfredi L. Effectiveness of full agonist opioids in patients stabilized on buprenorphine undergoing major surgery: a case series. Am J Ther. 2010;17(5):523-528. PubMed CrossRef
17.Jones HE, O’ Grady K, Dahne J, et al. Management of acute postpartum pain in patients maintained on methadone or buprenorphine during pregnancy. Am J Drug Alcohol Abuse. 2009;35(3):151-156. PubMed CrossRef
18.Macintyre PE, Russell RA, Usher KA, et al. Pain relief and opioid requirements in the first 24 hours after surgery in patients taking buprenorphine and methadone opioid substitution therapy. Anaesth Intensive Care. 2013;41(2):222-230. PubMed CrossRef
19.Vilkins AL, Bagley SM, Hahn KA, et al. Comparison of post-cesarean section opioid analgesic requirements in women with opioid use disorder treated with methadone or buprenorphine. J Addict Med. 2017;11(5):397-401. PubMed CrossRef
20.Meyer M, Paranya G, Keefer Norris A, et al. Intrapartum and postpartum analgesia for women maintained on buprenorphine during pregnancy. Eur J Pain. 2010;14(9):939-943. PubMed CrossRef
21.Höflich AS, Langer M, Jagsch R, et al. Peripartum pain management in opioid dependent women. Eur J Pain. 2012;16(4):574-584. PubMed CrossRef
22.Kögel B, Christoph T, Strassburger W, et al. Interaction of mu-opioid receptor agonists and antagonists with the analgesic effect of buprenorphine in mice. Eur J Pain. 2005;9(5):599-611. PubMed CrossRef
23.Oifa S, Sydoruk T, White I, et al. Effects of intravenous patient-controlled analgesia with buprenorphine and morphine alone and in combination during the first 12 postoperative hours: a randomized, double-blind, four-arm trial in adults undergoing abdominal surgery. Clin Ther. 2009;31(3):527-541. PubMed CrossRef
24.Mendelson J, Upton RA, Everhart ET, et al. Bioavailability of sublingual buprenorphine. J Clin Pharmacol. 1997;37(1):31-37. PubMed CrossRef
25.Zubieta J, Greenwald MK, Lombardi U, et al. Buprenorphine-induced changes in mu-opioid receptor availability in male heroin-dependent volunteers: a preliminary study. Neuropsychopharmacology. 2000;23(3):326-334. PubMed CrossRef
26.Greenwald MK, Johanson CE, Moody DE, et al. Effects of buprenorphine maintenance dose on mu-opioid receptor availability, plasma concentrations, and antagonist blockade in heroin-dependent volunteers. Neuropsychopharmacology. 2003;28(11):2000-2009. PubMed CrossRef
27.Greenwald M, Johanson CE, Bueller J, et al. Buprenorphine duration of action: mu-opioid receptor availability and pharmacokinetic and behavioral indices. Biol Psychiatry. 2007;61(1):101-110. PubMed CrossRef
28.Greenwald MK, Comer SD, Fiellin DA. Buprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: implications for clinical use and policy. Drug Alcohol Depend. 2014;144:1-11. PubMed CrossRef
29.Lembke A, Ottestad E, Schmiesing C. Patients maintained on buprenorphine for opioid use disorder should continue buprenorphine through the perioperative period. Pain Med. 2019;20(3):425-428. PubMed CrossRef
30.Volpe DA, McMahon Tobin GA, Mellon RD, et al. Uniform assessment and ranking of opioid μ receptor binding constants for selected opioid drugs. Regul Toxicol Pharmacol. 2011;59(3):385-390. PubMed CrossRef
31.Raffa RB, Haidery M, Huang HM, et al. The clinical analgesic efficacy of buprenorphine. J Clin Pharm Ther. 2014;39(6):577-583. PubMed CrossRef
32.Khanna IK, Pillarisetti S. Buprenorphine—an attractive opioid with underutilized potential in treatment of chronic pain. J Pain Res. 2015;8:859-870. PubMed
33.Brown SM, Holtzman M, Kim T, et al. Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active. Anesthesiology. 2011;115(6):1251-1260. PubMed
34.Highlights of Prescribing Information: Sublocade (buprenorphine extended-release) injection for subcutaneous use CIII. Sublocade website; p 30, line 766. https://www.sublocade.com/Content/pdf/prescribing-information.pdf. Revised April 2019.
*The suggested doses of 4 mg and 8 mg are influenced by the available dosing of sublingual products such as Suboxone.
This PDF is free for all visitors!
Save
Cite