Prophylactic Antidepressant Treatment of Interferon-Induced Depression in Chronic Hepatitis C: A Systematic Review and Meta-Analysis

ABSTRACT
Objective: To assess the utility of prophylactic administration of antidepressants in preventing a major depressive episode during antiviral treatment for chronic hepatitis C.
Data Sources: A computerized literature search was conducted in MEDLINE, PsycINFO, EMBASE, the Cochrane Library, and ClinicalTrials.gov to locate articles published in any language from the earliest available online year until October 2012, using the following phrase and Boolean logic algorithm: "hepatitis and c and (interferon-alpha OR peginterferon OR (pegylated and interferon)) and (depression OR mood) and (prevention OR prophylactic OR prophylaxis OR antidepressant)."
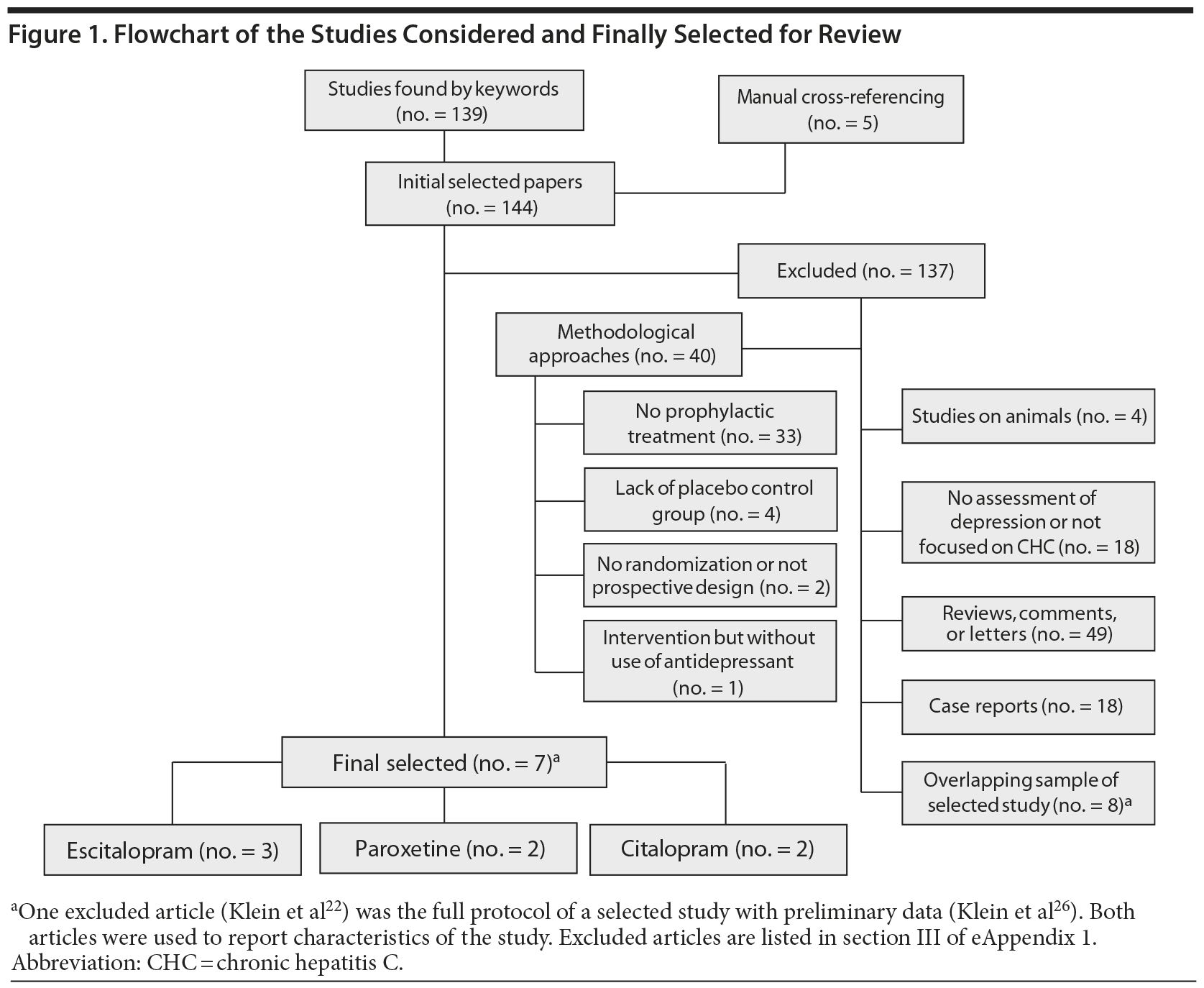
Study Selection: Double-blind, randomized, placebo-controlled trials using antidepressants prophylactically before starting antiviral therapy for chronic hepatitis C were included. At baseline, none of the patients in the trials presented depression (DSM-IV-TR criteria). Using keywords and cross-referenced bibliographies, 144 studies were identified and examined in depth. 137 articles were rejected because inclusion criteria were not met. Finally, 7 studies were included.
Data Extraction: Data were extracted independently by 2 investigators. The primary outcome measure was the onset of a major depressive episode during the antiviral treatment. Depressive symptoms, other side effects, and sustained virologic response were also examined. A full review and meta-analysis were performed. Odds ratios (ORs), mean differences, and estimated numbers needed to treat (NNTs) with 95% confidence intervals (CIs) were calculated.
Results: 591 patients were randomly assigned to antiviral treatment and another intervention: escitalopram (n = 197), paroxetine (n = 42), citalopram (n = 53), or placebo (n = 299). Selective serotonin reuptake inhibitors (SSRIs), as a group, reduced the incidence of a major depressive episode during antiviral treatment (OR = 0.53; 95% CI, 0.33 to 0.84). The NNT was 12 (95% CI, 7.0 to 37.9). SSRIs reduced depressive symptoms at 24 weeks of treatment (mean difference −2.18; 95% CI, −4.25 to −0.10). With regard to side effects, only dizziness was associated with administration of antidepressants (OR = 2.65; 95% CI, 1.46 to 4.80). There were no differences in sustained virologic response (OR = 1.22; 95% CI, 0.58 to 2.57).
Conclusions: Administration of SSRIs before starting antiviral treatment reduces the incidence of interferon-induced depression, with a relatively moderate prophylactic impact and good tolerability.
J Clin Psychiatry 2014;75(10):e1113-e1121
© Copyright 2014 Physicians Postgraduate Press, Inc.
Submitted: September 17, 2013; accepted February 28, 2014 (doi:10.4088/JCP.13r08800).
Corresponding author: Roc×o Mart×n-Santos, MD, PhD, Department of Psychiatry and Psychology, Institut Cl×nic de Neurociרncies, Hospital Clinic, IDIBAPS, Universitat de Barcelona, CIBERSAM, 170 Villarroel St, 08036-Barcelona, Spain ([email protected]).
Major depressive disorder is the leading cause of life disability and one of the most expensive illnesses for society, in terms of both direct and indirect costs.1,2 The prevalence of depression is important in patients with medical conditions related to inflammatory processes, such as cardiovascular diseases, rheumatoid arthritis, autoimmune disorders, obesity, or chronic hepatitis C (CHC).3 Moreover, there is substantial evidence for the role of cytokine therapies in inducing depressive symptoms in clinical populations.4,5
Hepatitis C virus infection is a significant public health problem that affects 130-170 million people worldwide.6,7 The approved treatment for CHC is the combination of pegylated interferon-α (IFN-α) and antiviral ribavirin for 24 or 48 weeks.8 Recently, the US Food and Drug Administration and the European Medicines Agency recommended the addition of a protease inhibitor in patients with viral genotype 1.9,10 The problem with antiviral treatment is that it has a high profile of side effects, including fatigue, insomnia, irritability, and low mood, with a full major depressive episode (MDE) being observed in about 25% of patients treated, according to a previous meta-analysis.11 Prevention or proper management of IFN-induced depression is therefore essential, because depressive patients often show a poor quality of life, suicidal ideation, a lack of treatment adherence, and alterations to their sustained virologic response (SVR).5
Although the exact neurobiological basis of IFN-induced depression is not known, there is evidence that administration of an exogenous cytokine such as IFN-α leads to the activation of certain proinflammatory cytokines, causing alterations in brain apoptotic mechanisms and neurotransmission.4 Cytokine-induced alterations within the central nervous system (CNS) may rely on different mechanisms, including passage of cytokines through leaky regions of the blood-brain barrier and activation of nervous pathways. A high concentration of proinflammatory cytokines with a CNS action may modulate the serotonergic system, a factor related to depression.12 Antidepressants such as selective serotonin reuptake inhibitors (SSRIs) selectively block the reuptake of serotonin into the presynaptic terminal and initiate their therapeutic effect through the increase of serotonin at the synaptic cleft.13 SSRIs have been proposed as a useful treatment for IFN-induced depression.14 Given the high incidence of depressive illness during antiviral treatment and its potential impact on quality of life and virologic response, the prevention of IFN-induced depression would be a useful intervention. However, prophylactic administration of antidepressants in all patients starting antiviral therapy for CHC is controversial.15,16
The aim of this study was to carry out a systematic review and meta-analysis of data that could help to assess the benefits of using prophylactic antidepressants during antiviral treatment for CHC.
METHOD
Data for this systematic review were collected with an advanced document protocol in accordance with the PRISMA guidelines17 (see eAppendix 1, section I).
All steps in the literature search, study identification, study selection, quality assessment, and data extraction were performed independently by 2 clinical researchers (M.U. and D.H.). Disagreements were resolved by discussion, and consensus was achieved in the selection of articles for analysis.
Study Identification
A comprehensive, computerized literature search was conducted in MEDLINE, PsycINFO, EMBASE, the Cochrane Library, and ClinicalTrials.gov. We searched for relevant studies published from the earliest available online year until October 2012, using the following phrase and Boolean logic algorithm: "hepatitis and c and (interferon-alpha OR peginterferon OR (pegylated and interferon)) and (depression OR mood) and (prevention OR prophylactic OR prophylaxis OR antidepressant)." We also searched for any additional studies in the reference lists of the articles identified and conference proceedings.

- Administration of selective serotonin reuptake inhibitors (SSRIs) before starting antiviral treatment for chronic hepatitis C reduces the incidence of interferon-induced depression.
- SSRIs did not alter sustained virologic response and showed good tolerability, except for increasing dizziness during antiviral treatment.
- Individual studies suggested that patients who had a history of depression or showed subthreshold depressive symptoms at baseline may benefit greatly from prophylactic treatment with SSRIs; however, this possibility could not be confirmed in the meta-analysis.
After titles and abstracts were examined, full-text articles of potentially relevant studies were obtained. Inclusion and exclusion criteria were then applied, and the selected articles were included in the systematic review.
Study Selection
Articles were reviewed using the following inclusion criteria: (1) randomized clinical trials using prophylactic antidepressants in patients receiving antiviral therapy for CHC; (2) inclusion of control group treated with placebo; (3) double-blind study design; (4) a detailed description of methods and methodological background that included assessment of MDD using a validated instrument or a semistructured interview performed by a trained clinician based on DSM-IV criteria; (5) psychiatric assessment before starting the treatment, and a good description of this; (6) euthymia at baseline (not fulfilling criteria for a DSM-IV/ICD depressive episode); and (7) initiation of antidepressants before starting antiviral therapy at therapeutic doses.
The following exclusion criteria were applied: (1) naturalistic, nonrandomized, or non-placebo-controlled design; (2) follow-up < 12 weeks; (3) articles concerning overlapping samples; and (4) sample size < 10.
Quality of sequence generation, allocation concealment, blinding, missing outcome data, selective reporting, and other biases were assessed with the Cochrane risk of bias method.18
Summary Measures (Outcomes)
The primary outcome measure was the onset of an MDE according to DSM-IV criteria during the antiviral treatment.
The secondary outcomes were (1) depressive symptomatology scores during antiviral treatment, based on a validated rating scale; (2) presence of potential side effects attributed to combination treatment (antidepressant and antiviral therapy); and (3) proportion of patients achieving SVR.
Data Extraction
For each article, we recorded the author, year of publication, design, characteristics of the sample, viral coinfection, adjunctive psychopharmacology, instruments for assessing depression, dose and type of IFN-α, adjunctive ribavirin follow-up time, and data on discontinuation and patients lost to follow-up. Outcomes of incidence of MDE, SVR, depressive symptoms, and potential side effects were abstracted for each group.
Statistical Analysis
The odds ratio (OR) with 95% confidence interval (CI) was used to estimate the strength of association of dichotomous variables. For statistically significant results, we calculated the number needed to treat (NNT) statistic and its 95% CI as the inverse of the risk difference. The mean difference with 95% CI was used to estimate the strength of association of quantitative variables.
Heterogeneity between trials was assessed using both the χ2 and I2 tests. Between-study heterogeneity was considered to be significant for a P value < .10 on the χ2 test. If there was no heterogeneity, a fixed model was used. In the event of heterogeneity, a random-effects model was used.
To establish the robustness of the primary outcome by sensitivity analyses, we applied a random-effects model and excluded studies with higher risk of bias and studies with short follow-up.
Publication bias was examined in a funnel plot of log OR against its standard error, using Begg test, while the degree of asymmetry was tested statistically using Egger unweighted regression asymmetry test.19,20
Statistical analyses were performed using Review Manager, Version 5.0 (The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen, Denmark).
RESULTS
With the use of keywords and cross-referenced bibliographies, 144 studies were identified and examined in depth. One hundred thirty-seven articles were rejected because inclusion criteria were not met (Figure 1 and eAppendix 1, section II). Finally, 7 different studies were selected for systematic review.15,16,21-25 Data were extracted from 6 full original articles15,16,21,23-25 and 1 poster presented at an international congress.26 The selected studies were published between 2007 and 2012, and all were reported in English. This review includes a total of 591 CHC patients who were randomly allocated to initiate antiviral treatment plus antidepressant (n = 292) or placebo (n = 299).
Characteristics of the Studies
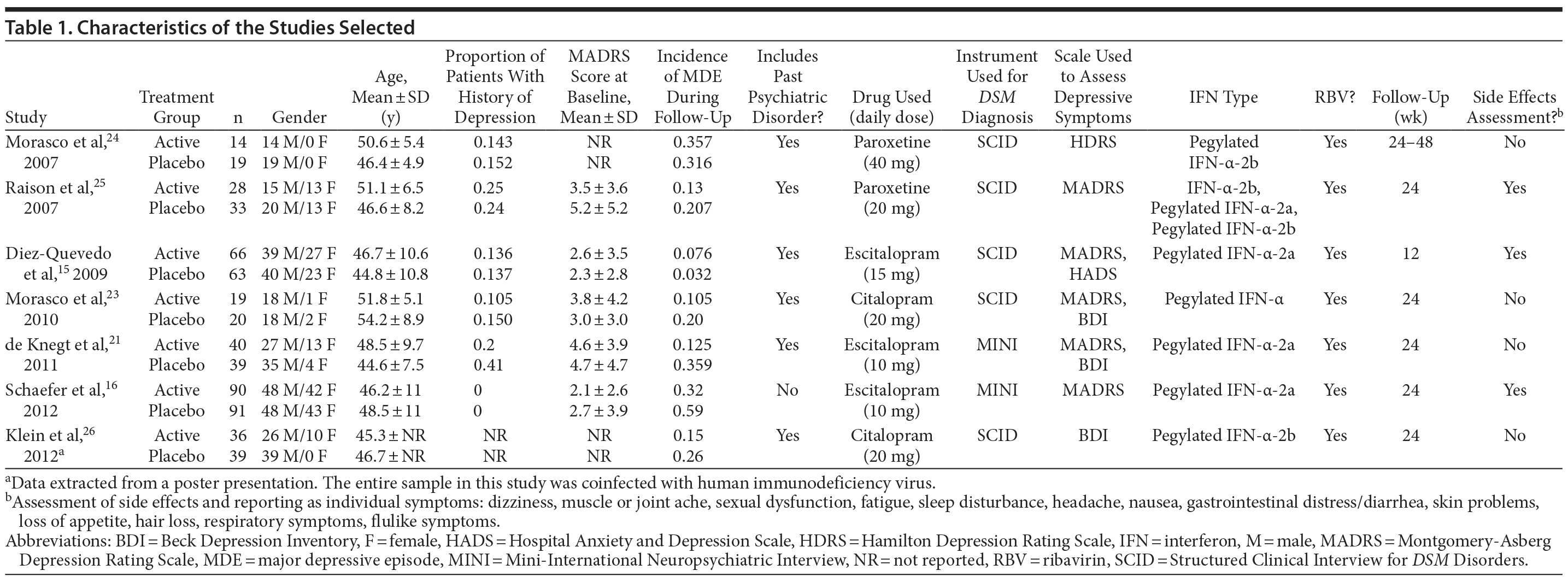
The characteristics of the selected studies are reported in Table 1. Three studies used escitalopram (10-15 mg/d),15,16,21 2 used citalopram (20 mg/d),22,23 and 2 used paroxetine (20-40 mg/d).24,25 All of the studies excluded patients with a current MDE and those treated with other antidepressants. Five studies15,21,23-25 included patients with a history of a depressive episode in both the case group and the control group (range, 10%-40% for each group). Twenty-eight patients treated with an SSRI (9.6%) and 39 treated with placebo (13.0%) had a past depressive episode.
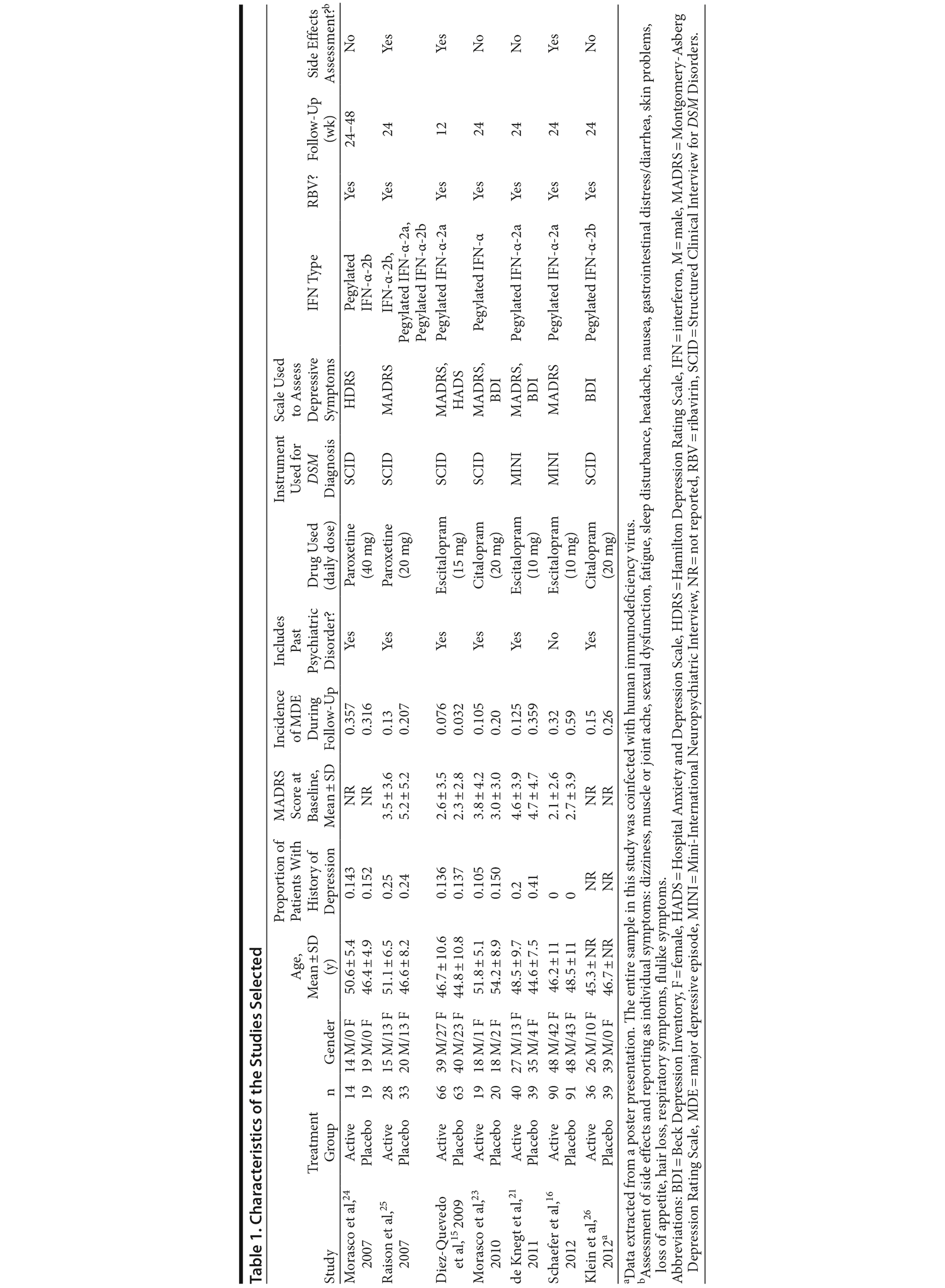
In terms of potential bias, all studies were randomized, but only 5 studies15,21,23,25,26 had an adequate description of randomization, and 4,15,21,25,26 of allocation concealment. All studies were described as double-blind. Some studies did not report loss of subjects to follow-up, SVR, or potential side effects. Other sources of bias were imbalance in risk factors for depression between groups and short follow-up (12 weeks) (eAppendix 1, sections III and IV).
Incidence of Depression
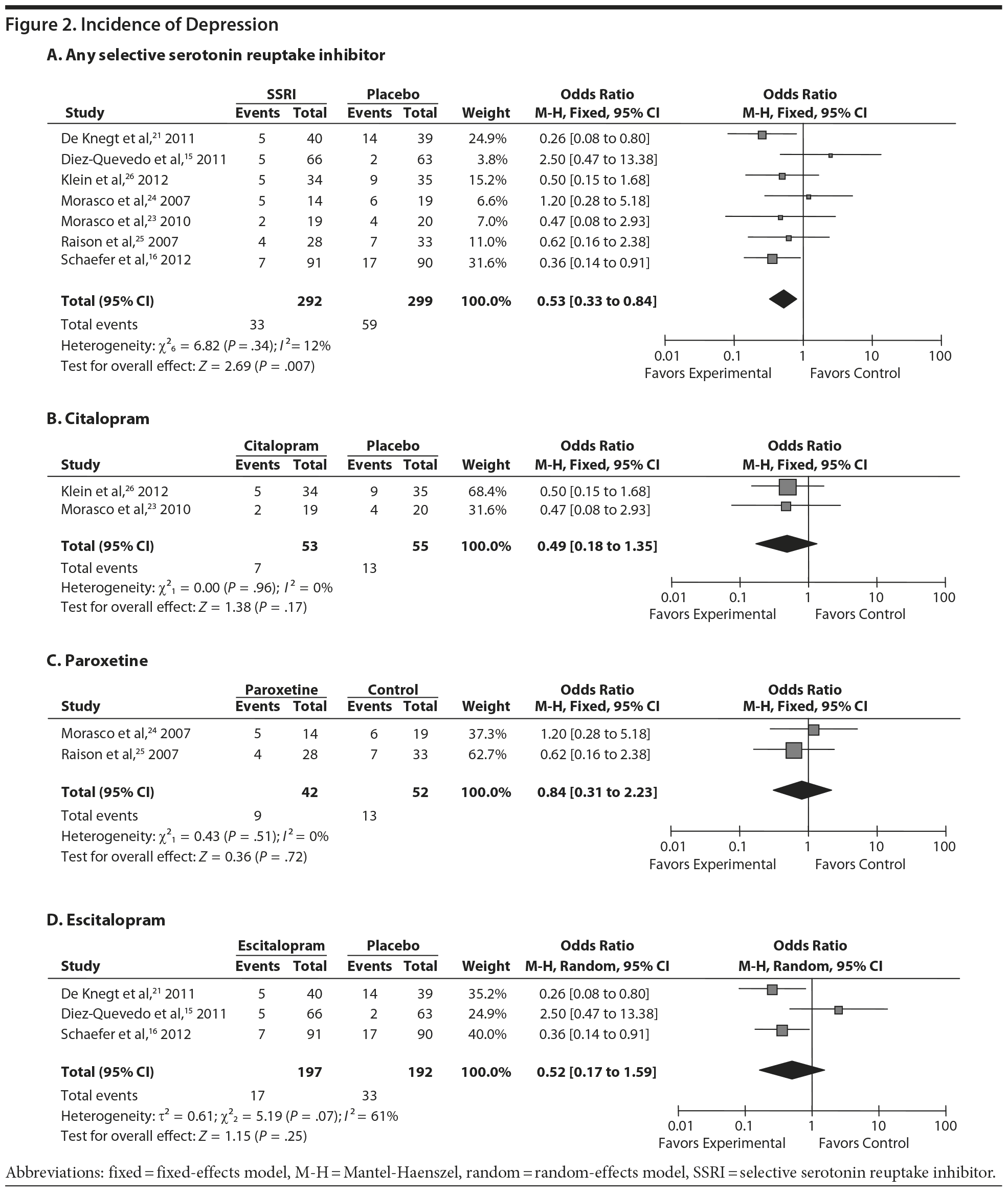
All selected studies reported the incidence of depression during follow-up. A total of 292 patients treated with an SSRI and 299 patients treated with placebo were included in the analysis of incidence. Thirty-three patients (11.3%) in the antidepressant group and 59 (19.7%) in the placebo group presented an MDE during follow-up. According to our meta-analysis, the use of any SSRI reduced the incidence of IFN-induced depression, as compared with placebo (OR = 0.53; 95% CI, 0.33 to 0.84) (Figure 2). The overall estimated NNT in order to prevent 1 MDE was 12 (95% CI, 7.0 to 37.9).
The meta-analysis was also performed for each type of antidepressant separately. Three studies that included 197 patients treated with escitalopram and 192 patients treated with placebo reported no significant differences in depression rates (OR = 0.52; 95% CI, 0.17 to 1.59). Neither were there any differences in depression rates according to the analyses of (1) 42 patients treated with paroxetine versus 52 patients treated with placebo (OR = 0.84; 95% CI, 0.31 to 2.23) or (2) 53 patients treated with citalopram versus 59 patients treated with placebo (OR = 0.49; 95% CI, 0.18 to 1.35) (Figure 2).
Changes in Depressive Symptoms
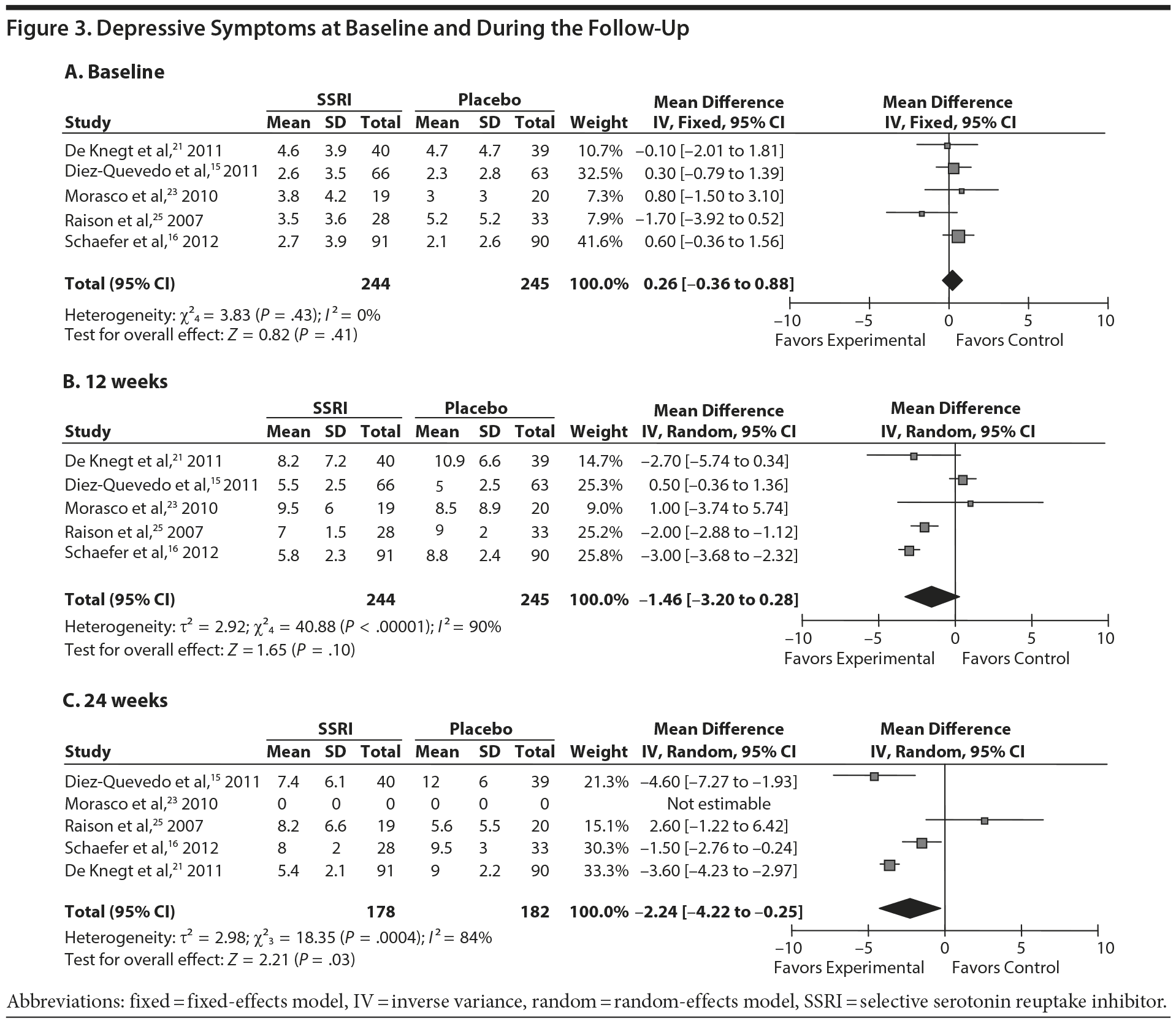
Five of the 7 studies reported mean scores with standard deviations of depressive symptoms at baseline and at least 1 follow-up point.15,16,21,24,25 All of these studies reported depression scores using the Montgomery-Asberg Depression Rating Scale (MADRS), and these data were extracted for meta-analysis. Five studies examined MADRS scores at baseline and at 12 weeks of antiviral treatment, while 4 of these also did so at 24 weeks.16,21,24,25 At baseline, MADRS scores did not differ between cases and controls (mean difference = 0.26; 95% CI, −0.36 to 0.88). However, the SSRI group presented significantly fewer depressive symptoms at 24 weeks of treatment (mean difference = −2.18; 95% CI, −4.25 to −0.10), although this was not evident at 12 weeks (mean difference = −1.45; 95% CI, −3.24 to 0.34) (Figure 3).
Side Effects
Three studies reported a list of the incidence of different symptoms attributed to the combination of antiviral treatment and SSRI or placebo.15,16,25 Two studies used escitalopram, and 1 used paroxetine, and together they assessed a total of 184 patients treated with SSRI and 187 patients treated with placebo.
Compared with placebo, administration of a prophylactic antidepressant increased symptoms of dizziness (OR = 2.61; 95% CI, 1.44 to 4.72). Conversely, patients treated with SSRIs reported less muscle or joint ache (OR = 0.63; 95% CI, 0.42 to 0.96). According to our meta-analysis, there were no differences between SSRI and placebo in relation to the following symptoms: sexual dysfunction (OR = 2.34; 95% CI, 0.97 to 5.61), fatigue (OR = 0.83; 95% CI, 0.56 to 1.25), sleep disturbance (OR = 0.76; 95% CI, 0.50 to 1.15), headache (OR = 0.81; 95% CI, 0.53 to 1.24), nausea (OR = 0.97; 95% CI, 0.63 to 1.48), gastrointestinal distress/diarrhea (OR = 1.55; 95% CI, 0.93 to 2.58), skin problems (OR = 0.92; 95% CI, 0.61 to 1.40), loss of appetite (OR = 1.09; 95% CI, 0.65 to 1.82), hair loss (OR = 1.10; 95% CI, 0.64 to 1.90), respiratory symptoms (OR = 0.64; 95% CI, 0.40 to 1.03), or flulike symptoms (OR = 0.81; 95% CI, 0.51 to 1.27) (eAppendix 1, section V).
Sustained Virologic Response
Four studies addressed SVR in patients treated with antidepressant (n = 190) and in those given a placebo (n = 192).15,16,23,24 According to our analysis, there were no differences in SVR between these 2 groups (OR = 1.22; 95% CI, 0.58 to 2.57) (eAppendix 1, section VI).
Discontinuation and Loss to Follow-Up
Six of the 7 studies reported the number of patients who discontinued antiviral treatment or were lost to follow-up.15,16,21,23,25,26 These patients accounted for 80 subjects in the placebo group (28.6%) and 65 subjects in the SSRI group (23.4%) (OR = 0.77; 95% CI, 0.52 to 1.13). Only 4 studies specified the reasons for discontinuation.15,16,21,25 Potential side effects were the reason for discontinuation in 20 patients of the placebo group (8.8%) and 19 patients of the SSRI group (8.4%) (OR = 0.98; 95% CI, 0.51 to 1.90) (eAppendix 1, section VII).
Subgroup Analysis, Sensitivity Analysis, and Meta-Regression
We were unable to perform a subanalysis examining the potential benefit of antidepressants among the subjects with a history of depression or those with subthreshold depressive symptoms at baseline.
Only 1 study21 reported full data about incidence of MDE during antiviral treatment in patients with a history of depression: 37.5% of patients treated with escitalopram and 66.6% treated with placebo developed depression (n = 23).
No studies reported the mean (SD) MADRS scores at baseline in the 2 groups (those with induced depression and those without depression during antiviral treatment and prophylactic antidepressant treatment). However, 2 studies reported that patients with higher scores on the MADRS before starting antiviral treatment may especially benefit from the prophylactic administration of antidepressants. Patients with a MADRS score at baseline > 4 presented an incidence of depression of 14% if treated with escitalopram and 44% if treated with placebo.23 Furthermore, in subjects with depression scores above the median (MADRS score > 3) at baseline, paroxetine was associated with a maximal reduction in MADRS scores compared with placebo at 20 weeks of antiviral treatment.25
Sensitivity analyses of the primary outcome showed that findings were similar when the random-effects model was used (OR = 0.53; 95% CI, 0.32 to 0.90), including only studies with at least 24 weeks of follow-up (OR = 0.45; 95% CI, 0.27 to 0.74) and excluding articles with higher risk of bias (OR = 0.48; 95% CI, 0.28 to 0.82) (eAppendix 1, section VIII).
Meta-regression was not performed because the number of selected articles was less than 10.18
Heterogeneity and Publication Bias
Significant heterogeneity was identified in the comparison of (1) escitalopram versus placebo (χ2 = 5.19, P = .07), (2) sustained virologic response (χ2 = 7.35, P = .06), and (3) MADRS scores at 12 weeks (χ2 = 36.44, P < .00001) and 24 weeks (χ2 = 16.97, P = .0007) of treatment, thereby justifying the use of random-effects models. We found no significant heterogeneity between studies with respect to the other variables evaluated.
The funnel plots revealed no publication bias among the selected studies (eAppendix 1, section IX).
DISCUSSION
In this systematic review, we report that prophylactic administration of any SSRI before starting antiviral treatment for CHC reduces the subsequent incidence of an MDE (DSM-IV) by 43%. However, the impact of the intervention was moderate (NNT = 12), and the reduction in depressive symptoms (MADRS) was not significant until week 24 of antiviral treatment. The NNT observed suggests a meaningful real-world effect; for example, it is lower than NNTs reported for mainstream medications such as statins for cardiovascular event reduction or antidepressants to prevent a depressive episode in bipolar disorder.27-29 Adding an SSRI to the antiviral treatment was generally well tolerated; antidepressants were associated with less muscle or joint ache, though also with more dizziness and with a trend toward more sexual side effects. The use of a prophylactic antidepressant was not related with changes in sustained virologic response to antiviral treatment.
Immunologic and neurotransmitter factors may play an important role in the development of depression during antiviral treatment. Administration of exogenous cytokines activates other proinflammatory cytokines such as interleukin 6 (IL-6), which is associated with a central nervous system (CNS) inflammatory response.4 A high concentration of proinflammatory cytokines with CNS action may modulate the serotonergic system through the up-regulation of enzymes such as indoleamine 2,3-dioxygenase, altering levels of serotonin and the expression of the serotonin transporter (SERT) or presynaptic receptors (5-HT1A).12 Cytokine-induced impaired serotonin synthesis may also be due to the effect of inflammatory factors on the synthesis and activity of tetrahydrobiopterin (BH4), a cofactor of amino acid monooxygenases including tryptophan hydroxylase.30 SSRIs may modulate the changes induced by cytokines by increasing the reuptake of serotonin at the synaptic cleft.13 One in vitro study31 showed that although administration of IFN-α produced a down-regulation of 5-HT1A receptors, this effect is attenuated if an antidepressant is administered previously. Clinical studies have also shown that certain genetic variants that determine the levels of expression of 5-HT1A receptors are related to IFN-induced depression.32 These biological findings support the results of this review, which found that administration of an SSRI can prevent depression during antiviral treatment for CHC.
The meta-analysis performed for types of SSRI separately found no differences between escitalopram, citalopram, or paroxetine and placebo. This finding is quite likely due to the small sample size in each group when analyzed separately, as well as to the relatively moderate impact of antidepressants in terms of preventing IFN-induced depression in the general population. Some of the selected studies excluded patients with a history of depression or those with a past severe psychiatric disorder16; this probably means that the patients included in this review were at lower risk of developing IFN-induced depression than are the patients usually found in clinical practice. According to previous studies and a recent systematic review with meta-analysis,11 a personal history of depressive disorder is one of the most widely reported risk factors for developing depression during antiviral treatment. In fact, CHC patients with a history of depressive disorder have a 4-fold higher risk of suffering a depressive episode during interferon treatment than do those without such a history.11 Prophylactic administration of antidepressants in this subgroup of patients may therefore be of particular benefit,33 although it should be mentioned that such patients represented a fairly small proportion of the subjects included in this review. Due to the small number of patients with a history of depression and the lack of data regarding clinical response in this subgroup, we were unable to perform a subanalysis examining the potential benefit of antidepressants among these subjects. Of note, it has been shown that patients with a psychiatric diagnosis at the initiation of interferon treatment do not necessarily exhibit reduced viral clearance and more frequent treatment discontinuation than patients free of psychiatric disorder at baseline.34
In conclusion, this review shows that the prophylactic administration of SSRIs does reduce depression during antiviral treatment for CHC. Considering that depression may be associated with major complications such as suicidal ideation or lack of treatment adherence, and also in view of the relatively good tolerability of antidepressants, this review supports the initiation of prophylactic treatment with SSRI to prevent IFN-induced depression. Nevertheless, although SSRI use was associated with a reduction in the risk of depression of around 43%, more than 11% of patients treated with SSRIs developed depression. This finding suggests a need to monitor these patients irrespective of SSRI use. In fact, recent guidelines recommend multidisciplinary monitoring for psychiatric symptoms during antiviral therapy for CHC in all patients and suggest that the decision to initiate antidepressant pretreatment should be based on a case-by-case approach and the patient’s own preferences, taking into account the presence of risk factors for depression.14 In line with previous reviews35 and in view of the reduction in depression rates and the good tolerability of SSRIs reported in this study, we hypothesize that prophylactic antidepressant treatment in subjects at risk, such as those with a past depressive episode or those with subthreshold depressive symptoms at baseline, will be highly effective. Future research should focus on these particular subgroups of patients, who may well benefit from this intervention. This review and future studies should help not only to optimize the management of patients with CHC, but also to develop clinical guidelines based on stronger evidence.
Limitations
This study does have certain limitations. First, there was a degree of heterogeneity between studies with respect to the sample characteristics. We sought to minimize this problem by reporting potential confounding variables such as age, gender, coinfection, exclusion of patients with psychiatric history, IFN dose and type, and cotreatment with ribavirin. Some of the selected studies excluded patients with a history of depression or a past severe psychiatric disorder, and this fact may limit the generalizability of our results to clinical practice. It is also acknowledged that randomized clinical trials are likely to present significant publication bias. We tried to minimize this bias by searching for reported trial designs before publication of results (ClinicalTrials.gov) and also by assessing potential publication bias using Begg-Egger funnel plots.
Drug names: citalopram (Celexa and others), escitalopram (Lexapro and others), paroxetine (Paxil, Pexeva, and others).
Author affiliations: Department of Psychiatry and Psychology, Hospital Cl×nic, IDIBAPS, CIBERSAM; and Department of Psychiatry and Clinical Psychobiology, Universitat de Barcelona, Catalonia (Drs Udina, Hidalgo, Navinés, Vieta, and Mart×n-Santos); Liver Unit, Hospital Cl×nic, IDIBAPS, CIBERehd, Barcelona, Catalonia (Dr Forns); Liver Section, Parc de Salut Mar, IMIM, Universitat Autײnoma de Barcelona, Barcelona, Catalonia (Dr Sol× ); Human Pharmacology and Clinical Neurosciences Research Group, Hospital del Mar Medical Research Institute (IMIM-Parc de Salut Mar), RETIC-RTA; and Pharmacology Department, Universitat Autײnoma de Barcelona, Barcelona, Catalonia (Dr Farré), Spain; and Laboratory of Nutrition and Integrative Neurobiology, NutriNeuro, INRA UMR 1286, University Victor Segalen Bordeaux 2, Bordeaux, France (Dr Capuron).
Author contributions: Drs Udina and Mart×n-Santos designed the review and extracted data. Drs Udina, Hidalgo, and Navinés selected studies to be included. Drs Udina and Farré did statistical analyses. Drs Udina, Forns, Sol× , Capuron, Vieta, and Mart×n-Santos collaborated on data interpretation. All authors wrote the report.
Potential conflicts of interest: Dr Forns has received grant support from Jansen, Gilead, and MSD. Dr Sol× has received grants from and served as a consultant, advisor, or CME speaker for Gilead, Janssen, and Roche. Dr Vieta has received grants from and served as consultant, advisor, or CME speaker for Almirall, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Forest, Gedeon Richter, GlaxoSmithKline, Janssen-Cilag, Jazz, Johnson & Johnson, Lundbeck, Merck, Novartis, Organon, Otsuka, Pfizer, Pierre-Fabre, Qualigen, Sanofi-Aventis, Servier, Schering-Plough, Solvay, Takeda, Spanish Ministry of Science and Innovation (CIBERSAM), Seventh European Framework Programme (ENBREC), Stanley Medical Research Institute, United Biosource Corporation, and Wyeth. Drs Udina, Hidalgo, Navinés, Farré, Capuron, and Mart×n-Santos report no potential conflict of interest.
Funding/support: This study was funded by the following Spanish grants: Ministerio de Econom×a y Competitividad, Instituto de Carlos III, FIS: PSICOCIT-VHC-P110/01827, and PSIGEN-VHC-EC08/00201 (principal investigator: Dr Mart×n-Santos). It was cofinanced by the European Regional Development Fund (ERDF), European Union, "A Way to Build Europe." It was also supported by the Generalitat de Catalunya (Support a les activitats dels Grups de Recerca): SGR2009/1435 and SGR2013/1411.
Role of the sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Supplementary material: See accompanying pages.
REFERENCES
1. World Health Organization (WHO). Initiative on depression in public health. http://www.who.int/mental_health/management/depression/depressioninph/en/. Accessed November 7, 2012.
2. Löthgren M. Economic evidence in affective disorders: a review. Eur J Health Econ. 2004;5(suppl 1):S12-S20. PubMed doi:10.1007/s10198-005-0283-4
3. Evans DL, Charney DS, Lewis L, et al. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005;58(3):175-189. PubMed doi:10.1016/j.biopsych.2005.05.001
4. Raison CL, Borisov AS, Majer M, et al. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry. 2009;65(4):296-303. PubMed doi:10.1016/j.biopsych.2008.08.010
5. Mart×n-Santos R, D×ez-Quevedo C, Castellv× P, et al. De novo depression and anxiety disorders and influence on adherence during peginterferon-alpha-2a and ribavirin treatment in patients with hepatitis C. Aliment Pharmacol Ther. 2008;27(3):257-265. PubMed doi:10.1111/j.1365-2036.2007.03568.x
6. Wasley A, Alter MJ. Epidemiology of hepatitis C: geographic differences and temporal trends. Semin Liver Dis. 2000;20(1):1-16. PubMed doi:10.1055/s-2000-9506
7. Ly KN, Xing J, Klevens RM, et al. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156(4):271-278. PubMed doi:10.7326/0003-4819-156-4-201202210-00004
8. Brok J, Gluud LL, Gluud C. Effects of adding ribavirin to interferon to treat chronic hepatitis C infection: a systematic review and meta-analysis of randomized trials. Arch Intern Med. 2005;165(19):2206-2212. PubMed doi:10.1001/archinte.165.19.2206
9. Pearlman BL. Protease inhibitors for the treatment of chronic hepatitis C genotype-1 infection: the new standard of care. Lancet Infect Dis. 2012;12(9):717-728. PubMed doi:10.1016/S1473-3099(12)70060-9
10. Chou R, Hartung D, Rahman B, et al. Comparative effectiveness of antiviral treatment for hepatitis C virus infection in adults: a systematic review. Ann Intern Med. 2013;158(2):114-123. PubMed doi:10.7326/0003-4819-158-2-201301150-00576
11. Udina M, Castellv× P, Moreno-Espa×±a J, et al. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry. 2012;73(8):1128-1138. PubMed doi:10.4088/JCP.12r07694
12. Raison CL, Dantzer R, Kelley KW, et al. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry. 2010;15(4):393-403. PubMed doi:10.1038/mp.2009.116
13. Stahl SM. Mechanism of action of serotonin selective reuptake inhibitors; serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord. 1998;51(3):215-235. PubMed doi:10.1016/S0165-0327(98)00221-3
14. Schaefer M, Capuron L, Friebe A, et al. Hepatitis C infection, antiviral treatment and mental health: a European expert consensus statement. J Hepatol. 2012;57(6):1379-1390. PubMed doi:10.1016/j.jhep.2012.07.037
15. Diez-Quevedo C, Masnou H, Planas R, et al. Prophylactic treatment with escitalopram of pegylated interferon alfa-2a-induced depression in hepatitis C: a 12-week, randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2011;72(4):522-528. PubMed doi:10.4088/JCP.09m05282blu
16. Schaefer M, Sarkar R, Knop V, et al. Escitalopram for the prevention of peginterferon-α2a-associated depression in hepatitis C virus-infected patients without previous psychiatric disease: a randomized trial. Ann Intern Med. 2012;157(2):94-103. PubMed doi:10.7326/0003-4819-157-2-201207170-00006
17. Moher D, Liberati A, Tetzlaff J, et al; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. PubMed doi:10.1371/journal.pmed.1000097
18. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.0.1. The Cochrane Collaboration; 2008. http://www.handbook.cochrane.org/. Updated September 2008. Accessed November 5, 2012.
19. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. PubMed doi:10.1136/bmj.315.7109.629
20. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088-1101. PubMed doi:10.2307/2533446
21. de Knegt RJ, Bezemer G, Van Gool AR, et al. Randomised clinical trial: escitalopram for the prevention of psychiatric adverse events during treatment with peginterferon-alfa-2a and ribavirin for chronic hepatitis C. Aliment Pharmacol Ther. 2011;34(11-12):1306-1317. PubMed doi:10.1111/j.1365-2036.2011.04867.x
22. Klein MB, Cooper C, Brouillette MJ, et al; CTN194 Investigators. CTN-194 (PICCO): design of a trial of citalopram for the prevention of depression and its consequences in HIV-hepatitis C co-infected individuals initiating pegylated interferon/ribavirin therapy. Contemp Clin Trials. 2008;29(4):617-630. PubMed doi:10.1016/j.cct.2008.01.001
23. Morasco BJ, Loftis JM, Indest DW, et al. Prophylactic antidepressant treatment in patients with hepatitis C on antiviral therapy: a double-blind, placebo-controlled trial. Psychosomatics. 2010;51(5):401-408. PubMed doi:10.1016/S0033-3182(10)70722-2
24. Morasco BJ, Rifai MA, Loftis JM, et al. A randomized trial of paroxetine to prevent interferon-alpha-induced depression in patients with hepatitis C. J Affect Disord. 2007;103(1-3):83-90. PubMed doi:10.1016/j.jad.2007.01.007
25. Raison CL, Woolwine BJ, Demetrashvili MF, et al. Paroxetine for prevention of depressive symptoms induced by interferon-alpha and ribavirin for hepatitis C. Aliment Pharmacol Ther. 2007;25(10):1163-1174. PubMed doi:10.1111/j.1365-2036.2007.03316.x
26. Klein MB, Singer J, Brouillette MJ, et al. A multicentre randomized double-blind placebo controlled trial of prophylactic citalopram for the prevention of interferon-alpha associated neurocognitive dysfunction in HIV-HCV co-infection: CTN 194 (PICCO). Presented at the 19th International AIDS Conference; July 22-27, 2012; Washington, DC. WEPE055.
27. Ghaemi SN, Wingo AP, Filkowski MA, et al. Long-term antidepressant treatment in bipolar disorder: meta-analyses of benefits and risks. Acta Psychiatr Scand. 2008;118(5):347-356. PubMed doi:10.1111/j.1600-0447.2008.01257.x
28. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1:CD004816. PubMed
29. LaRosa JC, He J, Vupputuri S. Effect of statins on risk of coronary disease: a meta-analysis of randomized controlled trials. JAMA. 1999;282(24):2340-2346. PubMed doi:10.1001/jama.282.24.2340
30. Capuron L, Schroecksnadel S, Féart C, et al. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol Psychiatry. 2011;70(2):175-182. PubMed doi:10.1016/j.biopsych.2010.12.006
31. Cai W, Khaoustov VI, Xie Q, et al. Interferon-alpha-induced modulation of glucocorticoid and serotonin receptors as a mechanism of depression. J Hepatol. 2005;42(6):880-887. PubMed doi:10.1016/j.jhep.2005.01.024
32. Kraus MR, Al-Taie O, Schפfer A, et al. Serotonin-1A receptor gene HTR1A variation predicts interferon-induced depression in chronic hepatitis C. Gastroenterology. 2007;132(4):1279-1286. PubMed doi:10.1053/j.gastro.2007.02.053
33. Schaefer M, Schwaiger M, Garkisch AS, et al. Prevention of interferon-alpha associated depression in psychiatric risk patients with chronic hepatitis C. J Hepatol. 2005;42(6):793-798. PubMed doi:10.1016/j.jhep.2005.01.020
34. Pariante CM, Orr×¹ MG, Baita A, et al. Treatment with interferon-alpha in patients with chronic hepatitis and mood or anxiety disorders. Lancet. 1999;354(9173):131-132. PubMed doi:10.1016/S0140-6736(98)04793-X
35. Lotrich FE. Major depression during interferon-alpha treatment: vulnerability and prevention. Dialogues Clin Neurosci. 2009;11(4):417-425. PubMed