This CME activity is expired. For more CME activities, visit cme.psychiatrist.com.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
Objective: To review the efficacy of antidepressants and other therapeutic agents for the treatment of cognitive impairment in adults with major depressive disorder (MDD).
Data Sources: We conducted a database search of MEDLINE, PsycINFO, and Embase through Ovid on May 7, 2019. The year of publication was not restricted. The search terms “Major Depressive Disorder,” “depress*,” “cognit*,” and “therapeutics” were used.
Study Selection: The studies included in this review were clinical trials of antidepressants and other therapeutic agents in MDD populations. Participants were aged between 18 and 65 years and had a DSM-III, -IV, or -5 diagnosis of MDD. In total, 2,045 research papers were screened, 53 full-text articles were assessed, and 26 articles were eligible to be included in this systematic review.
Data Extraction: The data and quality of research papers were assessed and screened by 2 independent reviewers. Discrepancies were resolved through a third reviewer.
Results: Overall, studies demonstrated that tricyclic antidepressants do not have procognitive effects, while vortioxetine and bupropion have demonstrated procognitive effects in MDD populations relative to selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors. Several non-antidepressant agents, such as modafinil, amphetamines, and erythropoietin, have also demonstrated significant positive effects on cognition in depression.
Conclusions: Present-day antidepressants and other agents have demonstrated procognitive effects in MDD, but the findings between various agents are mixed. Further research looking at objective measures of cognitive performance would be helpful to obtain more definitive results regarding the efficacy of therapeutics for cognitive impairment in MDD.
This CME activity is expired. For more CME activities, visit CMEInstitute.com.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders

CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation. A $10 processing fee will apply.
CME Objective
After studying this article, you should be able to:
- Try to address cognitive impairments in patients with major depressive disorder using the latest data to guide prescribing
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Nurses Credentialing Center (ANCC) and the American Academy of Physician Assistants (AAPA) accept certificates of participation for educational activities certified for AMA PRA Category 1 Credit™ from organizations accredited by the ACCME.
Release, Expiration, and Review Dates
This educational activity was published in July 2020 and is eligible for AMA PRA Category 1 Credit™ through August 31, 2022. The latest review of this material was July 2020.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Marlene P. Freeman, MD, Editor in Chief, has received research funding from JayMac and Sage; has been a member of the advisory boards for Otsuka, Alkermes, and Sunovion; has been a member of the Independent Data Safety and Monitoring Committee for Janssen; has been a member of the Steering Committee for Educational Activities for Medscape; and, as a Massachusetts General Hospital (MGH) employee, works with the MGH National Pregnancy Registry, which is sponsored by Teva, Alkermes, Otsuka, Actavis, and Sunovion, and works with the MGH Clinical Trials Network and Institute, which receives research funding from multiple pharmaceutical companies and the National Institute of Mental Health. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
ABSTRACT
Objective: To review the efficacy of antidepressants and other therapeutic agents for the treatment of cognitive impairment in adults with major depressive disorder (MDD).
Data Sources: We conducted a database search of MEDLINE, PsycINFO, and Embase through Ovid on May 7, 2019. The year of publication was not restricted. The search terms “Major Depressive Disorder,” “depress*,” “cognit*,” and “therapeutics” were used.
Study Selection: The studies included in this review were clinical trials of antidepressants and other therapeutic agents in MDD populations. Participants were aged between 18 and 65 years and had a DSM-III, -IV, or -5 diagnosis of MDD. In total, 2,045 research papers were screened, 53 full-text articles were assessed, and 26 articles were eligible to be included in this systematic review.
Data Extraction: The data and quality of research papers were assessed and screened by 2 independent reviewers. Discrepancies were resolved through a third reviewer.
Results: Overall, studies demonstrated that tricyclic antidepressants do not have procognitive effects, while vortioxetine and bupropion have demonstrated procognitive effects in MDD populations relative to selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors. Several non-antidepressant agents, such as modafinil, amphetamines, and erythropoietin, have also demonstrated significant positive effects on cognition in depression.
Conclusions: Present-day antidepressants and other agents have demonstrated procognitive effects in MDD, but the findings between various agents are mixed. Further research looking at objective measures of cognitive performance would be helpful to obtain more definitive results regarding the efficacy of therapeutics for cognitive impairment in MDD.
J Clin Psychiatry 2020;81(4):19r13200
To cite: Blumberg MJ, Vaccarino SR, McInerney SJ. Procognitive effects of antidepressants and other therapeutic agents in major depressive disorder: a systematic review. J Clin Psychiatry. 2020;81(4):19r13200.
To share: https://doi.org/10.4088/JCP.19r13200
© Copyright 2020 Physicians Postgraduate Press, Inc.
aCentre for Depression and Suicide Studies, St Michael’s Hospital, Toronto, Ontario, Canada
bInstitute of Medical Science, University of Toronto, Toronto, Ontario, Canada
cLi Ka Shing Knowledge Institute, St Michael’s Hospital, Toronto, Ontario, Canada
dDepartment of Psychiatry, University of Toronto, Toronto, Ontario, Canada
eUniversity Hospital Galway, Galway, Ireland
*Corresponding author: Sophie R. Vaccarino, BScH, Research Assistant, ASR Suicide & Depression Studies Program, St Michael’s Hospital, 193 Yonge St, 6th Fl, Toronto, ON M5B 1M4, Canada ([email protected]).
Major depressive disorder (MDD) affects over 300 million people and is currently the leading cause of disability worldwide.1 According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5),2 MDD is characterized by a marked change in mood and/or anhedonia and the presence of several other psychophysiological changes, such as disturbed sleep, changes in appetite, fatigue, and a diminished ability to think or concentrate.2 Cognitive impairment is estimated to affect approximately two-thirds of individuals with MDD.3 The DSM-5 characterizes cognitive impairment as difficulty with thinking, concentrating, or making decisions.2 However, impairments on neuropsychological tests in MDD populations have also been demonstrated in the domains of processing speed, attention, executive function, learning, and memory with moderate effect sizes.4,5 Moreover, these deficits remain present despite full or partial remission of MDD symptoms.6 In particular, executive dysfunction tends to persist, which may contribute to the psychosocial impairment7-9 commonly experienced by those with remitted MDD.10 Psychosocial functioning is understood as the degree to which individuals adequately interact with their environment across daily, occupational, and social domains.11 It is well established that cognitive deficits in MDD are associated with impaired psychosocial functioning.12 Additionally, studies show a positive relationship between self-reported cognitive impairment and loss of occupational productivity, impaired social functioning, and reduced daily functioning.7,13,14 Thus, there is strong evidence suggesting that MDD is associated with impairments in multiple cognitive domains and that these impairments are notably associated with difficulties in psychosocial functioning.
Pharmacologic Treatments in Major Depressive Disorder
While extensive research has been conducted on the effects of antidepressants on depressed mood, the procognitive effects of antidepressants and other therapeutic agents have not been thoroughly investigated. A systematic review and meta-analysis15 concluded that antidepressants, such as tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and norepinephrine-dopamine reuptake inhibitors (NDRIs), have a significant positive effect on psychomotor speed and delayed recall in adults with MDD, though no significant difference between the classes of antidepressants was found. Yet, that review included only studies of antidepressants with the primary mechanism of action being monoamine modulation in 1 or more of the following categories: SSRIs, SNRIs, serotonin antagonist and reuptake inhibitors, noradrenergic and specific serotonergic antidepressants, TCAs, and multimodal antidepressants. This led to the exclusion of certain antidepressants, such as bupropion, as well as non-antidepressant agents. Additionally, the paper did not investigate the relationship between cognition and functional outcomes. Finally, new clinical trials have been conducted since the publication of this previous review, which we will explore in the current review.
Vortioxetine, a multimodal serotonergic antidepressant, is the first antidepressant recognized by the US Food and Drug Administration for its procognitive effects.16 Moreover, there is evidence that bupropion improves cognitive functioning in patients with depression.17 More recently, adjunctive agents, such as amphetamines,18 erythropoietin, and modafinil,19 have been investigated for their cognitive effects in MDD. To date, clinical trials looking at the procognitive effects of therapeutic agents in MDD populations have obtained positive findings; however, these studies are limited by their small sample sizes, heterogeneous cognitive measures, and lack of objective measures of cognitive functioning.

- Despite the common persistence of cognitive impairment in populations with major depressive disorder (MDD), treatment options are understudied and limited.
- A review of all potential therapeutic agents is necessary to inform future research investigating the benefits of pharmaceutical agents for cognitive impairment in MDD.
- So far, vortioxetine seems to be a viable treatment option for MDD patients with cognitive impairment.
The Current Review
Cognitive impairment is an important yet understudied phenomenon in MDD and should be considered a treatment priority as it is strongly related to psychosocial impairment. Therefore, the primary objective of the current systematic review is to examine the overall efficacy of antidepressants and other therapeutic agents in MDD, with a focus on objective measures of cognition. Previous studies reporting cognitive improvements based on participant self-reports lend themselves to low validity, considering the weak relationship between reported and actual cognitive impairment20 and the strong relationship between cognitive complaints and depressive symptomatology.21 Consequently, the use of objective measures only is a key strength of this study and will give a more realistic assessment of the procognitive effects of the discussed pharmacologic agents. As a secondary outcome measure, we will examine functional outcomes and their relation to cognitive improvements. Thus, this review aims to offer a detailed overview of the procognitive efficacy of antidepressant and non-antidepressant agents. Accordingly, this article will provide practical information regarding the need for future studies assessing the efficacy of the most successful agents for treating cognitive impairment in MDD.
METHODS
Search Methods
A comprehensive database search of MEDLINE, PsycINFO, and Embase through Ovid was conducted on May 7, 2019, using the following search terms: “Major Depressive Disorder” OR “depress*” AND “cognit*” AND “therapeutics,” “antidepressant agent,” “antidepressant,” “procognitive treatment,” “clinical trial,” “psychostimulant,” OR “procognitive.” Duplicates were removed automatically by Ovid using the “deduplicate” function. Results were then screened manually for any remaining duplicates by author M.J.B. In addition, ClinicalTrials.gov was searched, using the terms “cognition” and “depression.” Additional papers were identified from the reference section of the included articles. Authors M.J.B. and S.R.V. independently assessed the search results for eligibility. The authors resolved all discrepancies through discussion with coauthor S.J.M.
Inclusion Criteria
- Randomized controlled and open-label trials assessing the effects of pharmacologic interventions (antidepressant and non-antidepressant agents) on cognition.
- Studies measuring objective cognitive functioning.
- Studies of human participants between 18 and 65 years of age who met DSM-III, -IV, or −5 or International Classification of Disease (ICD)-10 or –11 criteria for MDD.
- Articles written in English.
- There was no restriction on year of publication.
Exclusion Criteria
- Single-dose trials.
- Naturalistic studies.
- Studies in which psychotherapy was the primary intervention being investigated.
- Studies that included only subjective cognitive outcome measures.
- Studies including participants with concurrent suicide risk.
- Studies in which participants met criteria for other psychiatric disorders, such as bipolar disorder, attention-deficit/hyperactivity disorder (ADHD), psychotic disorders, substance use disorder, or posttraumatic stress disorder; comorbid anxiety was not excluded, as it is a common comorbidity of MDD.
Quality of Assessment
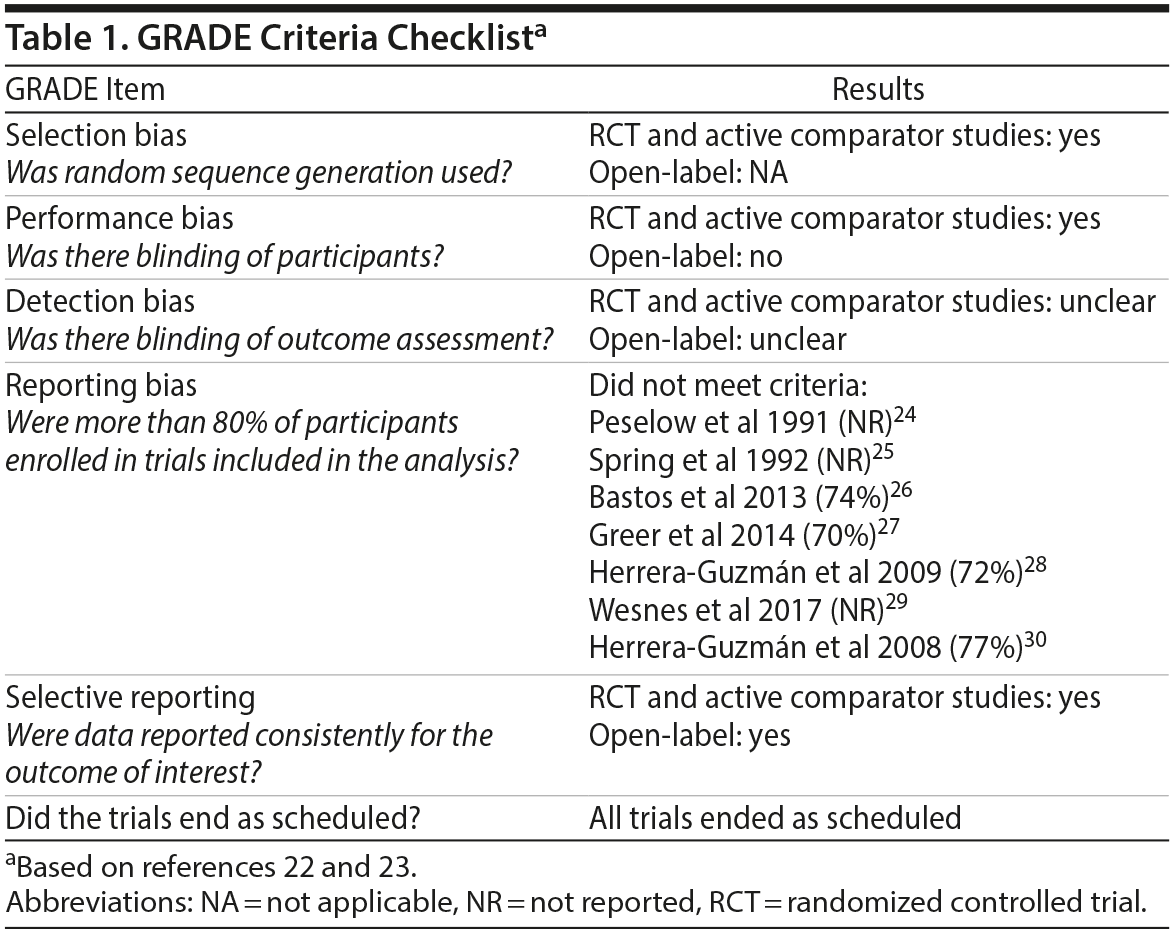
The quality of papers was assessed using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) checklist.22,23 All placebo-controlled and active-comparator studies had a low potential for selection bias and performance biases because they used double-blind, treatment randomization procedures. In the open-label studies, participants received the same treatment, which minimized selection bias; however, performance bias could not be excluded. Additionally, because details regarding the blinding of outcome assessments were not given, detection bias could not be considered. A minimum of 70% of participants enrolled in the studies completed the trial. Although the GRADE criteria deem 80% apt, we considered 70% an acceptable rate based on the study population. All studies reported significant and nonsignificant results, demonstrating a low selective reporting bias. None of the studies ended prematurely. Table 1 summarizes the GRADE criteria for the included studies.
RESULTS
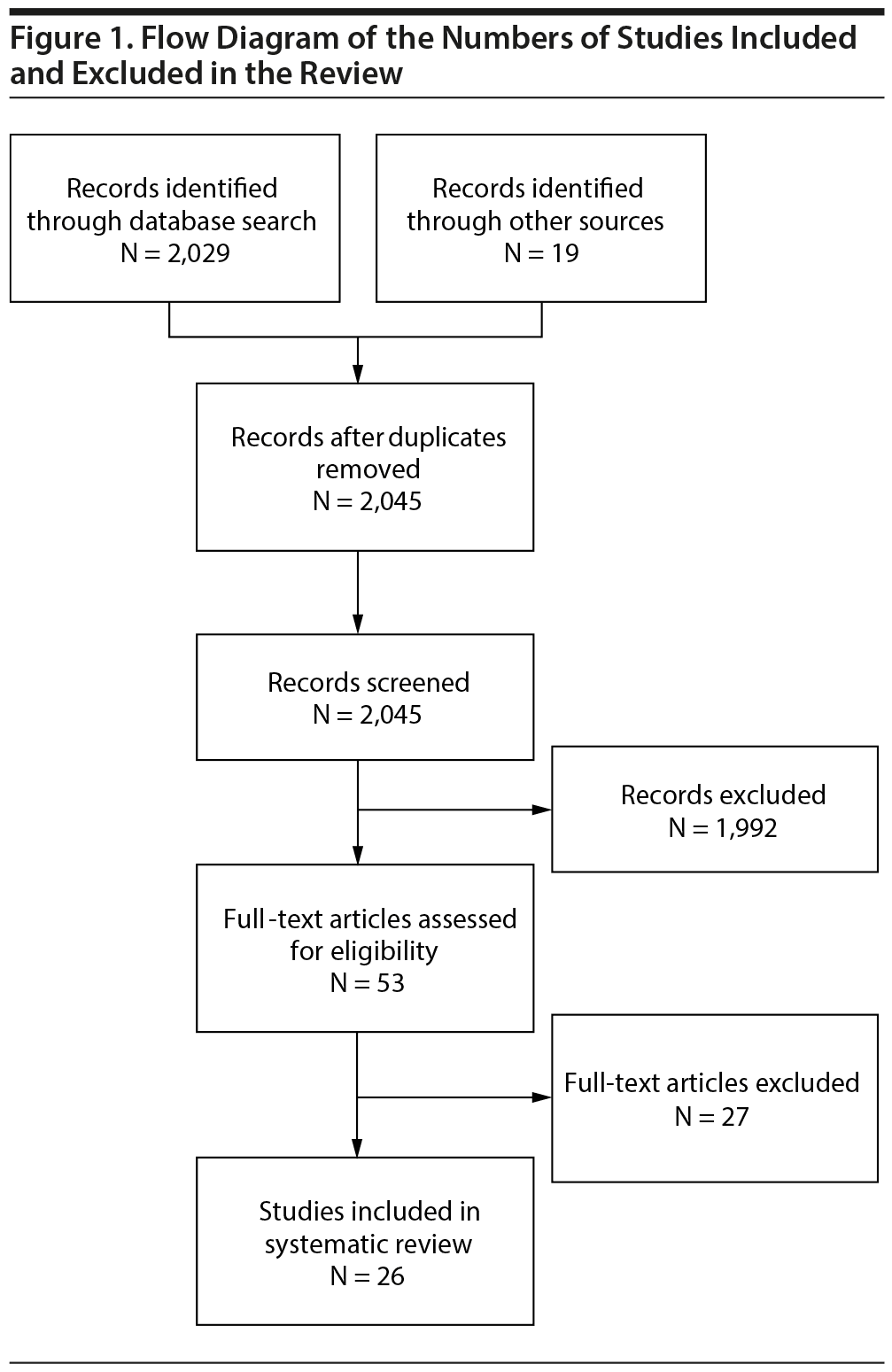
Of 2,045 articles identified through the database search and additional search methods, 53 full-text articles were assessed for eligibility, and 26 were included in the systematic review (Figure 1). Studies were excluded for reasons such as lack of objective measures of cognition, nondepressed study populations, and inappropriate age of study population.
Description of the Studies
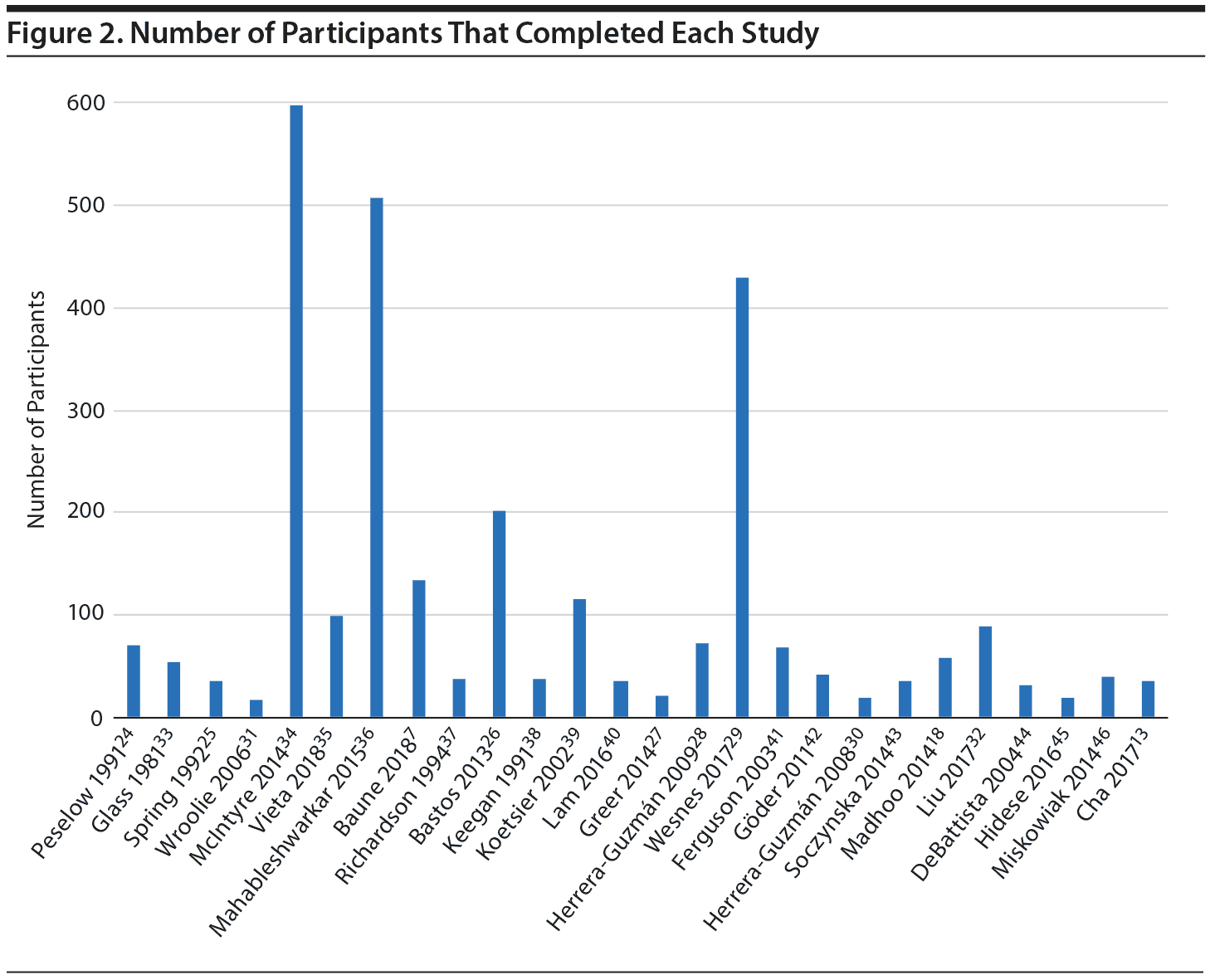
The included articles were published between 1982 and 2018. Of these 26 articles, 13 were randomized controlled trials (RCTs), 7 were open-label trials, and 6 were active comparator studies. Sample sizes varied from 17 to 598 MDD participants: 13 studies included a sample size of less than 50 people, 9 included a sample size between 50 and 200, and 4 had a sample size greater than 200 (Figure 2). Study duration ranged from 1 week to 24 months. The mean age of subjects ranged from 24 to 56 years, with an average age of 42. The average proportion of females was 65% across the included studies. It is important to note that one study31 included a female-only sample and another study32 included a male-only sample. We classified study medications as TCAs, SSRIs and other serotonergic agents, SNRIs, NDRIs, and non-antidepressant agents.
Tricyclic Antidepressants
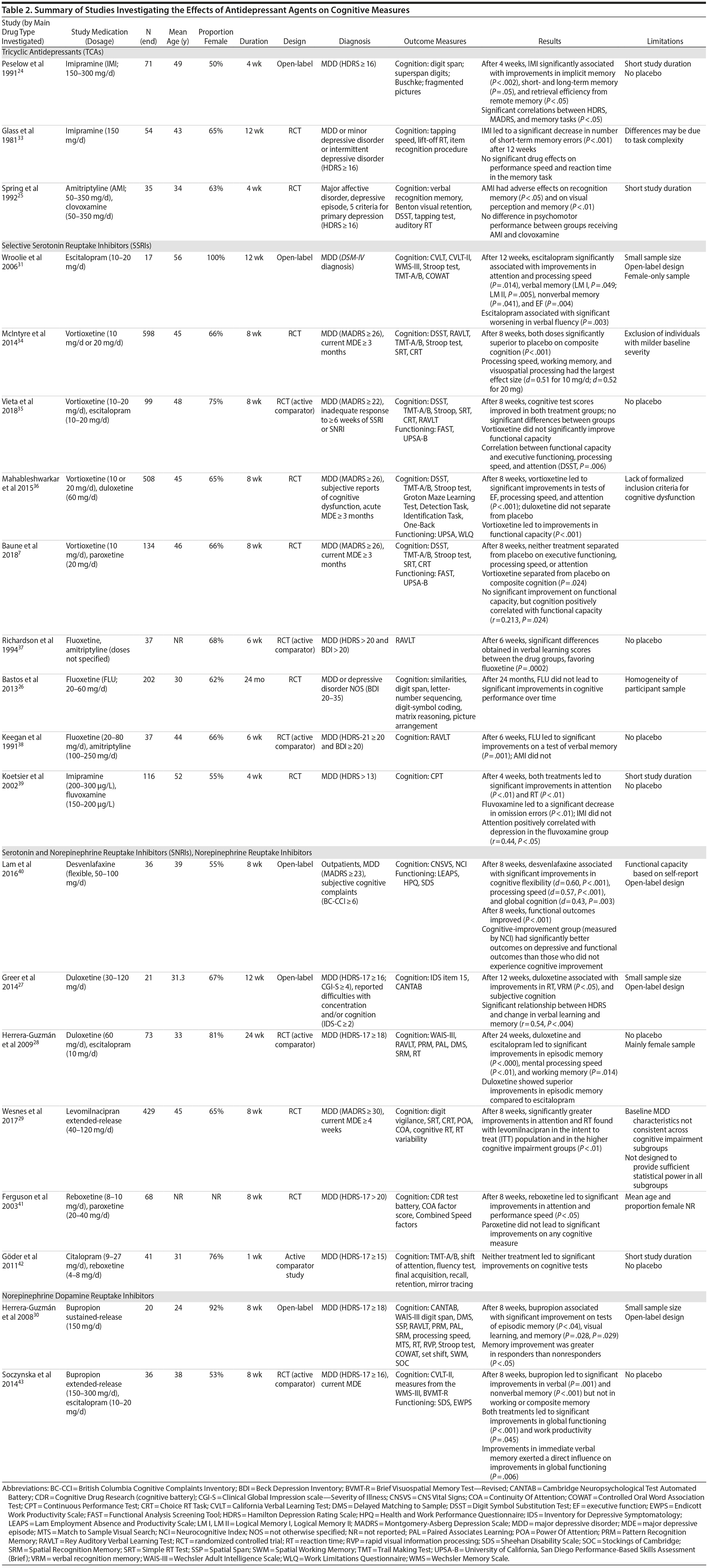
Cognition. In general, the studies included in this review were unable to conclude that TCAs are efficacious at improving cognition in MDD. One study33 (N = 54) found that 12 weeks of imipramine significantly improved short-term memory functioning, but not performance speed, compared to placebo in participants with moderate levels of depression (mean Hamilton Depression Rating Scale [HDRS] score = 20.0) at baseline. An open-label study24 (N = 71) found that 4 weeks of imipramine was significantly associated with improvements on memory tasks, but this was dependent on depression severity. The participants in this study had higher levels of depression (mean HDRS = 24.7; mean Montgomery-Asberg Depression Rating Scale [MADRS] score = 30.5) at baseline.
Conversely, amitriptyline was not found to be associated with improvements in cognitive functioning. An RCT25 (N = 35) found that following 4 weeks of amitriptyline, no significant improvements were found in psychomotor performance in individuals with severe depression (mean HDRS = 24.7) at baseline. In addition, 2 studies37,38 (both with N = 37) found that amitriptyline was associated with significantly worse performance on tests of verbal learning compared to fluoxetine. These 2 studies did not include information regarding baseline severities of depression. This makes it difficult to infer whether changes in cognition were related to changes in depressive severity.
Selective Serotonin Reuptake Inhibitors and Serotonergic Agents
Cognition. Two active comparator studies (both with N = 37)37,38 found that fluoxetine was associated with significant improvements on verbal learning scores compared to amitriptyline after 6 weeks of treatment. Conversely, a larger RCT26 (N = 202) found that 24 months of fluoxetine was not associated with a significant improvement in cognitive performance over time in a sample of moderately depressed individuals (mean Beck Depression Inventory [BDI] score = 26.7). These results hold more weight than the former studies as this study was placebo-controlled, included a large sample, and lasted 24 months. Therefore, these results suggest that fluoxetine does not have significant effects on cognition.
An RCT39 (N = 116) found that 4 weeks of fluvoxamine treatment significantly improved attention and reaction time (RT) in individuals with severe depression (mean HDRS = 27.3) at baseline. However, this improvement was mediated by depression severity (r = 0.44, P < .05).39
An open-label study31 (N = 17) found that 12 weeks of escitalopram treatment in individuals with moderate depression (mean HDRS = 21.2) was correlated with significant improvements in measures of attention and processing speed, verbal memory, nonverbal memory, and executive functioning. However, this study also found that escitalopram was associated with significant worsening of verbal fluency.31 These results should be interpreted with caution because this study included a small female-only sample and had no placebo-controlled group.
Finally, vortioxetine treatment was associated with significant improvements in multiple domains of cognitive functioning.7,34-36 Specifically, 1 study34 (N = 598) found that following 8 weeks of treatment in participants with moderate depression (mean MADRS = 31.5), 2 doses of vortioxetine (10 mg/d and 20 mg/d) were significantly superior to placebo in improving global cognition. The largest effect sizes (Cohen effect size, d = 0.51 for 10 mg and d = 0.52 for 20 mg) were obtained on the Digit Symbol Substitution Test (DSST), a measure of executive functioning, speed of processing, and attention.34 Additionally, half to two-thirds of the effect of vortioxetine was a direct effect on cognition, rather than a consequence of depressive symptom improvement.34 Other, more recent studies7,35,36 have supported these findings, demonstrating that vortioxetine is associated with improvements in cognitive functioning in individuals experiencing moderate levels of depression.
Functional outcomes. Paroxetine and escitalopram were not associated with functional improvement.7,35 After 8 weeks (N = 508), vortioxetine led to significant improvements in functional capacity (P < .001).36 However, another RCT (N = 134)7 and active comparator study (N = 99)35 did not obtain significant results for the effect of vortioxetine on functional capacity.
Despite contradicting results regarding the direct effect of vortioxetine on functional outcomes, it was found that change in cognitive performance was positively correlated with change in performance-based functional capacity for those taking vortioxetine (r = 0.21, P = .02).7 Further, after 8 weeks of vortioxetine or escitalopram treatment, a partial correlation was obtained (r = 0.31, P = .006) between the DSST and functioning, reflecting a moderate relationship between cognitive performance and functional capacity.35
Serotonin and Norepinephrine Reuptake Inhibitors and Norepinephrine Reuptake Inhibitors
Cognition. Conflicting results were found for the efficacy of reboxetine. After 1 week of reboxetine treatment (N = 41), no significant improvements in cognitive flexibility, declarative memory, or visual and motor skills were found in individuals with moderate depression (mean HDRS = 20.7).42 This could be due to the short study duration. Conversely, another study (N = 68) found that after 8 weeks, reboxetine was significantly associated with improvements on measures of attention and RT.41 Information regarding sample severity of depression was not provided.
Significant improvements in attention and RT measures were found in severely depressed individuals (mean MADRS = 35.2) treated with 8 weeks of levomilnacipran versus placebo (N = 429).29 These improvements were greater for individuals who were more cognitively impaired at baseline compared to those with less impairment.
Furthermore, 8 weeks of open-label treatment (N = 36) with desvenlafaxine was associated with significant improvements in cognitive flexibility, processing speed, and global cognition (d = 0.43, P = .003) in outpatients with moderate depression (mean MADRS = 28.5).40
Finally, duloxetine was associated with significant improvements in the domains of psychomotor speed, visual memory, decision making, and verbal learning and memory after 12 weeks of open-label treatment (N = 21) in individuals with moderate depression (mean HDRS = 19.1).27 However, improvements in verbal learning and memory were moderated by change in depressive severity (r = 0.54, P < .004).27 Duloxetine was also associated with significant improvements in episodic memory, compared to escitalopram, after 24 weeks (N = 73) of treatment in individuals with severe depression (mean HDRS = 25.2).28 Moreover, duloxetine was associated with significant increases in mental processing speed variables and working memory (WM); however, duloxetine did not significantly separate from escitalopram on these measures.28 Conversely, duloxetine did not separate from placebo on tests of executive functioning, processing speed, and attention in an 8-week RCT (N = 508) comparing its efficacy to vortioxetine and placebo.36 The results of the latter study should be taken into consideration as it is the only placebo-controlled study and included a large sample of participants with moderate depression (mean MADRS = 31.7).
Functional outcomes. Eight weeks (N = 36) of desvenlafaxine treatment resulted in significant improvements in functional outcomes (measured using the Lam Employment Absence and Productivity Scale [d = 1.35, P < .001]; Sheehan Disability Scale [d = 1.45, P < .001]; and Health and Work Performance Questionnaire [d = 0.89, P < .001]).40
Norepinephrine-Dopamine Reuptake Inhibitors
Cognition. Following 8 weeks of open-label bupropion treatment, participants (N = 20) with severe depression (mean HDRS = 24.8) significantly improved on measures of memory and some measures of mental processing speed; improvement was greater in responders versus nonresponders.30 A study (N = 36) comparing the effects of bupropion to escitalopram found that 8 weeks of bupropion in individuals with moderate depression (mean HDRS = 23.4) was associated with significant improvements on measures of verbal memory and nonverbal memory, but not WM or composite memory.43
Functional outcomes. Following 8 weeks of bupropion, significant improvements were observed on functional measures; however, this improvement was not significantly greater than that found with escitalopram.43 The same study found that change in immediate verbal memory directly influenced psychosocial functioning.43
The details of all studies assessing the efficacy of antidepressant agents are outlined in Table 2.
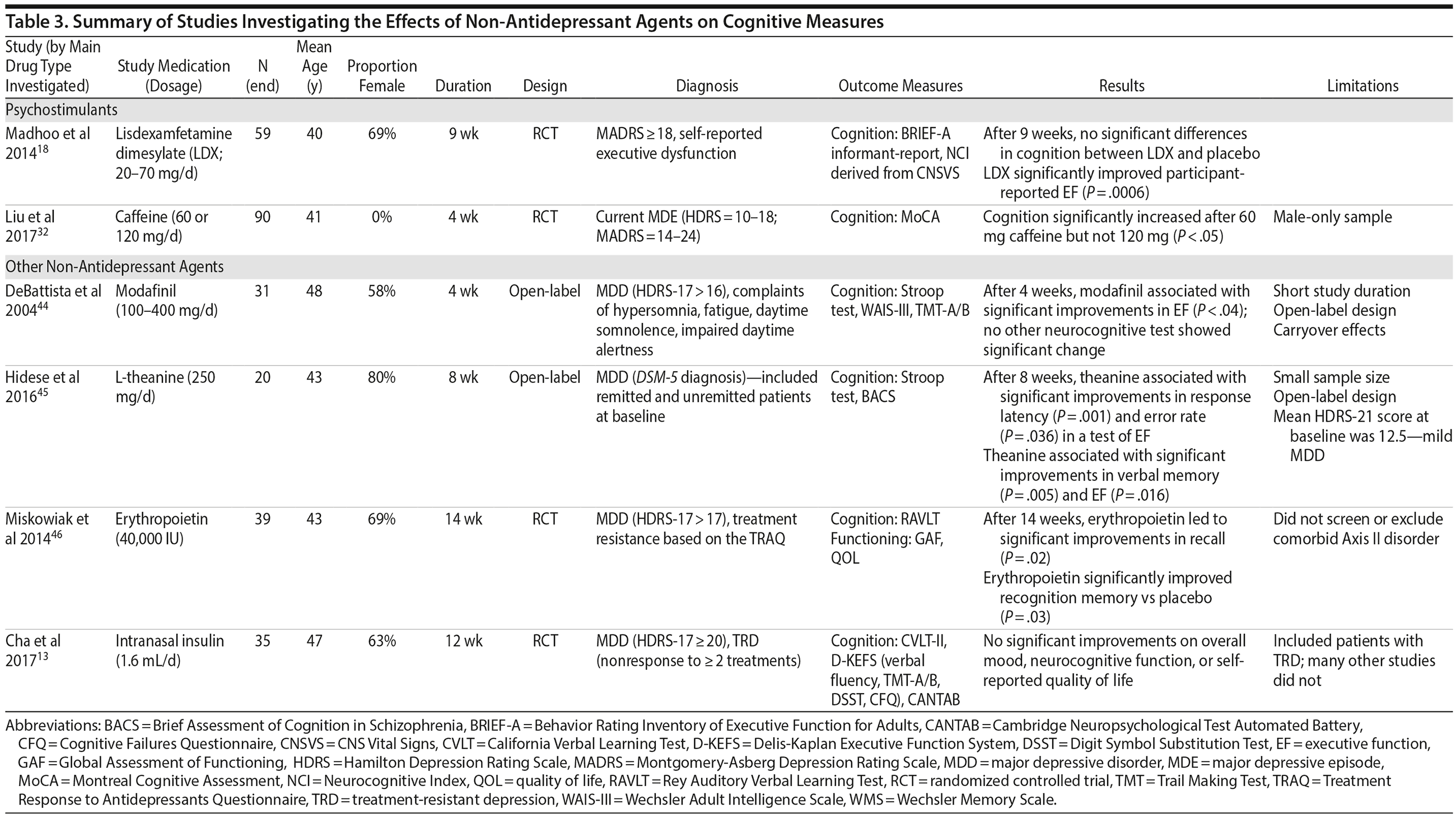
Non-Antidepressant Agents: Psychostimulants
Cognition. Following 9 weeks of lisdexamfetamine dimesylate augmentation to SSRI therapy in remitted MDD patients (N = 59), significant improvements in executive functioning were found compared to placebo; however, there was no difference between lisdexamfetamine dimesylate and placebo on composite cognition.18 The lack of positive findings on composite cognition could be due to the inclusion of a study population with mild levels of depression (mean MADRS = 12.3).
An open-label study (N = 90) found that 60 mg of caffeine, but not 120 mg, was associated with improved cognition.32 However, this study included a male-only sample, making it difficult to generalize these findings to females with MDD. Moreover, these participants experienced mild levels of depression (mean HDRS = 14.1; mean MADRS = 18.3).
Other Non-Antidepressant Agents
Cognition. An open-label study (N = 31) of modafinil augmentation therapy in moderately depressed participants (mean HDRS = 21.4; mean BDI = 20.6) found that 4 weeks of modafinil was associated with significant improvements on a test of executive functioning,44 though no other neurocognitive tests showed such improvements.44 The lack of positive findings could be attributed to the short study duration and small sample size.
Eight weeks of erythropoietin administration enhanced recall memory and recognition memory more than placebo in individuals with treatment-resistant depression (mean HDRS = 20); these effects were maintained over the entire 14 weeks of the study (N = 39).46
l-Theanine, an amino acid, was associated with significant improvements in executive functioning and verbal memory after 8 weeks of open-label administration (N = 20) in a sample with mild depression (mean HDRS = 12.5).45 Finally, following 12 weeks of intranasal insulin treatment in participants (N = 35) with treatment-resistant depression (mean MADRS = 25.9), no effects on cognition were found.13
The details of studies assessing the efficacy of non-antidepressant agents are outlined in Table 3.
DISCUSSION
The aim of this systematic review was to assess the efficacy of various antidepressants and other therapeutic agents for relieving cognitive impairment in MDD. Specifically, studies assessing the effects of pharmaceutical agents on objective measures of cognition in adults with MDD were assessed.
Tricyclic Antidepressants
Overall, studies assessing the efficacy of tricyclic antidepressants demonstrated that TCAs are not procognitive. While individuals taking imipramine experienced significantly improved memory, significant correlations were also obtained between memory tasks and depressive symptom measures, suggesting that imipramine did not directly improve memory.24 Furthermore, amitriptyline may have significant adverse effects on memory.25 Eligible studies that examined TCAs were limited by their relatively small sample size and short study duration, and none of the studies assessing the efficacy of TCAs investigated their effect on functional capacity. Moreover, these studies included differing objective measures of cognition which may explain different results across studies. Nevertheless, further research on the efficacy of TCAs for cognitive impairment is not warranted as studies have consistently demonstrated that TCAs, such as amitriptyline and clomipramine, have lower acceptability rates than other efficacious antidepressants.47
Selective Serotonin Reuptake Inhibitors and Serotonergic Agents
In general, the studies included in this review suggest that older SSRIs, such as fluoxetine and fluvoxamine, are not procognitive, but that newer serotonergic agents may exert positive effects on cognitive functioning. Escitalopram, a newer antidepressant, was correlated with improved executive functioning, attention, processing speed, verbal memory, and nonverbal memory, but worsened verbal fluency.31 However, recent studies have found that escitalopram is inferior to other new antidepressants, such as vortioxetine and duloxetine, at improving cognitive impairments in adults with MDD.28,35
The results obtained in the included studies extended the evidence of vortioxetine’s procognitive efficacy previously demonstrated in elderly patients with MDD.48 Vortioxetine’s effect on cognition appears to be largely direct and independent, rather than an epiphenomenon of depressive symptom improvement,34 suggesting that vortioxetine could be used to target cognitive impairment specifically. Vortioxetine’s procognitive efficacy is likely due to its ability to modulate a wide range of neurotransmitters (ie, dopamine [DA], norepinephrine [NE], γ-aminobutyric acid [GABA]),49 in addition to its multimodal serotonergic actions. Improvements in cognitive functioning with vortioxetine were positively correlated with improvements in functional capacity.7 This finding is important, as MDD is consistently associated with impaired global functioning.50 Thus, among serotonergic agents, vortioxetine has consistently demonstrated its superiority at improving cognitive and functional impairment in MDD.
Serotonin and Norepinephrine Reuptake Inhibitors and Norepinephrine Reuptake Inhibitors
Newer SNRIs are also superior to older SNRIs (eg, reboxetine) at improving cognition in MDD. For instance, both levomilnacipran and desvenlafaxine, newer antidepressants, resulted in significant improvements in measures of cognitive functioning. For levomilnacipran, greater improvements were found in those with higher baseline cognitive impairments,29 suggesting the utility of the drug for individuals with poor baseline cognitive functioning. Moreover, levomilnacipran and desvenlafaxine have shown efficacy in improving functional outcomes in individuals with MDD.29,51,52 Finally, 2 studies27,28 demonstrated that duloxetine improves multiple domains of cognitive functioning. However, in a study comparing its efficacy to vortioxetine and placebo, no significant improvements were found on measures of cognition.36 Discrepancies between studies could be due to the heterogeneity of the cognitive measures used and the fact that the first 2 studies27,28 did not include control groups. Nevertheless, another study (which did not meet inclusion criteria for this review due to improper age range) supports duloxetine’s procognitive effects: a double-blind, placebo-controlled trial, conducted in an elderly population with recurrent MDD, found that duloxetine resulted in a significant improvement in global cognition.53
Norepinephrine-Dopamine Reuptake Inhibitors
It has been hypothesized that antidepressants with potent noradrenergic effects enhance cognition.17 Among included studies, bupropion was shown to have positive effects on cognition, specifically on memory and processing speed. Other studies support these findings. One study, which did not meet criteria for this review, found that 8 weeks of bupropion improved neurocognitive performance in patients with MDD and concurrent suicide risk.54 This study was excluded due to the inclusion of individuals with suicide risk. Additionally, bupropion has been shown to have superior effects to SSRIs on memory in a naturalistic study.17
Multiple studies, including those in this review, have suggested that improvement in verbal memory following bupropion treatment is associated with greater improvement in global functioning.55,56 The mechanism of action of bupropion has been scarcely studied in human populations. Commonly suggested mechanisms are the reuptake inhibition of DA and NE by bupropion and its primary metabolite hydroxybupropion.57,58 One microdialysis study that used the Porsolt animal model of depression measured neurotransmitter levels in the nucleus accumbens of mice and found increased extracellular concentrations of DA and NE in response to bupropion administration.57 Another study found elevated levels of these neurotransmitters in the rat prefrontal cortex (PFC) in response to bupropion.57 Noradrenergic reuptake inhibition in the PFC is shown to improve executive deficits in MDD4; this was not found in either of the 2 included studies investigating bupropion. However, the first study43 included a small sample (N = 20) of younger individuals (mean age = 24). Younger individuals may not experience the same level of impairment as older individuals with MDD since cognitive impairments have been shown to be related to the cumulative duration of depressive episodes.5 Moreover, the second study30 did not include cognitive measures that assess executive functioning.
Non-Antidepressant Agents: Psychostimulants
Stimulant agents have also been investigated for their effectiveness at improving cognitive impairments in MDD. Executive function deficits are of particular interest in MDD59 as they result in significant problems in coping with stressful life events.18,60 NE and DA are important neurotransmitters involved in maintaining executive functions mediated by the PFC61; consequently, agents that modulate these neurotransmitters may improve executive functioning in MDD.18
There is evidence that lisdexamfetamine, a stimulant typically used to treat ADHD, is able to regulate these neurotransmitters. Specifically, lisdexamfetamine is converted to the active metabolite d-amphetamine, which blocks the reuptake of catecholamines, subsequently increasing their release.61,62 Consequently, lisdexamfetamine may be able to enhance cognitive functioning in the PFC. For instance, lisdexamfetamine has been shown to ameliorate subjective executive dysfunction in patients with residual depressive symptoms.63 However, among eligible studies, lisdexamfetamine did not significantly improve objective cognition. The absence of positive results on objective measures could be due to the use of self-reported executive dysfunction as an inclusion criterion.
Another stimulant, caffeine, is frequently used for its psychoactive properties.32 Caffeine was found to enhance the activity of 6 typical antidepressants in mice.64 Of eligible studies, one study found that low doses of caffeine enhanced cognition in adults with MDD.32 Specifically, improvements were seen in subtests of the Montreal Cognitive Assessment, which measures higher cognitive functions, such as executive functioning and WM, and lower cognitive abilities, such as attention. These findings are supported by the general consensus that low doses of caffeine improve lower cognitive functions, such as RT and attention.65 There is less research on the effects of caffeine on higher cognitive abilities.65 Future studies should further investigate the impact of stimulants on objective cognitive impairments and their relationship with improvements in work and life functioning.
Other Non-Antidepressant Agents
Finally, other therapeutic agents were investigated for their procognitive efficacy. Modafinil is an effective wake-promoting agent that has stimulant-like properties.44 One study (which did not meet inclusion criteria due to its use of a single dose of modafinil) found that modafinil significantly improved episodic memory and WM in individuals with remitted depression.66 Modafinil was also found to counteract cognitive impairments associated with sleep deprivation.67 DeBattista and colleagues44 found that modafinil was associated with significant improvements in executive functioning, but not in other cognitive domains. However, this study was limited by its small sample size (N = 31) and short study duration (4 weeks).
Erythropoietin has also been explored for its procognitive effects. Systemically administered erythropoietin is able to cross the blood-brain barrier68 and exert neuroprotective and neurotrophic effects in neuropsychiatric disorders.69 In one study, erythropoietin was found to enhance recall memory and recognition memory in MDD.46 Finally, l-theanine is an amino acid that is contained in green tea and has been suggested to have psychotropic effects.45 l-Theanine was correlated with improved cognition; however, the study was limited by its small sample (N = 20) and open-label design.45 It is difficult to make definitive conclusions regarding the procognitive efficacy of non-antidepressant agents as there are few clinical trials focusing on these. Future studies should continue to assess the effectiveness of these agents using larger samples, controlled trials, and objective cognitive measures as primary outcome measures.
Limitations
There were several limitations to the studies included in our review. Seven of the 26 studies were open-label. A lack of blinding to study medication can lead to patient and experimenter bias, which can subsequently influence the results. There was a substantial difference between sample sizes across studies. Moreover, many of the studies were short-term (ie, 4 weeks), which restricts the ability to make any conclusions regarding long-term treatment effects on cognition.
A key limitation to the review was the large diversity of objective measures used in the included studies. This heterogeneity makes it difficult to compare study results and to make definitive conclusions regarding treatment outcomes. In addition, the majority of studies did not include objective cognitive functioning as a primary outcome measure, resulting in the use of brief cognitive batteries. These batteries may not encompass the broad range of cognitive domains that may be impaired in MDD. A strict focus on studies that include objective cognitive functioning as a primary outcome measure would be ideal. However, very few of these studies currently exist.
Newer drugs, such as vortioxetine, are more likely to be studied for their procognitive efficacy because older drugs have low acceptability rates.47 Nevertheless, this may introduce bias favoring newer drugs over older ones. Specifically, newer drugs are more likely to be investigated, increasing the likelihood that positive results are obtained for these agents.
It is also important to note that patients’ symptom profiles may bias drug choice and response. For instance, those with psychomotor slowing are more likely to be prescribed and respond to bupropion.70 Consequently, sample populations in bupropion studies may have psychomotor slowing due to recruitment bias and may show greater efficacy compared to studies in which symptom profiles are not considered.
In addition, the included studies include heterogenous samples that may include participants without objective cognitive impairment. If unimpaired patients are included in trials for cognition, they are likely to weaken the treatment effect observed in participants who are impaired and who do show positive improvements. This introduces an important limitation to studies that did not limit their sample to participants with objective cognitive impairments.
In this systematic review, we excluded studies that included participants with bipolar disorder. This was done to ensure a homogenous study population and because bipolar patients are more cognitively impacted by their illness than unipolar depressed patients.71 However, this represents a limitation since information regarding the procognitive effects of certain drugs may be missing. For instance, ketamine has been studied for its procognitive efficacy in samples that include bipolar patients.72
Finally, while the current review is comprehensive, a meta-analysis would be ideal. However, the purpose of this systematic review was to synthesize the best available evidence regarding the procognitive efficacy of therapeutic agents. A meta-analysis with the use of statistical methods was not conducted as it was not within the expertise of our research team. Future studies should consider conducting a meta-analysis on the summarized studies.
In conclusion, the aim of this review was to investigate the effects of antidepressants and other agents on cognitive impairments in adults with MDD, with a strict focus on objective measures of cognitive functioning. Although some positive effects have been found for multiple cognitive domains, the results of different studies are contradictory and inconclusive. Overall, vortioxetine has the greatest support for its procognitive effects; SNRIs, NRIs, and bupropion also show promise, but more research is needed. Although some agents show promising results, the rates of cognitive impairment remain high despite remission.6 Nevertheless, the cause of cognitive impairment in MDD is complex: severity of symptoms,73 cumulative duration of depressive episodes,5 and presence of comorbidities4 all contribute to impaired cognition in MDD. Additionally, individuals differ in terms of experienced impairments, and therapeutic agents may also differ in regard to which cognitive impairments they target. Consequently, targeting cognitive deficits in current and residual MDD is difficult. Future studies should continue to investigate the effect of antidepressants and non-antidepressant agents using standardized and objective cognitive testing as a primary outcome measure. Furthermore, it would be beneficial to explore the impact of these therapies on functional outcomes and how these relate to improvements in cognitive functioning and depressive symptomatology. The current review offers a thorough summary of present-day pharmacologic treatments for cognitive impairment in MDD. The findings of this review will help inform clinicians for prescribing effective medications for patients with MDD and cognitive impairments.
Submitted: December 9, 2019; accepted May 6, 2020.
Published online: July 21, 2020.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, imipramine, amitriptyline, clovoxamine, escitalopram, vortioxetine, duloxetine, paroxetine, fluoxetine, fluvoxamine, desvenlafaxine, levomilnacipran, reboxetine, citalopram, bupropion, lisdexamfetamine, modafinil, erythropoietin, and intranasal insulin are not approved by the US Food and Drug Administration for the treatment of cognitive impairment in major depressive disorder.
Financial disclosure: Dr McInerney has received consulting fees from Janssen in the last 5 years. Mss Blumberg and Vaccarino have no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
Funding/support: None.
REFERENCES
1. Depression (fact sheet). Geneva, Switzerland: World Health Organization; 2018.
2. American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
3. Rock PL, Roiser JP, Riedel WJ, et al. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. 2014;44(10):2029-2040. PubMed CrossRef
4. Murrough JW, Iacoviello B, Neumeister A, et al. Cognitive dysfunction in depression: neurocircuitry and new therapeutic strategies. Neurobiol Learn Mem. 2011;96(4):553-563. PubMed CrossRef
5. Hasselbalch BJ, Knorr U, Hasselbalch SG, et al. The cumulative load of depressive illness is associated with cognitive function in the remitted state of unipolar depressive disorder. Eur Psychiatry. 2013;28(6):349-355. PubMed CrossRef
6. Bhalla RK, Butters MA, Mulsant BH, et al. Persistence of neuropsychologic deficits in the remitted state of late-life depression. Am J Geriatr Psychiatry. 2006;14(5):419-427. PubMed CrossRef
7. Baune BT, Sluth LB, Olsen CK. The effects of vortioxetine on cognitive performance in working patients with major depressive disorder: a short-term, randomized, double-blind, exploratory study. J Affect Disord. 2018;229:421-428. PubMed CrossRef
8. Godard J, Grondin S, Baruch P, et al. Psychosocial and neurocognitive profiles in depressed patients with major depressive disorder and bipolar disorder. Psychiatry Res. 2011;190(2-3):244-252. PubMed CrossRef
9. Cambridge OR, Knight MJ, Mills N, et al. The clinical relationship between cognitive impairment and psychosocial functioning in major depressive disorder: a systematic review. Psychiatry Res. 2018;269:157-171. PubMed CrossRef
10.Lam RW, Kennedy SH, Mclntyre RS, et al. Cognitive dysfunction in major depressive disorder: effects on psychosocial functioning and implications for treatment. Can J Psychiatry. 2014;59(12):649-654. PubMed CrossRef
11.Ro E, Clark LA. Psychosocial functioning in the context of diagnosis: assessment and theoretical issues. Psychol Assess. 2009;21(3):313-324. PubMed CrossRef
12.Knight MJ, Baune BT. Cognitive dysfunction in major depressive disorder. Curr Opin Psychiatry. 2018;31(1):26-31. PubMed CrossRef
13.Cha DS, Best MW, Bowie CR, et al. A randomized, double-blind, placebo-controlled, crossover trial evaluating the effect of intranasal insulin on cognition and mood in individuals with treatment-resistant major depressive disorder. J Affect Disord. 2017;210:57-65. PubMed CrossRef
14.IsHak WW, James DM, Mirocha J, et al. Patient-reported functioning in major depressive disorder. Ther Adv Chronic Dis. 2016;7(3):160-169. PubMed CrossRef
15.Rosenblat JD, Kakar R, McIntyre RS. The cognitive effects of antidepressants in major depressive disorder: a systematic review and meta-analysis of randomized clinical trials. Int J Neuropsychopharmacol. 2015;19(2):pyv082. PubMed CrossRef
16.Al-Sukhni M, Maruschak NA, McIntyre RS. Vortioxetine: a review of efficacy, safety and tolerability with a focus on cognitive symptoms in major depressive disorder. Expert Opin Drug Saf. 2015;14(8):1291-1304. PubMed CrossRef
17.Gualtieri CT, Johnson LG. Bupropion normalizes cognitive performance in patients with depression. MedGenMed. 2007;9(1):22. PubMed
18.Madhoo M, Keefe RS, Roth RM, et al. Lisdexamfetamine dimesylate augmentation in adults with persistent executive dysfunction after partial or full remission of major depressive disorder. Neuropsychopharmacology. 2014;39(6):1388-1398. PubMed CrossRef
19.Vaccarino SR, McInerney SJ, Kennedy SH, et al. The potential procognitive effects of modafinil in major depressive disorder: a systematic review. J Clin Psychiatry. 2019;80(6):19r16767. PubMed CrossRef
20.Mowla A, Ashkani H, Ghanizadeh A, et al. Do memory complaints represent impaired memory performance in patients with major depressive disorder? Depress Anxiety. 2008;25(10):E92-E96. PubMed CrossRef
21.Fischer C, Schweizer TA, Atkins JH, et al. Neurocognitive profiles in older adults with and without major depression. Int J Geriatr Psychiatry. 2008;23(8):851-856. PubMed CrossRef
22.Guyatt GH, Oxman AD, Schünemann HJ, et al. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380-382. PubMed CrossRef
23.Meader N, King K, Llewellyn A, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Syst Rev. 2014;3(1):82. PubMed CrossRef
24.Peselow ED, Corwin J, Fieve RR, et al. Disappearance of memory deficits in outpatient depressives responding to imipramine. J Affect Disord. 1991;21(3):173-183. PubMed CrossRef
25.Spring B, Gelenberg AJ, Garvin R, et al. Amitriptyline, clovoxamine and cognitive function: a placebo-controlled comparison in depressed outpatients. Psychopharmacology (Berl). 1992;108(3):327-332. PubMed CrossRef
26.Bastos AG, Guimar×£es LS, Trentini CM. Neurocognitive changes in depressed patients in psychodynamic psychotherapy, therapy with fluoxetine and combination therapy. J Affect Disord. 2013;151(3):1066-1075. PubMed CrossRef
27.Greer TL, Sunderajan P, Grannemann BD, et al. Does duloxetine improve cognitive function independently of its antidepressant effect in patients with major depressive disorder and subjective reports of cognitive dysfunction? Depress Res Treat. 2014;2014:627863. PubMed CrossRef
28.Herrera-Guzmán I, Gudayol-Ferré E, Herrera-Guzmán D, et al. Effects of selective serotonin reuptake and dual serotonergic-noradrenergic reuptake treatments on memory and mental processing speed in patients with major depressive disorder. J Psychiatr Res. 2009;43(9):855-863. PubMed CrossRef
29.Wesnes KA, Gommoll C, Chen C, et al. Effects of levomilnacipran extended-release on major depressive disorder patients with cognitive impairments: post-hoc analysis of a phase III study. Int Clin Psychopharmacol. 2017;32(2):72-79. PubMed CrossRef
30.Herrera-Guzmán I, Gudayol-Ferré E, Lira-Mandujano J, et al. Cognitive predictors of treatment response to bupropion and cognitive effects of bupropion in patients with major depressive disorder. Psychiatry Res. 2008;160(1):72-82. PubMed CrossRef
31.Wroolie TE, Williams KE, Keller J, et al. Mood and neuropsychological changes in women with midlife depression treated with escitalopram. J Clin Psychopharmacol. 2006;26(4):361-366. PubMed CrossRef
32.Liu QS, Deng R, Fan Y, et al. Low dose of caffeine enhances the efficacy of antidepressants in major depressive disorder and the underlying neural substrates. Mol Nutr Food Res. 2017;61(8):1-11. PubMed CrossRef
33.Glass RM, Uhlenhuth EH, Hartel FW, et al. Cognitive dysfunction and imipramine in outpatient depressive. Arch Gen Psychiatry. 1981;38(9):1048-1051. PubMed CrossRef
34.McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol. 2014;17(10):1557-1567. PubMed CrossRef
35.Vieta E, Sluth LB, Olsen CK. The effects of vortioxetine on cognitive dysfunction in patients with inadequate response to current antidepressants in major depressive disorder: a short-term, randomized, double-blind, exploratory study versus escitalopram. J Affect Disord. 2018;227:803-809. PubMed CrossRef
36.Mahableshwarkar AR, Zajecka J, Jacobson W, et al. A randomized, placebo-controlled, active-reference, double-blind, flexible-dose study of the efficacy of vortioxetine on cognitive function in major depressive disorder. Neuropsychopharmacology. 2015;40(8):2025-2037. PubMed CrossRef
37.Richardson JS, Keegan DL, Bowen RC, et al. Verbal learning by major depressive disorder patients during treatment with fluoxetine or amitriptyline. Int Clin Psychopharmacol. 1994;9(1):35-40. PubMed CrossRef
38.Keegan D, Bowen RC, Blackshaw S, et al. A comparison of fluoxetine and amitriptyline in the treatment of major depression. Int Clin Psychopharmacol. 1991;6(2):117-124. PubMed CrossRef
39.Koetsier GC, Volkers AC, Tulen JH, et al. CPT performance in major depressive disorder before and after treatment with imipramine or fluvoxamine. J Psychiatr Res. 2002;36(6):391-397. PubMed CrossRef
40.Lam RW, Iverson GL, Evans VC, et al. The effects of desvenlafaxine on neurocognitive and work functioning in employed outpatients with major depressive disorder. J Affect Disord. 2016;203:55-61. PubMed CrossRef
41.Ferguson JM, Wesnes KA, Schwartz GE. Reboxetine versus paroxetine versus placebo: effects on cognitive functioning in depressed patients. Int Clin Psychopharmacol. 2003;18(1):9-14. PubMed
42.Göder R, Seeck-Hirschner M, Stingele K, et al. Sleep and cognition at baseline and the effects of REM sleep diminution after 1 week of antidepressive treatment in patients with depression. J Sleep Res. 2011;20(4):544-551. PubMed CrossRef
43.Soczynska JK, Ravindran LN, Styra R, et al. The effect of bupropion XL and escitalopram on memory and functional outcomes in adults with major depressive disorder: results from a randomized controlled trial. Psychiatry Res. 2014;220(1-2):245-250. PubMed CrossRef
44.DeBattista C, Lembke A, Solvason HB, et al. A prospective trial of modafinil as an adjunctive treatment of major depression. J Clin Psychopharmacol. 2004;24(1):87-90. PubMed CrossRef
45.Hidese S, Ota M, Wakabayashi C, et al. Effects of chronic l-theanine administration in patients with major depressive disorder: an open-label study. Acta Neuropsychiatr. 2017;29(2):72-79. PubMed CrossRef
46.Miskowiak KW, Vinberg M, Christensen EM, et al. Recombinant human erythropoietin for treating treatment-resistant depression: a double-blind, randomized, placebo-controlled phase 2 trial. Neuropsychopharmacology. 2014;39(6):1399-1408. PubMed CrossRef
47.Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391(10128):1357-1366. PubMed CrossRef
48.Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol. 2012;27(4):215-223. PubMed CrossRef
49.Salagre E, Grande I, Solé B, et al. Vortioxetine: A new alternative for the treatment of major depressive disorder [in Spanish]. Rev Psiquiatr Salud Ment. 2018;11(1):48-59. PubMed CrossRef
50.Motter JN, Pimontel MA, Rindskopf D, et al. Computerized cognitive training and functional recovery in major depressive disorder: a meta-analysis. J Affect Disord. 2016;189:184-191. PubMed CrossRef
51.Dunlop BW, Reddy S, Yang L, et al. Symptomatic and functional improvement in employed depressed patients: a double-blind clinical trial of desvenlafaxine versus placebo. J Clin Psychopharmacol. 2011;31(5):569-576. PubMed CrossRef
52.Soares CN, Kornstein SG, Thase ME, et al. Assessing the efficacy of desvenlafaxine for improving functioning and well-being outcome measures in patients with major depressive disorder: a pooled analysis of 9 double-blind, placebo-controlled, 8-week clinical trials. J Clin Psychiatry. 2009;70(10):1365-1371. PubMed CrossRef
53.Raskin J, Wiltse CG, Siegal A, et al. Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: an 8-week, double-blind, placebo-controlled trial. Am J Psychiatry. 2007;164(6):900-909. PubMed CrossRef
54.Gorlyn M, Keilp J, Burke A, et al. Treatment-related improvement in neuropsychological functioning in suicidal depressed patients: paroxetine vs bupropion. Psychiatry Res. 2015;225(3):407-412. PubMed CrossRef
55.Baune BT, Miller R, McAfoose J, et al. The role of cognitive impairment in general functioning in major depression. Psychiatry Res. 2010;176(2-3):183-189. PubMed CrossRef
56.Rothschild AJ, Raskin J, Wang CN, et al. The relationship between change in apathy and changes in cognition and functional outcomes in currently non-depressed SSRI-treated patients with major depressive disorder. Compr Psychiatry. 2014;55(1):1-10. PubMed CrossRef
57.Stahl SM, Pradko JF, Haight BR, et al. A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Prim Care Companion J Clin Psychiatry. 2004;6(4):159-166. PubMed CrossRef
58.Ascher JA, Cole JO, Colin JN, et al. Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry. 1995;56(9):395-401. PubMed
59.Reppermund S, Ising M, Lucae S, et al. Cognitive impairment in unipolar depression is persistent and non-specific: further evidence for the final common pathway disorder hypothesis. Psychol Med. 2009;39(4):603-614. PubMed CrossRef
60.Marazziti D, Consoli G, Picchetti M, et al. Cognitive impairment in major depression. Eur J Pharmacol. 2010;626(1):83-86. PubMed CrossRef
61.Arnsten AFT, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57(11):1377-1384. PubMed CrossRef
62.Solanto MV. Dopamine dysfunction in AD/HD: integrating clinical and basic neuroscience research. Behav Brain Res. 2002;130(1-2):65-71. PubMed CrossRef
63.Harvey PD, et al. Assessment of executive dysfunction in adults with major depressive disorder receiving lisdexamfetamine dimesylate augmentation of escitalopram. Presented at the 165th Annual Meeting of the American Psychiatric Association. 2012; New York, NY.
64.Szopa A, Poleszak E, Wyska E, et al. Caffeine enhances the antidepressant-like activity of common antidepressant drugs in the forced swim test in mice. Naunyn Schmiedebergs Arch Pharmacol. 2016;389(2):211-221. PubMed CrossRef
65.McLellan TM, Caldwell JA, Lieberman HR. A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci Biobehav Rev. 2016;71:294-312. PubMed CrossRef
66.Kaser M, Deakin JB, Michael A, et al. Modafinil improves episodic memory and working memory cognition in patients with remitted depression: a double-blind, randomized, placebo-controlled study. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2(2):115-122. PubMed
67.Batéjat DM, Lagarde DP. Naps and modafinil as countermeasures for the effects of sleep deprivation on cognitive performance. Aviat Space Environ Med. 1999;70(5):493-498. PubMed
68.Brines ML, Ghezzi P, Keenan S, et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 2000;97(19):10526-10531. PubMed CrossRef
69.Sirén AL, Fasshauer T, Bartels C, et al. Therapeutic potential of erythropoietin and its structural or functional variants in the nervous system. Neurotherapeutics. 2009;6(1):108-127. PubMed CrossRef
70.Randall PA, Lee CA, Podurgiel SJ, et al. Bupropion increases selection of high effort activity in rats tested on a progressive ratio/chow feeding choice procedure: implications for treatment of effort-related motivational symptoms. Int J Neuropsychopharmacol. 2014;18(2):1-11. PubMed CrossRef
71.Gualtieri CT, Morgan DW. The frequency of cognitive impairment in patients with anxiety, depression, and bipolar disorder: an unaccounted source of variance in clinical trials. J Clin Psychiatry. 2008;69(7):1122-1130. PubMed CrossRef
72.Lara DR, Bisol LW, Munari LR. Antidepressant, mood stabilizing and procognitive effects of very low dose sublingual ketamine in refractory unipolar and bipolar depression. Int J Neuropsychopharmacol. 2013;16(9):2111-2117. PubMed CrossRef
73.Jaeger J, Berns S, Uzelac S, et al. Neurocognitive deficits and disability in major depressive disorder. Psychiatry Res. 2006;145(1):39-48. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite