ABSTRACT
Objective: To examine whether a positive urine drug of abuse screen in youth who receive medical care is associated with subsequent risk of external mortality (eg, overdose, suicide, homicide, accident).
Methods: This was a population-based retrospective cohort study of all Olmsted County (Minnesota) residents who were 13–18 years of age at the time of urine drug screen (UDS) testing (January 1, 1999, to November 28, 2012). Cox regression models were used to examine the relationships between having a positive UDS and external mortality, adjusted for sex, race, age, alcohol exposure, psychiatric diagnoses as defined by the International Classification of Diseases (ICD-9/ICD-10), and medical setting of UDS testing. Separate analyses were done for (1) overall UDS results, (2) tetrahydrocannabinol (THC), and (3) cocaine.
Results: Of the 2,772 teenagers included in this study (47.2% male), a total of 26 died of external causes during a median follow-up period of 11.8 years. Testing positive for any illicit substance was not associated with significantly increased risk of external mortality (hazard ratio [HR] = 1.9; 95% CI, 0.9–4.2). Testing positive for cocaine was associated with significantly increased risk of external mortality (HR = 7.0; 95% CI, 1.9–25.0). Testing positive for THC was associated with a marginally significantly increased risk of external mortality (HR = 2.1; 95% CI, 1.0–4.7); however, when cocaine was added as a covariate in the analysis, the relationship between THC-positive UDS and mortality was still elevated but was no longer statistically significant (HR = 1.8; 95% CI, 0.8–4.1).
Conclusions: History of cocaine-positive UDS may help identify a population of young people who are at high risk of premature death.
J Clin Psychiatry 2022;83(1):20m13729
To cite: Markota M, Croarkin PE, Bobo WV. Positive urine drug screens and external mortality in teenagers who present for medical care. J Clin Psychiatry. 2022;83(1):20m13729.
To share: https://doi.org/10.4088/JCP.20m13729
© Copyright 2022 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry & Psychology, Mayo Clinic, Rochester, Minnesota
bDepartment of Psychiatry & Psychology, Mayo Clinic, Jacksonville, Florida
*Corresponding author: Matej Markota, MD, Department of Psychiatry & Psychology, Mayo Clinic, 200 First St SW, Rochester MN, 55905 ([email protected]).
Over the past decade, deaths from accidental drug overdoses have increased to the extent that life expectancy in the US has started to decline.1,2 The high prevalence of illicit substance use among teenagers makes it challenging to ascertain which substance-using youth are at high risk for early mortality.3,4 Even for cannabis—the most frequently used illicit drug—several reviews have concluded that there is insufficient evidence to determine whether an association exists between cannabis use and an increased risk for all-cause mortality.5,6 More recently, increased mortality was reported among cannabis-using youth with mood disorders.7 Moreover, a recent meta-analysis8 raised additional concerns for a specific association between cannabis use and suicidal behavior in youth. Significantly increased risk of cardiovascular disease mortality in adults has also been reported, particularly in those who started using cannabis as teenagers.9 A clearer understanding of the relationship between substance use and mortality risk in youth is needed.
Current practices for estimating the risk for mortality in teenage substance users rely heavily on clinical intuition. There is an urgent need for evidence-based methods to stratify the risk of early mortality in youth who are abusing substances, including which specific substances are associated with the greatest risk.10 Patient-reported patterns of substance use have shown only limited success in predicting mortality among people who use substances.11 Among teenagers, substance use disclosure is often unreliable, and corroboration by routine biochemical tests has been recommended in this population.12–14 Recent studies on drug overdoses call for improved efforts to screen for drug use and better understand the implications of screening results.15
At present, it is unknown if results of biochemical drug testing can be used to predict mortality in teenagers. The aim of this study was to examine the association between testing positive for any illicit substance, for cocaine, and for tetrahydrocannabinol (THC) on a routine urinary drug screen (UDS) and early mortality from drug overdose, suicide, homicide, or accidents (subsequently referred to as “external mortality”) in a geographically defined population-based cohort of youth, aged 13–18 years, who present for medical care.
METHODS
Data Source
The medical records linkage system of the Rochester Epidemiology Project (REP; https://rochesterproject.org/) was used to identify all Olmsted County residents between the ages of 12 and 18 years who had a UDS between January 1, 1998, and November 28, 2012. Adolescents with no parental research authorizations on file were excluded (< 4% of the overall population).16 Data on causes and dates of death were collected electronically through REP. The REP captures nearly the entire population of Olmsted County and has been described in detail elsewhere.16–19 Of note, no additional research authorization is required for REP to receive death certificates; these are sent to the REP by the state and are linked to the REP record for all county residents. This study was approved by the Institutional Review Boards of the Mayo Clinic and the Olmsted Medical Center.
Study Cohort
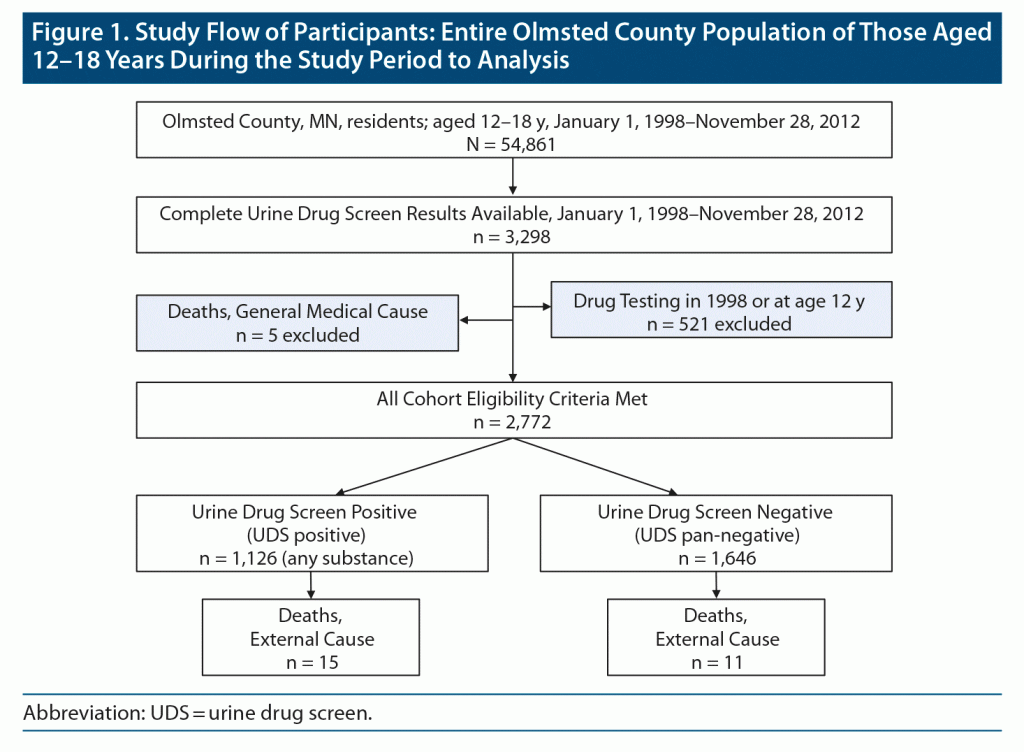
The initial cohort consisted of 3,298 Olmsted County residents who had a UDS completed between January 1, 1998, and November 28, 2012, and were 12–18 years old at the time of UDS testing. The beginning of follow-up was defined as the date of the first qualifying UDS test (see the “Follow-Up and Endpoints” section later in the Methods). To increase the likelihood that the follow-up period would start on the date of the first UDS test (rather than on the date of a repeat UDS test), we introduced a 1-year “washout period” that excluded all cohort members who had a UDS between January 1, 1998, and December 31, 1998 (n = 393), and those who were tested at the age of 12 years (n = 128). The average age at the first UDS in this cohort was 16.3 years, and the average time window between the first UDS and end of testing eligibility for this study was 2.6 years. Given a mean of 3.2 tests per cohort member, the washout period of 1 year in this study approximated the average time between 2 UDS tests in this cohort (1.2 tests per year). Cohort members who died of general medical causes (eg, malignancy, heart disease, lung disease) were also excluded (n = 5). The final cohort consisted of 2,772 youth (Figure 1).
Urine Drug Screens
Results of all UDSs between ages 13 and 18 years were electronically abstracted from automated REP laboratory result files that contained the dates, times, and results of all UDSs. Cohort members with any positive UDS during this time window were classified as being positive UDS cohort members. Cohort members with no positive UDS between ages 13 and 18 years were classified as being pan-negative UDS cohort members. Routine UDSs with results available for amphetamines, barbiturates, benzodiazepines, cocaine, opiates, phencyclidine, and THC (most cohort members also had alcohol results available; see the “Covariates” section later in the Methods) were included in this study, whereas targeted UDSs for only one substance were not, given our goal of examining the association between a positive routine UDS and mortality. However, if a positive UDS was followed by a confirmatory test, the latter test was included as a final result (eg, if a screening UDS was positive for opioids but confirmatory testing for opioids was negative, this was counted as a negative result).
REP prescription records were ascertained for prescriptions of medications to cohort members that could cause false-positive drug screens, as specified by Moeller and colleagues in 200820 and 2017.21 All cohort members who received a prescription for a medication that could potentially yield false-positive results on a UDS and who received enough medications to last until 3 months prior to testing positive were classified as UDS negative (n = 52) (Supplementary Table 1 lists the percentages of patients with positive UDS screens). Two secondary analyses were performed using original data without prescription record–based reclassification as a test of robustness (see the “Substance-Specific UDS Results and External Mortality” section in the Results).
Follow-Up and Endpoints
The beginning of follow-up was defined as the date of the first qualifying UDS test between 13 years 0 days and 18 years 364 days of age. The end of follow-up was defined as November 15, 2017, or the date of death, whichever came first. Electronic death certificates were reviewed to determine death from external causes versus death from general medical conditions. Death certificates were available for 28 of the 31 deceased cohort members. Of these, 23 died of external causes and 5 died of general medical conditions (Figure 1). All available diagnosis codes and medical records were reviewed for the 3 deceased cohort members with missing death certificates. All 3 were included in the final cohort, given the absence of any evidence of a medical illness that could have explained their premature deaths.
Covariates
Psychiatric comorbidity data were collected electronically from REP using International Classification of Diseases (ICD) diagnosis codes. ICD-9 codes were available for diagnoses made between January 1, 1998, and June 1, 2015, whereas ICD-10 codes were available for diagnoses made after September 29, 2016. Between June 1, 2015, and September 29, 2016, there was a transition period during which patients received either ICD-9 or ICD-10 codes. Diagnosis codes that appeared in the REP electronic files from each cohort member’s 13th birthday until 364 days after their 18th birthday were used to define diagnosed mental health conditions (Supplementary Table 2). Cohort members were divided into 2 groups defined by having (n = 2,357) or not having (n = 415) at least one ICD-9/10 diagnosis. Because substance-related ICD disorders and UDS results are closely intertwined, we did not include substance-related disorders as covariates. From the REP electronic indices, we also collected data on sex, age at each UDS, self-identified race, and results of urine, blood, or breathalyzer screening tests for alcohol (available for 2,641 cohort members, classified as positive or negative) between ages 13 and 18 years. As a proxy for the medical setting in which the index UDS was performed, we grouped cohort members into those with UDS done in an academic setting (Mayo Clinic, the largest medical provider in the county) and those with UDS done by other community providers.
Statistical Analysis
Sequential tests of the associations between UDS results and external mortality were performed. First, unadjusted associations between UDS results (positive, negative) and external mortality were estimated using Cox proportional hazard models (model #1). The analyses were repeated using the following models: model #2, adjusting for age at qualifying UDS, sex, and race; model #3, adjusting for covariates in model 2 plus alcohol use; and model #4, adjusting for covariates in model 3 plus ICD-9/10 evidence of psychiatric diagnosis and type of medical setting. Kaplan-Meier methods were used to compute survival estimates and curves. Kaplan-Meier curves were examined to ensure proportional hazards assumption was met. JMP statistical software (version 14.1.0; SAS Corporation; Cary, North Carolina) was used to perform all statistical analyses. The threshold for statistical significance level was adjusted to .02 to account for multiple (m = 3) comparisons using the method of Bonferroni.
RESULTS
Demographic and Clinical Characteristics
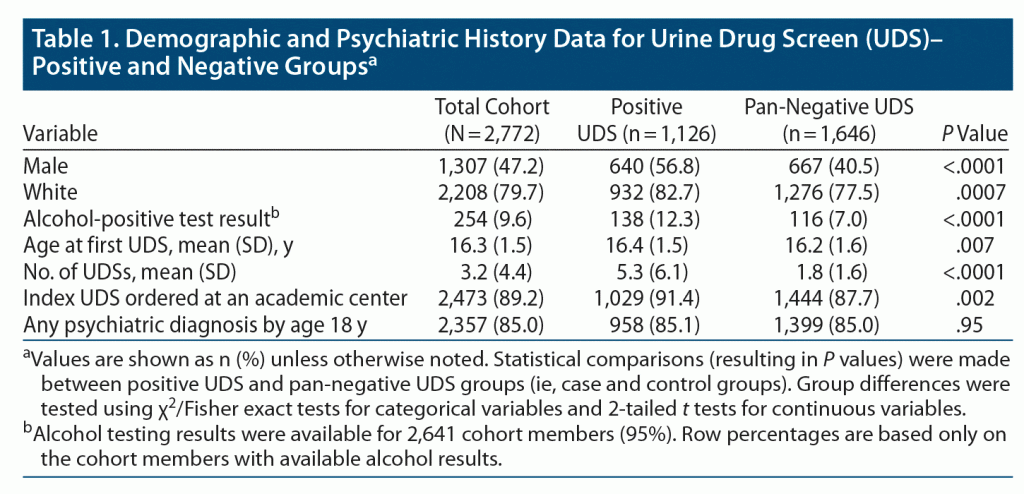
The 2,772 cohort members in this study contributed 32,694 person-years of follow-up (average duration of follow-up of 11.8 years). The mean age of cohort members at the time of index UDS was 16.3 years (Table 1). A total of 1,126 cohort members (40.6%) were classified as being UDS-positive, and 1,646 were classified as being pan-negative.
Mortality and Causes of Death
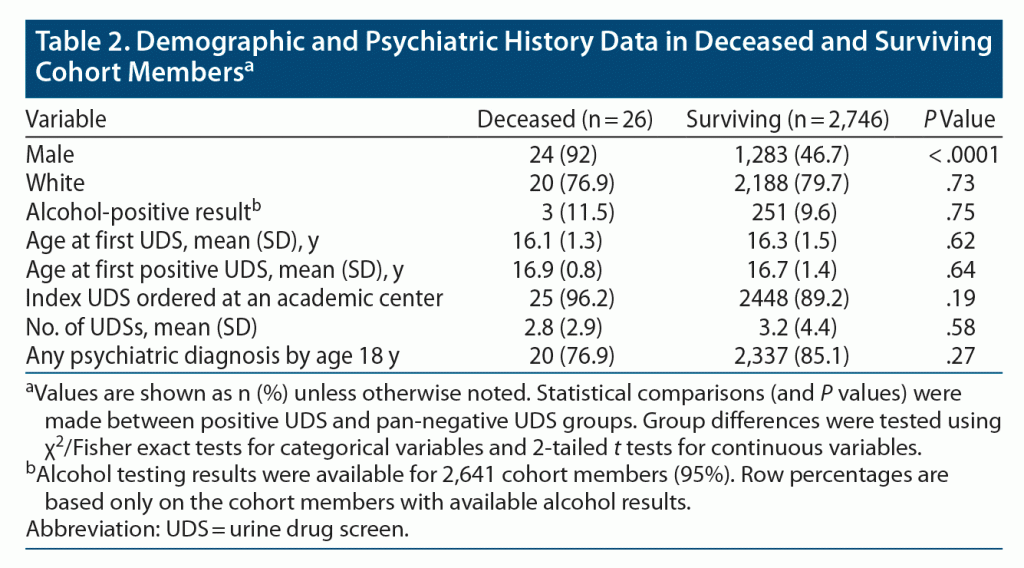
Twenty-six cohort members were classified as having died of external causes during the follow-up period, with a mortality incidence rate of 79.5 per 100,000 person-years. The mean time between the first positive UDS to death was 6 years. The majority of deaths (24/26; 92%) occurred in males (Table 2). Both of the deceased females had negative UDS results during the study period. There were no significant differences with regard to race, alcohol results, age at first UDS, age at first positive UDS, setting of medical testing, number of UDSs, or psychiatric comorbidity between surviving and deceased cohort members (Table 2). External causes of death were from suicides (n = 9, all male), lethal accidents (n = 7, 6 male), overdoses on drugs of abuse (n = 6, 5 male), and other types of external deaths (n = 4, all male). Five of the 6 lethal drug overdoses involved concurrent toxicities from multiple substances. Four overdose deaths included opioid toxicity, though only 1 of these cohort members had a positive UDS for opioids prior to death; interestingly, 2 opioid-related deaths occurred in youth who previously tested positive for cocaine. Fifteen (57.7%) of the 26 deceased cohort members had a UDS positive for THC, and 3 (11.5%) were positive for cocaine.
UDS Results and External Mortality
Testing positive for any substance between the ages of 13 and 18 years (1,126 positive and 1,646 negative) was not associated with significantly increased risk of external mortality (hazard ratio [HR] = 1.9; 95% CI, 0.9–4.2).
Two secondary analyses were conducted to address cohort members who were receiving prescription drugs that can produce a positive UDS. First, we tested for an association between mortality and UDS results without reclassification of positive UDS from prescribed substances (1,178 positive and 1,594 negative), which yielded similar findings as the main analysis (HR = 1.8; 95% CI, 0.8–3.9). We then entirely excluded 52 cohort members with a positive UDS and a concurrent prescription for medications that can produce a positive UDS and compared 1,126 UDS-positive to 1,594 UDS-negative cohort members and again obtained similar results (HR = 1.9; 95% CI, 0.9–4.1). There were no significant associations between UDS-positive results and external mortality in any of the multivariable models (data not shown).
Substance-Specific UDS Results and External Mortality
Three of 58 cohort members with a positive UDS result for cocaine died of external causes during follow-up (all male). None of these 3 decedents had evidence of cocaine ingestion at the time of death. Two of the 3 deaths in cocaine-positive youth were due to lethal drug overdoses that included opioids, and 1 was by a motor vehicle accident. All 3 cocaine-positive youth who died had also previously tested positive for THC, but not any other substance. Testing positive for cocaine (n = 58) was associated with a large and significantly increased risk of external mortality compared to negative-UDS (n = 1,646) cohort members (HR = 7.0; 95% CI, 1.9–25.0). Testing positive for cocaine remained significantly associated with external mortality after adjusting for age at first positive UDS, sex, and race (HR = 6.3; 95% CI, 1.7–23.1) (model #2); after further adjustment for alcohol results (HR = 6.1; 95% CI, 1.7–22.4) (model #3); and further adjustment for psychiatric comorbidity and medical setting of testing (HR = 6.1; 95% CI, 1.6–22.7) (model #4) (see Supplementary Table 3 for demographic and psychiatric history data for cocaine-positive and -negative youth).
Of the 1,019 cohort members with a positive UDS for THC, 15 died of external causes during follow-up. Testing positive for THC (n = 1,019) was associated with a marginally significant increase in external mortality compared to UDS-negative cohort members (n = 1,646) (HR = 2.1; 95% CI, 1.0–4.7); however, 176 cohort members who tested positive for THC also had positive UDS for other substances, including cocaine (n = 48) (see Supplementary Table 4 for demographic and clinical data for THC-positive and THC-negative youth). When cocaine was added as a covariate in the analysis, THC-positive UDS was still elevated but was no longer significantly associated with increased mortality (HR = 1.8; 95% CI, 0.8–4.1), while cocaine remained significantly associated with external mortality (HR = 4.7; 95% CI, 1.3–16.8). Similar results were obtained after restricting the analysis to the 843 cohort members with a positive UDS only for THC (HR = 1.9; 95% CI, 0.8–4.4).
Of note, the mean age for testing positive for THC was 16.7 years, and 17.3 for cocaine. On average in those who tested positive for THC and cocaine by 18 years of age, THC-positive UDS preceded cocaine-positive UDS by an average of 187 days. Those with THC- and cocaine-positive UDS compared to those with THC only–positive UDS were marginally more likely to have a psychiatric diagnosis (odds ratio [OR] = 4.2; 95% CI, 1.0–17.6); there was no difference between the 2 groups in age at first THC-positive UDS, sex, race, setting of UDS testing, or alcohol results.
Drug-specific associations between external mortality and positive UDS results for amphetamine, opioid, benzodiazepine, barbiturate, and phencyclidine were not tested due to low frequencies of deaths in teenagers who tested positive for the aforementioned substances and/or low frequencies of positive UDSs for these substances (see Supplementary Table 1).
DISCUSSION
This population-based study of youth who received drug testing as part of their routine medical care found no statistically significant association between testing positive for any substance and external mortality. However, cohort members who tested positive for cocaine had a pronounced increase in risk of premature death. There was no statistically significant effect of testing positive for THC and external mortality after accounting for the effects of testing positive for cocaine.
Several of our findings are consistent with results of prior research on substance use in youth. For example, the proportion of cohort members with at least 1 positive UDS during the follow-up period in this study (approximately 40%) is consistent with previously reported substance use rates in the general teenage population, and the age at first THC-positive UDS was similar to self-reported age at first cannabis use in the US population.3,22
This study provides new data on the risk of external mortality in youth with a positive UDS for cocaine. We found significantly increased risk of external mortality in cohort members who tested positive for cocaine as compared to youth with negative UDS results. The association between having a UDS-positive result for cocaine and external mortality remained both large and statistically significant after adjusting for important potential sources of confounding, including sex, race, age at UDS, alcohol results, psychiatric comorbidity, and medical setting of UDS testing. In broad strokes, results presented here are complimentary to findings from previous studies on mortality in cocaine users. A recent study based on national data15 found that rates of cocaine use in the general population do not mirror rates of cocaine-related deaths, suggesting a complex relationship between the two. Our findings of cocaine users’ being at higher risk of dying from non–cocaine-related external causes further add to this complexity. Cocaine is perceived as a dangerous drug by the majority of US teenagers; therefore, it may be the case that cocaine use occurs more commonly in individuals who engage in risky and potentially life-threatening cocaine- and non–cocaine-related behaviors.23 Consistent with previous studies, the present REP study also observed the role opioids have in causing death in cocaine users.15,24,25 Combined, this study and others suggest that, in trying to identify individuals at risk of lethal overdose (including opioid-related overdose), the focus should include not only evidence of opioid use but also a history of cocaine use, the latter of which may be a “red flag” for future opioid-related deaths.15,24,26
This study also provides novel data on the relationship between having a positive UDS for THC and external mortality in youth. As a group, THC-positive teenagers were at an increased risk of external mortality in our study; however, this association was no longer significant after controlling for cocaine UDS results. While this study does not document a statistically significant increase in external mortality associated with a positive THC UDS, it is important to note that there was a (nonsignificant) trend toward increase in mortality (eg, as evidenced by HR = 1.9; 95% CI, 0.8–4.4 in 843 cohort members with THC-only positive UDS), and it is quite possible that in a larger cohort a significant difference could be found.
Limitations
Previous studies have shown superiority of biochemical tests over clinical self-reporting for detecting substance use in teenagers, particularly for cocaine.12–14 However, testing by UDS is not “routine” in the same sense that measuring height/weight is, and only a minority of Olmsted County youth were tested with a UDS (Figure 1). UDS testing tends to be done in the setting of diagnosing or treating psychiatric or chemical dependency–related problems. The control group of pan-negative UDS cohort members was therefore not representative of the general teenage population, as it was “higher risk” just by virtue of having had a clinical concern leading to a UDS test. The design and findings of this study are relevant primarily to clinicians working in settings where UDSs of adolescents are part of the “routine” clinical care, such as inpatient or outpatient child and adolescent psychiatrists. Finally, Olmsted County, Minnesota, had some of the lowest rates of opioid use in the US during the study period.27 This may have contributed to the relatively low number of opioid-positive teenagers in this study and may limit the generalizability of our results to regions with higher rates of opioid misuse. Of note, we observed slightly more opioid-positive UDS cohort members than cocaine-positive UDS cohort members in our study, yet there were more deaths in cocaine-positive youth. Conversely, at time of death, opioid toxicity was more common than evidence of cocaine toxicity. Overall, this suggests that to identify youth who are at high risk of death by overdose, it is insufficient to focus only on identifying substance use that was deemed lethal at time of overdose. Instead, identifying other “red flags” may also be helpful.
Our findings raise the question of whether broad UDS screening across multiple environments, including non-clinical settings or clinical settings where they are historically not commonly used, can be of benefit for reducing early mortality. We do not believe that our results should be interpreted as supporting such an expansion. As with any other medical test, using drug toxicology tests can have unintended consequences.28 The authors believe that effects of UDS testing are heavily dependent on the context within which they are used. UDSs can provide diagnostic clarification and aid in treatment and recovery, which would be beneficial for the tested individual; however, same tests can yield false-positive results leading to unnecessary care or have undue consequences (eg, restriction of employment or educational opportunities) without offering access to help and treatment.
In conclusion, in a geographically defined population of youth who received a UDS during routine health care, having a UDS positive for cocaine was associated with increased risk of premature death, whereas testing positive for any illicit substance or for THC was not. History of cocaine-positive UDS may help clinicians identify youth who are at higher risk of premature death.
Submitted: October 22, 2020; accepted August 18, 2021.
Published online: January 11, 2022.
Potential conflicts of interest: Dr Markota has received a training research grant from the National Institute on Drug Abuse and American Academy of Child and Adolescent Psychiatry (NIDA-AACAP). Dr Croarkin has received research grant support from Pfizer, the National Institute of Mental Health (NIMH), the Brain and Behavior Research Foundation, and Mayo Clinic. He has received equipment support from Neuronetics and MagVenture for investigator-initiated studies. He received supplies and genotyping services from Assurex Health for investigator-initiated studies. He is a primary investigator for a multicenter study funded by Neuronetics and a site primary investigator for a study funded by NeoSync. He has served as a paid consultant for Engrail Therapeutics, Myriad Neuroscience, Procter & Gamble, and Sunovion. Dr Bobo has received research support from NIMH, Agency for Healthcare Research and Quality, and the Mayo Foundation for Medical Education and Research.
Funding/support: This work was funded by the 2017 NIDA-AACAP Resident Training Award in Substance Use Disorders awarded to Dr Markota. Mentorship and guidance on this research project was received at the 2018 Research Colloquium for Junior Investigators, which was partially funded by the NIDA of the National Institutes of Health (NIH) under Award Number R13DA042568. This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the NIH under Award Number R01AG034676 (Co-PIs: Dr Walter A. Rocca and Dr Jennifer St. Sauver). Statistical support was received by Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS). Research reported in this publication was also supported by the NIH under award R01 MH113700 by supporting research time for Dr Croarkin The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, National Institute on Drug Abuse, Rochester Epidemiology Project, or the American Academy of Child and Adolescent Psychiatry.
Role of the sponsor: NIDA-AACAP funding was used for data collection and processing; NIDA-AACAP had no influence on the conduct and publication of the study after the grant was accepted for funding. The NIH and NCATS had no direct influence on the conduct and publication of the study.
Previous presentation: Preliminary results of this study were presented as an abstract at the American Academy of Child and Adolescent Psychiatry’s 65th Annual Meeting; October 22–27, 2018; Seattle, Washington.
Additional information: Access to data presented in this article is not publicly available, as data contain identifiable and protected information.
Supplementary material: Available at Psychiatrist.com.
CLINICAL POINTS
- Urine drug screens are frequently used, but the prognostic value of positive drug screens is not fully understood, particularly among youth.
- These results suggest that youth who test positive for cocaine are at a higher risk of external mortality, whereas positive drug screens for cannabis did not predict mortality in youth.
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Childhood and Adolescent Mental Health section. Please contact Karen D. Wagner, MD, PhD, at [email protected].
References (28)

- Redfield RR. CDC Director’s Media Statement on US Life Expectancy. CDC website. https://www.cdc.gov/media/releases/2018/s1129-US-life-expectancy.html. Published 2018. Accessed June 29, 2019.
- Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959–2017. JAMA. 2019;322(20):1996–2016. PubMed CrossRef
- Johnston LDM, O’Malley RA, Bachman PM, et al. Monitoring the Future National Survey Results on Drug Use 1975–2018: Overview, Key Findings on Adolescent Drug Use. http://www.monitoringthefuture.org//pubs/monographs/mtf-overview2018.pdf. Published January 2019. Accessed June 10, 2020.
- Eaton DK, Kann L, Kinchen S, et al; Centers for Disease Control and Prevention (CDC). Youth risk behavior surveillance—United States, 2011. MMWR Surveill Summ. 2012;61(4):1–162. PubMed
- National Academies of Sciences Engineering and Medicine (US). Committee on the Health Effects of Marijuana. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press; 2017. https://www.ncbi.nlm.nih.gov/books/NBK423845/.
- Calabria B, Degenhardt L, Hall W, et al. Does cannabis use increase the risk of death? systematic review of epidemiological evidence on adverse effects of cannabis use. Drug Alcohol Rev. 2010;29(3):318–330. PubMed CrossRef
- Gobbi G, Atkin T, Zytynski T, et al. Association of cannabis use in adolescence and risk of depression, anxiety, and suicidality in young adulthood: a systematic review and meta-analysis. JAMA Psychiatry. 2019;76(4):426–434. PubMed CrossRef
- Fontanella CA, Steelesmith DL, Brock G, et al. Association of cannabis use with self-harm and mortality risk among youths with mood disorders. JAMA Pediatr. 2021;175(4):377–384. PubMed CrossRef
- Sun Y, Liu B, Wallace RB, et al. Association of cannabis use with all-cause and cause-specific mortality among younger- and middle-aged US adults. Am J Prev Med. 2020;59(6):873–879. PubMed CrossRef
- Clark DB, Martin CS, Cornelius JR. Adolescent-onset substance use disorders predict young adult mortality. J Adolesc Health. 2008;42(6):637–639. PubMed CrossRef
- Gjersing L, Bretteville-Jensen AL. Patterns of substance use and mortality risk in a cohort of ‘hard-to-reach’ polysubstance users. Addiction. 2018;113(4):729–739. PubMed CrossRef
- Dillon FR, Turner CW, Robbins MS, et al. Concordance among biological, interview, and self-report measures of drug use among African American and Hispanic adolescents referred for drug abuse treatment. Psychol Addict Behav. 2005;19(4):404–413. PubMed CrossRef
- Williams RJ, Nowatzki N. Validity of adolescent self-report of substance use. Subst Use Misuse. 2005;40(3):299–311. PubMed CrossRef
- Harrison L, Hughes A. Introduction–the validity of self-reported drug use: improving the accuracy of survey estimates. NIDA Res Monogr. 1997;167:1–16. PubMed
- Cano M, Oh S, Salas-Wright CP, et al. Cocaine use and overdose mortality in the United States: evidence from two national data sources, 2002-2018. Drug Alcohol Depend. 2020;214:108148. PubMed CrossRef
- St Sauver JL, Grossardt BR, Yawn BP, et al. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173(9):1059–1068. PubMed CrossRef
- Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. PubMed CrossRef
- St Sauver JL, Grossardt BR, Finney Rutten LJ, et al. Rochester Epidemiology Project Data Exploration Portal. Prev Chronic Dis. 2018;15:E42. PubMed CrossRef
- St Sauver JL, Grossardt BR, Leibson CL, et al. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151–160. PubMed CrossRef
- Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66–76. PubMed CrossRef
- Moeller KE, Kissack JC, Atayee RS, et al. Clinical interpretation of urine drug tests: what clinicians need to know about urine drug screens. Mayo Clin Proc. 2017;92(5):774–796. PubMed CrossRef
- Ahrnsbrak RBJ, Hedden SL, Lipari RN, et al. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: Results from the 2016 National Survey on Drug Use and Health Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. SAMHSA website. https://www.samhsa.gov/data/. Published 2017. Accessed December 17, 2018.
- Miech RA, Johnston LD, O’Malley PM, et al. Monitoring the Future National Survey Results on Drug Use, 1975–2017. Volume I. Ann Arbor: Institute for Social Research, The University of Michigan. http://www.monitoringthefuture.org//pubs/monographs/mtf-vol1_2017.pdf. Published 2018. Accessed March 7, 2019.
- O’Driscoll PT, McGough J, Hagan H, et al. Predictors of accidental fatal drug overdose among a cohort of injection drug users. Am J Public Health. 2001;91(6):984–987. PubMed CrossRef
- McCall Jones C, Baldwin GT, Compton WM. Recent increases in cocaine-related overdose deaths and the role of opioids. Am J Public Health. 2017;107(3):430–432. PubMed CrossRef
- Peacock A, Tran LT, Larney S, et al. All-cause and cause-specific mortality among people with regular or problematic cocaine use: a systematic review and meta-analysis. Addiction. 2020;116(4)725–742. PubMed
- Centers for Disease Control and Prevention. US County Opioid Dispensing Rates, 2006. CDC website. https://www.cdc.gov/drugoverdose/rxrate-maps/county2006.html. Accessed December 23, 2021.
- Phan HM, Yoshizuka K, Murry DJ, et al. Drug testing in the workplace. Pharmacotherapy. 2012;32(7):649–656. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite