Major depressive disorder (MDD) is a highly prevalent and recurrent mental disorder that can also affect women in the perinatal period.1 As antidepressants are regarded as effective treatments for MDD,2 use during the perinatal period is common, with prevalence rates ranging between 1.6% and 5.5%.3 Many women are reluctant to continue antidepressants during pregnancy and express a strong preference for nonpharmacologic treatment,4,5 as possible consequences for the unborn child cannot be ruled out.6,7 Approximately half of women therefore decide to discontinue their antidepressants prior to or during pregnancy.8-10
While the wish to avoid in utero antidepressant exposure to the fetus is understandable, discontinuation of medication may lead to relapse.11 Depressive relapse in the perinatal period can have a profound negative impact on the health of both the mother and the child.12 Currently, most women who decide to discontinue antidepressants are not offered a substitute nonpharmacologic treatment to prevent relapse, while in the general population, there is evidence that continuation of antidepressants is not superior to Preventive Cognitive Therapy (PCT) or Mindfulness Based Cognitive Therapy (MBCT).13-17 We present the first randomized controlled trial that evaluated the efficacy of PCT with gradual, guided discontinuation of antidepressants during pregnancy as compared to continuation of antidepressants.
Methods
Details about the study design can be found elsewhere.18 We included 44 women between 12 and 16 weeks pregnant, with a history of MDD (assessed with the Structured Clinical Interview for DSM-IV Axis I Disorders [SCID-I]),19 currently remitted, and with use of an antidepressant (Supplementary Methods). Comorbid medication was permitted. Women with a history of bipolar or psychotic disorder were excluded. The study was approved by the Medical Ethical Committee of the Erasmus Medical Center. Participants were randomized with a stratified block-randomization allocation to either discontinuation of antidepressants with PCT (STOP; n = 24) or continuation of antidepressant and care as usual (GO; n = 20). Women were followed throughout their pregnancy and up to 3 months postpartum to evaluate relapse of MDD using the SCID-I. Trained assessors masked to treatment allocation conducted all follow-up assessments. Continuous longitudinal self-report outcome measures, including the Edinburgh (Postnatal) Depression Scale,20 State-Trait Anxiety Scale,21 and the Hamilton Depression Rating Scale,22 were collected at baseline, 24 and 36 weeks of pregnancy, and 4 and 12 weeks postpartum. Analyses were carried out according to the intention-to-treat principle. Relapse risk was compared using a 1-tailed Fisher exact test. Odds ratio (OR) and 95% confidence interval (CI) are reported. The difference in time-related proportions of participants with relapse or recurrence by treatment was tested using the log-rank test. We deployed linear mixed models with random intercepts for the continuous variables (Supplementary Methods).
Results
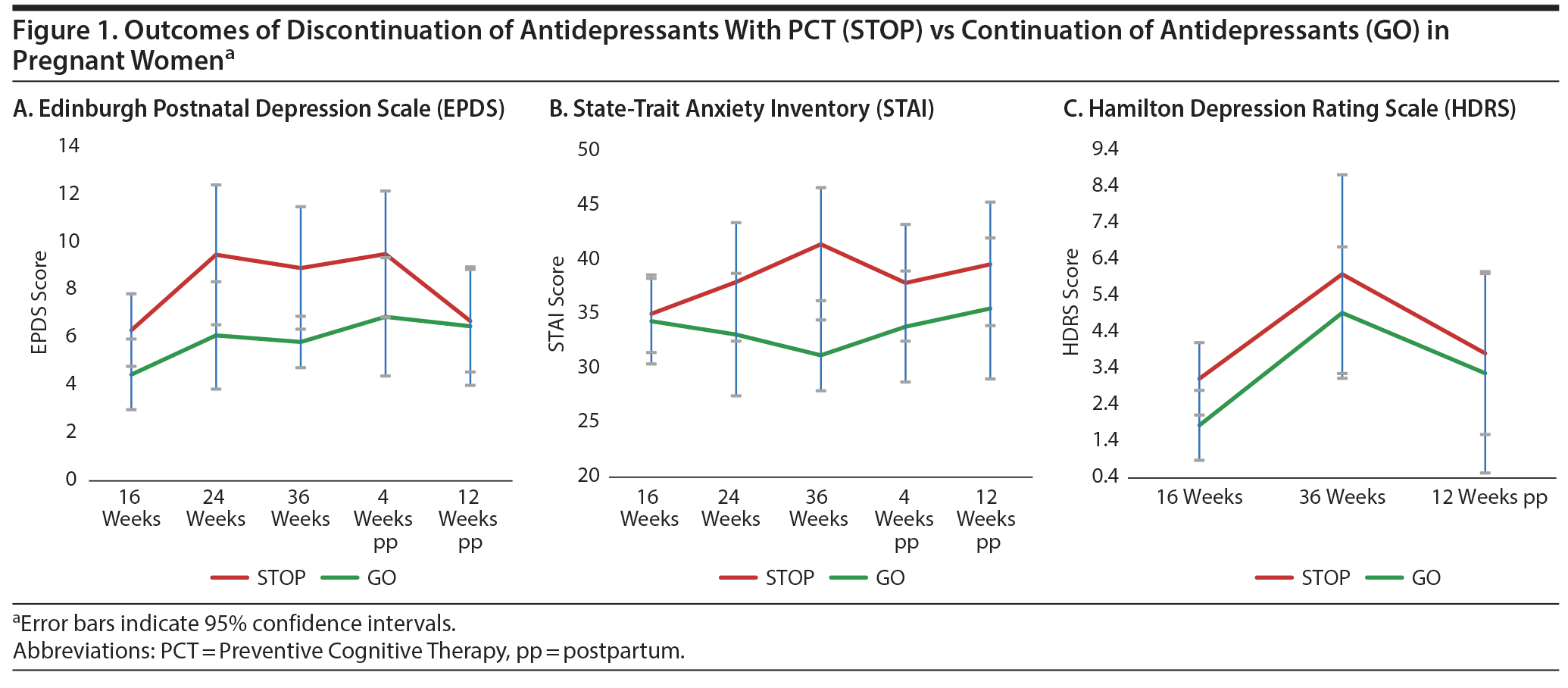
Demographic and clinical data of participants are presented in Supplementary Table 1. Six women (13.6%) experienced a recurrence of depression during study participation, 3 in the GO group (15.0%) and 3 in the STOP group (12.5%; OR = 1.24; 95% CI = 0.22-6.92; P = .58). Overall mean time to relapse from baseline (T0) was 143 days (STOP: 101 days, GO: 185 days). The log–rank test showed no statistically significant difference in time to recurrence (P = .93). We observed no differences in mood symptoms between the two groups over time (Figure 1 and Supplementary Table 2).
Conclusion
We found no evidence that Preventive Cognitive Therapy with gradual discontinuation of antidepressants during pregnancy altered the risk of maternal relapse of depression as compared to continuation of antidepressants. Although our findings need to be replicated in a larger trial, our study is a first step to study continuation versus discontinuation of antidepressants during pregnancy and should encourage further research on alternative strategies to continuation of antidepressants for pregnant women who have recovered from depression.
Published online: June 23, 2020.
Potential conflicts of interest: The authors report no financial or other relationship relevant to the subject of this report.
Funding/support: This work was supported by a grant from the Netherlands Organization for Health Research and Development (836021011), Den Haag, The Netherlands. Additional funding was available from the Erasmus Medical Center, Department of Psychiatry, Rotterdam, The Netherlands.
Role of the sponsor: The funding sources were not involved in the design and conduct of the study, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; or decision to submit the manuscript for publication.
Supplementary material: See accompanying pages.
aDepartment of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, New York
bDepartment of Psychiatry, Erasmus University Medical Center, Rotterdam, The Netherlands
cDepartment of Psychiatry, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, The Netherlands
dDepartment of General Practice and Elderly Care Medicine, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands
eEpidemiological and Social Psychiatric Research Institute, Erasmus Medical Center, Rotterdam, the Netherlands
fFaculty of Science, School of Psychology, The University of New South Wales, Sydney, Australia
gDepartment of Child and Adolescent Psychiatry, Erasmus Medical Center, Sophia’s Children Hospital, Rotterdam, The Netherlands
‘ ¡Shared first authorship.
*Corresponding author: Nina M. Molenaar, MD, PhD, Icahn School of Medicine at Mount Sinai, Department of Psychiatry, 1425 Madison Ave, New York, NY 10029 ([email protected]).
J Clin Psychiatry 2020;81(4):19l13099
To cite: Molenaar NM, Brouwer ME, Burger H, et al. Preventive cognitive therapy with antidepressant discontinuation during pregnancy: results from a randomized controlled trial. J Clin Psychiatry. 2020;81(4):19l13099.
To share: https://doi.org/10.4088/JCP.19l13099
© Copyright 2020 Physicians Postgraduate Press, Inc.
REFERENCES
1.Burcusa SL, Iacono WG. Risk for recurrence in depression. Clin Psychol Rev. 2007;27(8):959-985. PubMed CrossRef
2.National Institutes of Excellence (NICE). Depression in Adults: Recognition and Management. https://www.guidelines.co.uk/mental-health/depression-in-adults-recognition-and-management/454977.article. Published April 1, 2018.
3.Molenaar NM, Bais B, Lambregtse-van den Berg MP, et al. The international prevalence of antidepressant use before, during, and after pregnancy: a systematic review and meta-analysis of timing, type of prescriptions and geographical variability. J Affect Disord. 2020;264:82-89. PubMed CrossRef
4.Battle CL, Salisbury AL, Schofield CA, et al. Perinatal antidepressant use: understanding women’s preferences and concerns. J Psychiatr Pract. 2013;19(6):443-453. PubMed CrossRef
5.Kothari A, de Laat J, Dulhunty JM, et al. Perceptions of pregnant women regarding antidepressant and anxiolytic medication use during pregnancy. Australas Psychiatry. 2019;27(2):117-120. PubMed CrossRef
6.Campagne DM. Antidepressant use in pregnancy: are we closer to consensus? Arch Women Ment Health. 2019;22(2):189-197. PubMed CrossRef
7.Hendrick V, Stowe ZN, Altshuler LL, et al. Placental passage of antidepressant medications. Am J Psychiatry. 2003;160(5):993-996. PubMed CrossRef
8.Charlton RA, Jordan S, Pierini A, et al. Selective serotonin reuptake inhibitor prescribing before, during and after pregnancy: a population-based study in six European regions. BJOG. 2015;122(7):1010-1020. PubMed CrossRef
9.Hanley GE, Mintzes B. Patterns of psychotropic medicine use in pregnancy in the United States from 2006 to 2011 among women with private insurance. BMC Pregnancy Childbirth. 2014;14(1):242. PubMed CrossRef
10.Molenaar NM, Lambregtse-van den Berg MP, Bonsel GJ. Dispensing patterns of selective serotonin reuptake inhibitors before, during and after pregnancy: a 16-year population-based cohort study from the Netherlands. Arch Women Ment Health. 2020;23(1):71-79. PubMed CrossRef
11.Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499-507. PubMed CrossRef
12.Alder J, Fink N, Bitzer J, et al. Depression and anxiety during pregnancy: a risk factor for obstetric, fetal and neonatal outcome? a critical review of the literature. J Matern Fetal Neonatal Med. 2007;20(3):189-209. PubMed CrossRef
13.Bockting CLH, Klein NS, Elgersma HJ, et al. Effectiveness of preventive cognitive therapy while tapering antidepressants versus maintenance antidepressant treatment versus their combination in prevention of depressive relapse or recurrence (DRD study): a three-group, multicentre, randomised controlled trial. Lancet Psychiatry. 2018;5(5):401-410. PubMed CrossRef
14.Kuyken W, Byford S, Taylor RS, et al. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. J Consult Clin Psychol. 2008;76(6):966-978. PubMed CrossRef
15.Kuyken W, Hayes R, Barrett B, et al. Effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antidepressant treatment in the prevention of depressive relapse or recurrence (PREVENT): a randomised controlled trial. Lancet. 2015;386(9988):63-73. PubMed CrossRef
16.Fava GA, Ruini C, Rafanelli C, et al. Six-year outcome of cognitive behavior therapy for prevention of recurrent depression. Am J Psychiatry. 2004;161(10):1872-1876. PubMed CrossRef
17.Segal ZV, Bieling P, Young T, et al. Antidepressant monotherapy vs sequential pharmacotherapy and mindfulness-based cognitive therapy, or placebo, for relapse prophylaxis in recurrent depression. Arch Gen Psychiatry. 2010;67(12):1256-1264. PubMed CrossRef
18.Molenaar NM, Brouwer ME, Bockting CLH, et al. Stop or go? preventive cognitive therapy with guided tapering of antidepressants during pregnancy: study protocol of a pragmatic multicentre non-inferiority randomized controlled trial. BMC Psychiatry. 2016;16(1):72. PubMed CrossRef
19.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002.
20.Bergink V, Kooistra L, Lambregtse-van den Berg MP, et al. Validation of the Edinburgh Depression Scale during pregnancy. J Psychosom Res. 2011;70(4):385-389. PubMed CrossRef
21.Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI). Br J Clin Psychol. 1992;31(3):301-306. PubMed CrossRef
22.Hamilton M. Rating depressive patients. J Clin Psychiatry. 1980;41(12 Pt 2):21-24. PubMed
Save
Cite
Advertisement
GAM ID: sidebar-top