Prevalence of Negative Symptoms in Outpatients With Schizophrenia Spectrum Disorders Treated With Antipsychotics in Routine Clinical Practice: Findings From the CLAMORS Study
Objective: To analyze the prevalence of negative symptoms in antipsychotic-treated outpatients with schizophrenia spectrum disorders.
Method: A cross-sectional, retrospective multicenter study was carried out between May 2004 and April 2005 in 1,704 adult psychiatric outpatients meeting DSM-IV criteria for schizophrenia, schizophreniform, or schizoaffective disorder. We used 5 items of the Positive and Negative Syndrome Scale (PANSS) negative symptoms subscale to individually determine the presence of a negative symptom when the score on the item was > 3. Primary negative symptoms were considered present when patients fulfilled all of the following: > 3 score on the corresponding item; < 3 score on any positive item; no extrapyramidal symptoms; ≤ 3 score on anxiety and depression items; dose of haloperidol, when applicable, ≤ 15 mg/d; and no antiparkinsonian treatment.
Results: A total of 1,452 evaluable patients (863 men, 60.9%), 40.7 ± 12.2 (mean ± SD) years of age, were included. One or more negative symptoms were present in 57.6% of patients, with primary negative symptoms in 12.9% of subjects. The most frequent negative symptom items were social withdrawal (45.8%), emotional withdrawal (39.1%), poor rapport (35.8%), and blunted affect (33.1%). Negative symptoms (1-blunted affect, 2-emotional withdrawal, 3-poor rapport, 4-social withdrawal, 5-verbal fluency) were most associated with maleness (symptom 4); age > 40/45 years (men/women; symptoms 1,2,4); single/unmarried status (symptoms 2-4); unemployment (symptoms 3,4); higher score on the Clinical Global Impressions (CGI) scale and PANSS total score (symptoms 1-5); lower score on the PANSS positive symptoms subscale (symptoms 1,3); more than 52 weeks of treatment (symptoms 1-3,5); and high antipsychotic dose (symptom 2).
Conclusions: The prevalence of negative symptoms in patients with schizophrenia spectrum disorders treated with antipsychotics in routine clinical practice not only is still considerably high but also seems to be related to poorer functioning, unemployment, greater severity, and less positive symptomatology and higher antipsychotic dose.
J Clin Psychiatry 2010;71(3):280-286
© Copyright 2010 Physicians Postgraduate Press, Inc
Submitted: March 28, 2008; accepted December 9, 2008.
Online ahead of print: November 3, 2009 (doi:10.4088/JCP.08m04250yel).
Corresponding author: Julio Bobes, MD, PhD, Medicine Department, Psychiatry Area, University of Oviedo, Avenida Julián Claver×a 6, 33006, Oviedo, Asturias, Spain ([email protected]).
Notice of correction 6/9/2011: The key to Figure 1 on page 283 has been corrected—the gray square represents "No negative symptom" and the black square represents "At least one negative symptom."
For a long time, research efforts in schizophrenia were generally directed toward improvement of positive symptoms, those that are more directly related to the safety of the patient and those around him or her. However, negative symptoms are undoubtedly critical to a patient’s quality of life and particularly crucial to his or her social life. The psychiatric scientific community is now focusing efforts on improving knowledge and appropriate management of these types of symptoms. Negative symptoms are intrinsic to the pathology of schizophrenia and are associated with significant deficits in motivation, verbal and nonverbal communication, affect, and cognitive and social functioning,1 which, in turn, contribute to poor outcome and functioning in schizophrenia.2,3 The underlying mechanisms of negative symptoms are not well understood, which complicates the search for a therapeutic arsenal with a known mechanism of action. Investigation of the different entities characterized by negative symptoms, such as persistent primary negative symptoms, in addition to enhancing our understanding of the pathophysiology of this core dimension of the disease, may help to unravel the psychopathologic and biologic heterogeneity of schizophrenia.4
Persistence of negative symptoms has received recent attention in terms of developing clinical trials to assess different mechanisms of action to treat a dimension of the disease.5,6 One of the major problems when assessing drug efficacy in negative symptoms is the lack of distinction between primary and secondary negative symptoms.5,7 On the basis of some studies, the scientific literature has been claiming that second generation antipsychotics (SGAs) are more effective than conventional antipsychotics in the treatment of negative symptoms.8 However, the initial optimism that SGAs would prove to be powerful agents to improve negative symptoms has given way to relative pessimism.4,9-11 Although SGAs seem to be effective for secondary negative symptoms, they have shown no efficacy for primary negative symptoms, and new mechanisms of drug action appear to be required to address this aspect of the disease syndrome.12 Therefore, we expect a high prevalence of negative symptoms, even in patients treated with SGAs.
This article examines the prevalence of negative symptoms within the framework of a large cross-sectional study assessing cardiovascular, lipid, and metabolic outcomes in patients with schizophrenia, schizophreniform, or schizoaffective disorders treated with the antipsychotics most commonly used in daily practice, most of them SGAs (Cardiovascular, Lipid, and Metabolic Outcomes Research in Schizophrenia [CLAMORS] Study13).
METHOD
Investigators, Patients, and Design
The methods of the CLAMORS study have been published in detail elsewhere.13 Briefly, this was a cross-sectional, retrospective, multicenter study enrolling consecutive adult outpatients of both sexes, ages 18-74 years, with a diagnosis of schizophrenia, schizophreniform, or schizoaffective disorder according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) classification, and who received oral antipsychotic treatment for at least 12 weeks with any 1 of the following antipsychotic drugs: risperidone, olanzapine, quetiapine, ziprasidone, amisulpride, or haloperidol. Patients receiving treatment with 2 or more antipsychotics at the time of evaluation and/or those admitted to the hospital were excluded. An accredited Clinical Research Ethics Committee from 1 of the participating centers approved the study protocol. Written informed consent was obtained from all patients prior to enrollment.
According to the study protocol, each participating center was to recruit at least the first 12 consecutive patients meeting the selection criteria. The first 2 patients receiving treatment with each of the most commonly used antipsychotic drugs in our health care setting (risperidone, olanzapine, quetiapine, ziprasidone, amisulpride, and haloperidol) were consecutively invited to participate in the study.
Variables and Measurement Instruments
Prevalence of negative symptoms. Clinical severity and, particularly, the prevalence of negative symptoms were determined using the schizophrenia Positive and Negative Syndrome Scale (PANSS).14,15 Negative symptoms were assessed using 5 items of the PANSS negative symptoms subscale16: 1—blunted affect; 2—emotional withdrawal; 3—poor rapport; 4—social withdrawal; and 5—verbal fluency. Presence of a negative symptom was defined as severity score > 3 (on a response scale ranging from 1—absence of symptom to 7—extremely severe symptom). In addition to a severity score > 3, a primary negative symptom was considered present when patients fulfilled all of the following criteria: absence of positive symptoms (defined as a score < 3 for any positive symptom); absence of extrapyramidal symptoms (EPS) as assessed by psychiatrist interview; items 2 (anxiety) and 6 (depression) on the General Psychopathology PANSS subscale ≤ 3; dose of haloperidol not higher than 15 mg/d; and no antiparkinsonian treatment. In addition to the presence of negative symptoms, an overall negative symptoms PANSS subscore was calculated by the sum of the scores of these 5 items. The decision to use 5 items of the PANSS negative symptoms subscale was taken because 2 of the symptoms on the original 7-item subscale—difficulty in abstract thinking and stereotyped thinking—are consistently found not to be part of a negative symptoms factor.16
In order to ensure the accuracy of the data, psychiatrists were trained in the use of the PANSS scale. Accordingly, the study patient recruitment process began with clear instructions on the diagnostic criteria for the different schizophrenia spectrum disorders. This was done for all participating psychiatrists. These instructions dealt mainly with the standard psychiatric interview of the DSM-IV diagnostic criteria (anamnesis and exploration of mental condition), the PANSS scale, and the different study inclusion/exclusion criteria, along with other aspects of the study. Also, recruitment and diagnosis was carried out only by experienced psychiatrists belonging to the Spanish National Health System, in which all patients were followed. In Spain, National Health System positions require passing a high-level standard examination, which ensures that personnel have uniform expertise. In addition, all the physician participants in the CLAMORS study fulfilled the following criteria: certified psychiatrist working in out-patient mental health center facility for 3-4 years with previous experience in trials in the field of schizophrenia disorders.
Other measurements. Sociodemographic and clinical data were recorded including marital status, occupational status, body mass index (BMI), disease duration, and number of previous hospital admissions. These data were collected by the psychiatrists from the medical record (BMI, disease duration, and number of previous hospital admissions) or by patient interview (marital status, occupational status). Clinical severity was also determined using the Clinical Global Impressions-Severity scale (CGI-S).17
Statistical Analysis
A safety-only sample of evaluable patients was used for the analyses, including all patients receiving 1 of the 6 aforementioned antipsychotic drugs. Frequencies and percentages were used for qualitative variables and to estimate the prevalence of overall and primary negative symptoms. The mean, standard deviation (SD), and range were calculated for quantitative variables.
Sociodemographic and clinical characteristics of patients were compared by sex using the χ2 test and the Mann- Whitney U test. Prevalence of negative symptoms was compared by diagnosis of psychotic disorder, employment status, dose, and antipsychotic treatment using the χ2 test. Mean negative symptoms scores were compared by diagnosis of psychotic disorder using an analysis of variance (ANOVA). Bonferroni corrections were used for all multiple comparisons of negative symptoms. Logistic regression analyses were used to determine those factors associated with the presence of negative symptoms, including sex, age, marital status, work status, BMI, treatment time, type and dose of antipsychotic treatment, CGI severity, and total and positive PANSS scores, as potential factors associated with the presence of negative symptoms.
The P values correspond to the statistical significance of 2-tailed tests. A P value ≤ .05 was considered statistically significant. The statistical package SPSS version 13.0.1 (SPSS Inc, Chicago, Illinois) was used throughout.
RESULTS
Patients and Distribution by Groups
A total of 1,704 patients were recruited by 117 psychiatrists at 91 different outpatient centers. Of these, 252 (14.8%) who failed to meet the study selection criteria were excluded. The main reason for exclusion (202 patients, 11.9%) was current treatment with any antipsychotic for less than 12 weeks. Thus, 1,452 patients were considered evaluable.
Sociodemographic, Clinical, and Lifestyle Characteristics
Table 1 shows the main sociodemographic and general clinical characteristics of the patients. The diagnosis was schizophrenia in 1,108 patients (77.1%), schizophreniform disorder in 61 patients (4.2%), and schizoaffective disorder in 268 patients (18.6%) of the 1,437 patients with these data available. The mean duration (SD) of the psychotic disorder was 15.5 years (10.8 years), with a mean of 2.6 (3.0) hospital admissions attributable to the psychotic disorder since onset of the disease. The current treatment had been initiated due to a change in the previous antipsychotic therapy in 727 patients (51.7%), due to an exacerbation of symptoms in 508 (36.1%), and to manage a first episode in 171 patients (12.2%) of the 1,406 patients with these data available. The type of antipsychotic drug was distributed as follows: amisulpride (n = 213, 14.7%), haloperidol (n = 202, 13.9%), olanzapine (n = 306, 21.1%), quetiapine (n = 218, 15.0%), risperidone (n = 268, 18.5%), and ziprasidone (n = 240, 16.5%). The mean duration of antipsychotic treatment was 136.4 weeks (210.1 weeks). Most of the patients enrolled in the study did not have healthy lifestyle habits: few patients were on a diet of some kind (16.5%), controlling calorie or salt intake (21.2% and 14.3%), avoiding saturated fats/ cholesterol (23.8%), or eating a high fiber diet (26.2%).
Prevalence of Negative Symptoms
At least 1 negative symptom was present in 57.6% of patients, and all negative symptoms were present in 17.8% of subjects only. As shown in Table 2, the most frequent negative symptoms were social withdrawal (45.8% of cases) and emotional withdrawal (39.1%). The mean (SD) scores on social (3.2 [1.4]) and emotional (3.1 [1.2]) withdrawal items were significantly higher (P < .05) than the corresponding values for the rest of the negative items evaluated: 2.9 (1.2), 2.9 (1.3), and 2.8 (1.3) for blunted affect, poor rapport, and verbal fluency, respectively. One or more primary negative symptoms were present in 12.9% of subjects, with social withdrawal (9.4%), poor rapport (7.2%), and emotional withdrawal (6.9%) being the most frequent primary negative symptoms, followed by blunted affect in 6.0% and verbal fluency in 5.9% of subjects.
Mean scores for each negative symptom and the overall PANSS negative symptoms subscore were compared by diagnosis of psychotic disorder. This analysis found higher average scores for schizophrenia (mean = 20.9 [SD = 8.0]) than for schizophreniform (mean = 17.9 [SD = 7.1]) and schizoaffective (mean = 17.8 [SD = 7.6]) disorders (ANOVA, P < .05). The presence of each negative symptom and all negative symptoms was also compared by diagnosis of psychotic disorder, and we found more patients with negative symptoms for schizophrenia than for schizophreniform and schizoaffective disorders (χ2 test, P < .05, Table 2).
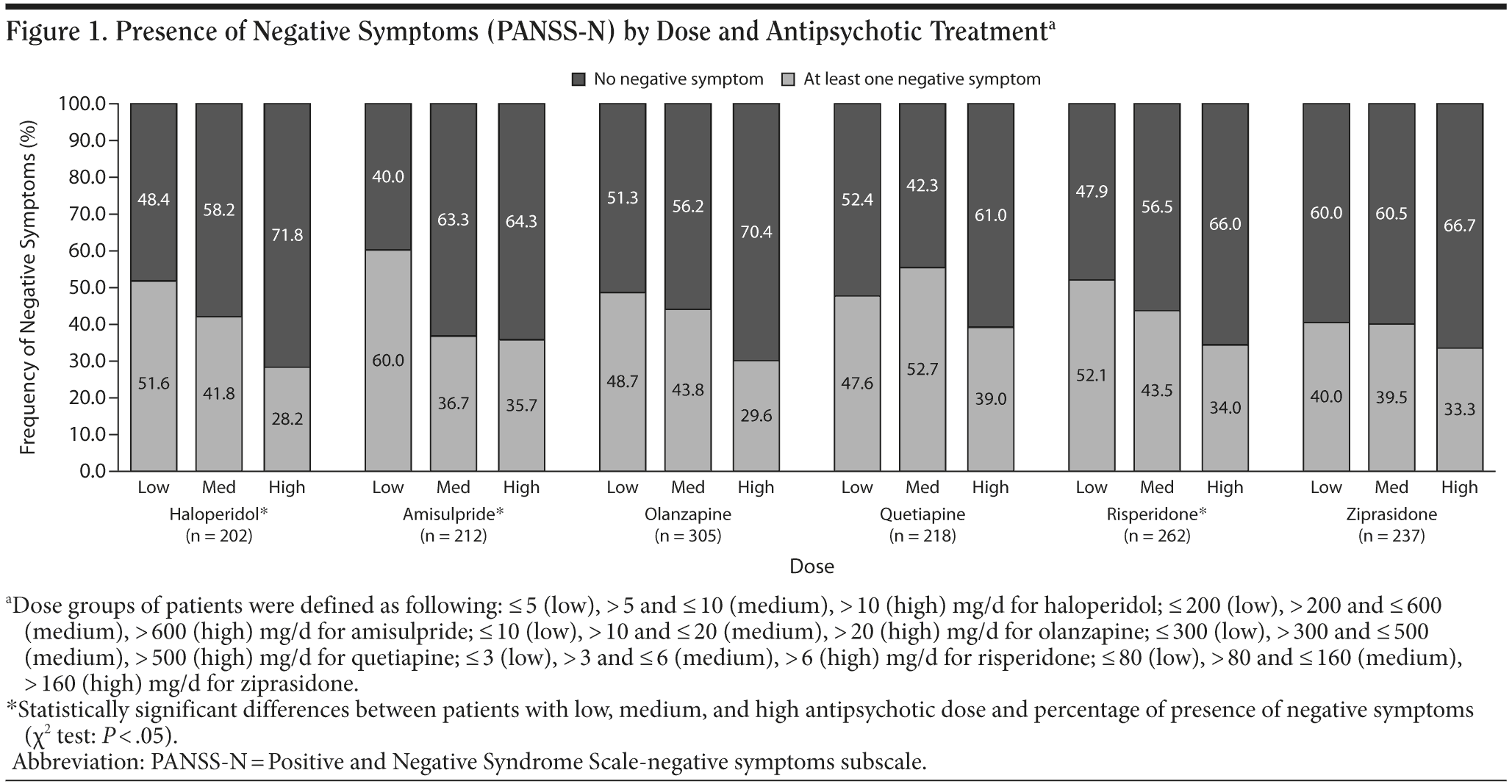
Negative symptoms, overall and also symptom-by- symptom, were significantly more prevalent among unemployed/inactive patients than among working patients (χ2 test, P < .001, in all cases). For all negative symptom comparisons, there was a significant association with employment status; that is, a higher level of negative symptoms was related to being unemployed. Among those working, 37.8% had at least 1 negative symptom compared with 62.3% of those patients who were not working, while the largest difference was seen for social withdrawal; 23.7% of employed patients had this symptom, compared with 51.2% of those who were not working (P < .001). Presence of at least 1 negative symptom was statistically nondifferent between antipsychotic treatments. However, presence of at least 1 negative symptom was associated with drug doses for haloperidol, amisulpride, and risperidone, which showed significant direct linear association; the lower the dose, the lowest the prevalence (χ2 test, P < .05, Figure 1).
Factors Associated With the Presence of Negative Symptoms
Table 3 displays all the factors that were associated with the presence of negative symptoms and the corresponding odds ratio (with its 95% confidence interval) of the association for each of these factors. Presence of negative symptoms varied diversely according to sex, age, marital status, working status, overall clinical severity, general and positive symptomatology, and duration of antipsychotic treatment. Clinical severity, as assessed by CGI and PANSS total (above median value) scores, showed a significant higher association with the presence of all negative symptoms individually, and with the presence of at least 1 negative symptom. This association was more robust with increased severity of disease as perceived by the clinician on the CGI scale. More than 1-year duration of antipsychotic treatment was also significantly associated with the presence of all negative symptoms individually (except for social withdrawal) and with the presence of at least 1 negative symptom.
Social withdrawal was the only negative symptom associated with being female (protective factor). Poor rapport, social withdrawal, and presence of all negative symptoms were significantly associated with work status (unemployed/inactive). Hence, for example, presence of poor rapport increases the risk for unemployed/inactive status by a mean of 88.9%, or presence of all negative symptoms increases the risk for this same working situation to a mean of 281.5%.
DISCUSSION
In this cross-sectional study, we estimated the frequency of negative symptoms in a large cohort of subjects with schizophrenia spectrum disorders treated with the antipsychotics most frequently used in daily practice, 5 different SGAs and 1 classic antipsychotic. Even though no studies similar to this have been found in the literature review,18 our results confirm that the prevalence of negative symptoms in patients receiving antipsychotic treatment in routine clinical practice for at least 12 weeks is considerably high, with a presence of 1 or more symptoms in 57.6% and a presence of all symptoms in 12.9% of patients. The most frequently present negative symptoms were those related to the integration of emotion and cognition: social withdrawal (45.8%), emotional withdrawal (39.1%), poor rapport (35.8%), and blunted affect (33.1%). These results are consistent with those obtained in previous studies,11 emphasizing these abnormalities as the hallmark characteristics of schizophrenia.
Furthermore, the results of this study confirm a strong association between the presence of negative symptoms and psychosocial outcomes, such as employment status or severity of illness, which may be reflected in the day-to-day life of patients, although raters were not instructed to ignore negative symptoms when using the CGI scale and this must be considered a potential confounding factor. Our results agree with those of a previous study19 in which negative symptoms and internal locus of control together accounted for 47% of the variance in occupational engagement. The results of this study also suggest that subjects with the highest positive symptoms scores (> 26) were less likely to have negative symptoms. This result may be controversial. However, in most studies, the positive correlation between positive and negative symptoms is during acute episodes. In this study, we were dealing with a stable outpatient population in which the positive correlation found in most studies with acute population may not be in place as we may be dealing with 2 types of stable patients: those with positive and those with negative residual symptoms. In that regard, some authors have supported the idea of a distinct schizophrenia subtype based on negative symptoms, as there are neurobiologic correlates of the negative symptom typology. However, these hypotheses have yielded mixed results.2,9,20 Further research should be undertaken on the potential usefulness of clinical dimensions such as negative symptoms, which may be more informative for the clinician than the use of classic subtypes.
Our study shows that schizophrenia seems to be more associated with the presence of any negative symptom than with a diagnosis of schizophreniform or schizoaffective disorder, as has been pointed out in previous studies.4,21,22
Negative symptoms of schizophrenia are still considered controversial because of the difficulty defining and measuring them, and because of the higher failure rate of available therapies. It seems necessary to conduct more research on the evaluation and treatment of negative symptoms, particularly with studies of longitudinal and prospective design, which should produce additional knowledge on managing and treating patients with these types of symptoms. However, this additional research would probably benefit from additional refinement and innovation in the methodology to be used, so that better treatments may be realized.2 In this regard, the use of mixed tools has been the subject of recent criticism, as in the case of the negative symptoms PANSS score used in the present study—it being argued that social, cognitive, and emotional measures do not interact properly.23
Current rating scales "capture" key domains of negative symptoms, in spite of considerable overlap between these domains. It has also been pointed out that negative symptoms can be difficult to evaluate objectively, particularly if researchers wish to balance rigorousness and brevity of assessment and ease of use.9 Additionally, although all psychiatrists were trained in the use of the PANSS scale, due to the observational nature of the study, no interrater reliability analysis could be carried out. This lack is another limitation of this study (as it is for most studies in this field). Therefore, the proposal of new methods for measuring these symptoms more accurately but feasibly, such as the "proxy" case identification tool using standardized symptom ratings instead of the Schedule for the Deficit Syndrome,24 which requires an independent clinical assessment, suggested by Kirkpatrick et al,25-27 may be powerful for future research in this field.
Prevalence of negative symptoms with haloperidol, amisulpride, and risperidone was sensitive to antipsychotic dose, presenting significant dose-dependent associations (the lower the dose, the lower the prevalence of negative symptoms). This result is controversial when compared with previous studies.28 However, the findings in the study relative to patients treated with haloperidol, amisulpride, or risperidone for at least 12 weeks might indicate that higher doses of selective D2 antagonists may worsen or induce negative symptoms, which would be in agreement with the hypodopaminergic pathophysiology postulated as being responsible for negative symptoms and with negative symptoms caused by D2 antagonists in healthy volunteers.4,29 Recent results with lower doses of olanzapine on negative symptoms may support this postulate.30 In our study, although a linear numerical trend was also observed between dose classification and presence of negative symptoms in subjects treated with olanzapine, dosage was statistically unrelated to frequency of negative symptoms. The cross-sectional nature of the study confirms that there was an association between higher doses of haloperidol, amisulpride, and risperidone and higher prevalence of negative symptoms. However, no conclusion can be reached as to whether higher doses of these antipsychotics actually induce negative symptoms. Nor can we establish whether this high prevalence of negative symptoms is due to the fact that clinicians use higher doses in patients with negative symptoms or due to the fact that the time of evolution of illness may be longer in these patients.
In conclusion, these findings from the CLAMORS study have shown that the prevalence of negative symptoms in patients treated with antipsychotics in daily clinical practice is considerably high. We consider this to be a source of concern for public health. The results of this study have shown the presence of negative symptoms to be associated with poor employment status and severity of disease. Increasing our understanding of the variables associated with negative symptoms in this vulnerable population may help to establish preventive and therapeutic programs for higher risk groups. Improving our methods for measuring these devastating symptoms, coupled with the ongoing development of novel antipsychotic agents, may fuel renewed interest in the evaluation of negative symptoms and optimism that better treatments for negative symptoms can be found.
Drug names: haloperidol (Haldol and others), olanzapine (Symbyax, Zyprexa, and others), quetiapine (Seroquel), risperidone (Risperdal and others), ziprasidone (Geodon).
Author affiliations: Medicine Department, Psychiatry Area, University of Oviedo, Asturias (Dr Bobes); Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM) (Drs Bobes and Arango); Psychiatry Department, Hospital General Universitario Gregorio Mara×±ón, Madrid (Dr Arango); the Operations Department, Biometria Clinica Clinical Research Organization (CRO), Barcelona (Ms Garcia-Garcia); and the Health Outcomes Research Department, Medical Unit, Pfizer Espa×±a, Alcobendas (Madrid) (Dr Rejas), Spain.
Financial disclosure: Dr Arango is a consultant for AstraZeneca, Bristol-Meyers Squibb, Janssen-Cilag, Pfizer, and Servier; has received grant/research support from AstraZeneca and Bristol-Myers Squibb; and is a member of the speakers/advisory boards for AstraZeneca, Bristol-Myers Squibb, Janssen-Cilag, Lundbeck, and Pfizer. Ms Garcia-Garcia is an employee of Biometria Clinica, a CRO contracted by Pfizer Espa×±a. Dr Rejas is an employee of Pfizer Espa×±a. Dr Bobes has no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
Funding/support: Funding for this study was provided by Pfizer Espa×±a; Pfizer Espa×±a participated in the idea and design of the study and provided an unrestricted grant for this study.
Disclaimer: All authors contributed to and approved the design and the protocol of the study, the literature searches and analyses, and the different versions of the manuscript.
Acknowledgment: A full list of principal investigators and collaborators of the CLAMORS Study Collaborative Group was published in Schizophrenia Research (2007;90[1]:162-173).
REFERENCES
1. Lindenmayer JP, Khan A, Iskander A, et al. A randomized controlled trial of olanzapine versus haloperidol in the treatment of primary negative symptoms and neurocognitive deficits in schizophrenia. J Clin Psychiatry. 2007;68(3):368-379. PubMed
2. Buckley PF, Stahl SM. Pharmacological treatment of negative symptoms of schizophrenia: therapeutic opportunity or cul-de-sac? Acta Psychiatr Scand. 2007;115(2):93-100. PubMed doi:10.1111/j.1600-0447.2007.00992.x
3. Milev P, Ho BC, Amdt S, et al. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162(3):495-506. PubMed doi:10.1176/appi.ajp.162.3.495
4. Artaloytia JF, Arango C, Lahti A, et al. Negative signs and symptoms secondary to antipsychotics: a double-blind, randomized trial of a single dose of placebo, haloperidol, and risperidone in healthy volunteers. Am J Psychiatry. 2006;163(3):488-493. PubMed doi:10.1176/appi.ajp.163.3.488
5. Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull. 2007;33(4):1013-1022. PubMed doi:10.1093/schbul/sbl057
6. Buchanan RW, Davis M, Goff D, et al. A summary of the FDA-NIMH-MATRICS workshop on clinical trial design for neurocognitive drugs for schizophrenia. Schizophr Bull. 2005;31(1):5-19. PubMed doi:10.1093/schbul/sbi020
7. Peralta V, Cuesta MJ. The deficit syndrome of the psychotic illness: a clinical and nosological study. Eur Arch Psychiatry Clin Neurosci. 2004;254(3):165-171. PubMed
8. Möller HG. Management of the negative symptoms of schizophrenia: new treatment options. CNS Drugs. 2003;17(11):793-823. PubMed doi:10.2165/00023210-200317110-00003
9. Stahl SM, Buckley PF. Negative symptoms of schizophrenia: a problem that will not go away. Acta Psychiatr Scand. 2007;115(1):4-11. PubMed doi:10.1111/j.1600-0447.2006.00947.x
10. Carpenter W Jr. Unmet therapeutic needs. Schizophr Bull. 2005;31(4):793-794. PubMed doi:10.1093/schbul/sbi065
11. Lewis S, Lieberman J. CATIE and CUtLASS: can we handle the truth? Br J Psychiatry. 2008;192(3):161-163. PubMed doi:10.1192/bjp.bp.107.037218
12. Arango C, Buchanan RW, Kirkpatrick B, et al. The deficit syndrome in schizophrenia: implications for the treatment of negative symptoms. Eur Psychiatry. 2004;19(1):21-26. PubMed doi:10.1016/j.eurpsy.2003.10.004
13. Bobes J, Arango C, Aranda P, et al. Cardiovascular and metabolic risk in outpatients with schizophrenia treated with antipsychotics: results of the CLAMORS Study. Schizophr Res. 2007;90(1-3):162-173. PubMed doi:10.1016/j.schres.2006.09.025
14. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276. PubMed
15. Peralta Martin V, Cuesta Zorita MJ. Validation of positive and negative symptom scale (PANSS) in a sample of Spanish schizophrenic patients. Actas Luso Esp Neurol Psiquiatr Cienc Afines. 1994;22(4):171-177. PubMed
16. Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry. 1997;58(12):538-546. PubMed
17. Guy W. Early Clinical Drug Evaluation Unit (ECDEU) Assessment Manual for Psychopharmacology. Rockville, MD: Nat Inst Ment Health; 1976.
18. van Os J, Walsh E, van Horn E, et al. Changes in negative symptoms and the risk of tardive dyskinesia: a longitudinal study. UK700 Group. Acta Psychiatr Scand. 2000;101(4):300-306. PubMed doi:10.1034/j.1600-0447.2000.101004300.x
19. Bejerholm U, Eklund M. Occupational engagement in persons with schizophrenia: relationships to self-related variables, psychopathology, and quality of life. Am J Occup Ther. 2007;61(1):21-32. PubMed
20. Kirkpatrick B, Buchanan RW, Ross DE, et al. A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry. 2001;58(2):165-171. PubMed doi:10.1001/archpsyc.58.2.165
21. Pini S, de Queiroz V, Dell’ Osso L, et al. Cross-sectional similarities and differences between schizophrenia, schizoaffective disorder and mania or mixed mania with mood-incongruent psychotic features. Eur Psychiatry. 2004;19(1):8-14. PubMed doi:10.1016/j.eurpsy.2003.07.007
22. Azorin JM, Kaladjian A, Fakra E. Current issues on schizoaffective disorder. Encephale. 2005;31(3):359-365. PubMed doi:10.1016/S0013-7006(05)82401-7
23. Kirkpatrick B, Fenton WS, Carpenter WT, et al. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32(2):214-219. PubMed doi:10.1093/schbul/sbj053
24. Kirkpatrick B, Buchanan RW, McKenny PD, et al. The schedule for the deficit syndrome: an instrument for research in schizophrenia. Psychiatry Res. 1989;30(2):119-123. PubMed doi:10.1016/0165-1781(89)90153-4
25. Kirkpatrick B, Buchanan RW, Breier A, et al. Case identification and stability of the deficit syndrome of schizophrenia. Psychiatry Res. 1993;47(1):47-56. PubMed doi:10.1016/0165-1781(93)90054-K
26. Goetz RR, Corcoran C, Yale S, et al. Validity of a ‘ proxy’ for the deficit syndrome derived from the Positive and Negative Syndrome Scale (PANSS). Schizophr Res. 2007;93(1-3):169-177. PubMed
27. Carpenter WT Jr, Arango C, Buchanan RW, et al. Deficit psychopathology and a paradigm shift in schizophrenia research. Biol Psychiatry. 1999;46(3):352-360. PubMed doi:10.1016/S0006-3223(99)00088-8
28. Leucht S. Amisulpride a selective dopamine antagonist and atypical antipsychotic: results of a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2004;7(suppl 1):S15-S20. PubMed doi:10.1017/S1461145704004109
29. Carpenter WT Jr. Serotonin-dopamine antagonists and treatment of negative symptoms. J Clin Psychopharmacol. 1995;15(suppl 1):30S-35S. PubMed
30. Lecrubier Y, Quintin P, Bouhassira M, et al. The treatment of negative symptoms and deficit states of chronic schizophrenia: olanzapine compared to amisulpride and placebo in a 6-month double-blind controlled clinical trial. Acta Psychiatr Scand. 2006;114(5):319-327. PubMed doi:10.1111/j.1600-0447.2006.00887.x