A Randomized Controlled Trial of Long-Acting Injectable Risperidone vs Continuation on Oral Atypical Antipsychotics for First-Episode Schizophrenia Patients: Initial Adherence Outcome
Objective: Nonadherence for first-episode schizophrenia is a major unsolved challenge. The long-acting injectable route is an appealing strategy, but there are concerns about acceptability. We report on acceptance and initial adherence outcomes with risperidone long-acting injection (RLAI) in first-episode schizophrenia patients.
Method: We conducted a prospective randomized controlled trial in which we enrolled patients defined by appropriate Structured Clinical Interview for DSM-IV diagnosis and ≤ 16 weeks of lifetime antipsychotic exposure. Participants were randomly assigned (2:1 ratio) to a recommendation of changing to RLAI versus continuing on oral therapy (ORAL). Nonadherence behavior was defined as a medication gap ≥ 14 days. Adherence attitudes were determined by the Rating of Medication Influences (ROMI) scale. A priori analysis defined treatment groups as intent-to-treat (ITT) and as-actually-treated (AAT) for the first 12 weeks after initial randomization. Participants were enrolled from December 2004 to March 2007.
Results: Of 46 eligible patients, 37 were randomly assigned, 11 to ORAL and 26 to RLAI. Nineteen of 26 patients (73%) accepted the RLAI recommendation. There were no differences in adherence behavior at 12 weeks based on initial randomization (Kaplan-Meier survival for ITT: 76% [95% CI, 35%-90%] adherent for RLAI vs 72% [95% CI, 55%-89%] for ORAL; log-rank P = .78), but patients accepting RLAI were significantly more likely to be adherent than patients staying on ORAL (AAT: 89% [95% CI, 64%-97%] adherent for RLAI vs 59% [95% CI, 32%-78%] for ORAL; log-rank P = .035). There were no ROMI attitude differences between either treatment group comparison at 12 weeks.
Conclusions: Most first-episode patients taking oral antipsychotics will accept a recommendation of RLAI therapy. On the basis of initial randomization status, an RLAI recommendation did not affect adherence behavior at 12 weeks. However, acceptance of RLAI was associated with significantly better adherence. Regardless of whether RLAI is recommended or accepted, there is no adverse impact on subsequent medication attitudes at 12 weeks. These results support the feasibility and acceptability of introducing RLAI as a treatment option for first-episode schizophrenia patients.
Trial Registration: clinicaltrials.gov Identifier: NCT00220714
J Clin Psychiatry 2009;70(10):1397-1406
© Copyright 2009 Physicians Postgraduate Press, Inc.
Submitted: April 14, 2009; accepted July 14, 2009 (doi:10.4088/JCP.09m05284yel).
Corresponding author: Peter J. Weiden, MD, Center for Cognitive Medicine, Department of Psychiatry, University of Illinois, 912 S Wood St, MC 913, Chicago, IL 60612 ([email protected]).
Maintenance antipsychotic treatment is as important for first-episode patients as it is for chronic or "revolving door" schizophrenia patients.1 Therefore, once a diagnosis of first-episode schizophrenia is established, continuation of maintenance antipsychotic medication to prevent relapse is also necessary. Even when only those first-episode patients who initially agree to maintenance antipsychotics are considered, many will stop antipsychotic medication within the first year. For most first-episode patients, then, the central adherence question is not "Will this patient stay on medication?" but rather "How long will this patient stay on medication before stopping?" Unfortunately, the literature on the effectiveness of pharmacologic or psychosocial interventions has not been encouraging. For example, compared to the older first-generation antipsychotics, the newer second-generation antipsychotics are only marginally better in increasing the duration of adherence in initial maintenance treatment.2-5 Therefore, it may be more realistic to shift the research focus to better tracking of adherence or to ways to reduce the consequences of nonadherence when it occurs.
The long-acting injectable route of medication delivery is often considered to be a "gold standard" of pharmacologic intervention for nonadherence in persistently ill patients,6,7 but it has not been extensively studied in first-episode schizophrenia. In principle, long-acting injectable antipsychotics can be used anytime after a diagnosis of schizophrenia is established. In practice, clinicians usually consider long-acting therapy as a last resort in persistently ill patients with established patterns of nonadherence and relapse.8-10 Before the availability of a long-acting second-generation antipsychotic, another issue had been a concern about using first-generation antipsychotics in first-episode patients. This concern is no longer an issue since 2004, when a long-acting version of risperidone became available in the United States.
We conducted a prospective randomized controlled trial (RCT) of maintenance antipsychotic treatment in a cohort of recently stabilized, first-episode schizophrenia patients who were embarking on their initial maintenance outpatient treatment. Eligible patients were randomly assigned to a clinical recommendation of staying on their current oral second-generation antipsychotic (ORAL) vs a recommendation of changing to risperidone long-acting injection (RLAI). Treatment continued for up to 2 years. We now report the initial results of the trial: the likelihood of patients’ accepting the long-acting injectable route if recommended and the subsequent effects on adherence attitudes and behavior in the first 12 weeks after randomization.
METHOD
This is a randomized, open-label, parallel study of remaining on treatment with ORAL vs changing to RLAI in recent-onset schizophrenia. Participants were enrolled from December 2004 to March 2007 at 2 affiliated sites: State University of New York (SUNY) Downstate Medical Center and Kings County Hospital Center (Brooklyn, New York). Institutional review board approval was obtained at each site. Because of diagnostic and treatment uncertainties inherent in treatment of first-episode psychosis, the study involved 2 phases: an initial evaluation and stabilization phase and the RCT. Separate informed consent was obtained for the evaluation and for the RCT. Figure 1, a CONSORT flowchart, shows the flow of subjects from the evaluation phase to the RCT.
Evaluation Phase
Subjects. Subjects between the ages of 16 and 40 years who were admitted to the inpatient and outpatient services of Kings County Hospital Center or SUNY Downstate Medical Center and who had a provisional clinical diagnosis of schizophreniform disorder, schizophrenia, or schizoaffective disorder and ≤ 16 weeks of lifetime total antipsychotic medication exposure were included. Medication exposure criteria were evaluated using hospital records and family/patient reports. Informed consent was obtained either during hospitalization or shortly after initial inpatient treatment and admission to outpatient treatment.
Procedures. Consenting subjects were treated clinically for up to 12 weeks before being randomly assigned into the RCT. Research procedures included Structured Clinical Interview for DSM-IV (SCID) diagnosis and psychopathology assessment using the Positive and Negative Syndrome Scale,11 the Calgary Depression Scale for Schizophrenia,12 and the Clinical Global Impressions-Severity of Illness subscale.13 Clinical procedures included optimizing the oral medication regimen within the U.S. Food and Drug Administration (FDA)-approved dosage for second-generation antipsychotics, conduct of a baseline patient and family psychoeducation session, and establishment of a therapeutic alliance with the clinical treatment team. Baseline physical examination included vital signs, weight and body mass index, and ratings for the Abnormal Involuntary Movement Scale,14 the Barnes Akathisia Rating Scale,15 and the Simpson-Angus Scale for antipsychotic-induced parkinsonism.16
Randomized Controlled Trial
Subjects. Subjects who met the following eligibility criteria were invited to participate: SCID-confirmed diagnosis of schizophrenia, schizophreniform disorder, or schizoaffective disorder; clinical indication for long-term maintenance antipsychotic treatment; clinical response to oral antipsychotic medication; willingness to attend outpatient treatment services; and completion of at least 1 dedicated baseline psychoeducation session that included a key family member.
Procedures. Consent procedures emphasized that agreeing to medication adherence for the duration of the trial was not a requirement of study participation and that acceptance of the long-acting injection was not required for those randomly assigned to the long-acting recommendation. Randomization was to a recommendation of either (1) remaining on oral medication (ORAL group) or (2) changing from oral to RLAI (RLAI group). Since we anticipated that some patients would refuse the RLAI recommendation, the initial randomization to RLAI or ORAL group assignment was in a 2:1 ratio, so twice as many subjects received a recommendation for RLAI as compared to remaining on ORAL. Study duration was up to 2 years for individual subjects. Treating clinicians were informed of the randomization; they were responsible for informing the patient (and family) of the patient’s randomized recommendation status during up to 2 follow-up psychoeducation sessions, which usually occurred within a week after randomization. The follow-up psychoeducation sessions began with the general rationale for maintenance antipsychotic medication, and the recommendation was tailored to patient-specific life goals. For all subjects, psychoeducation always emphasized medication decisions as voluntary, without overt or covert pressure. For the RLAI group, additional information and discussion of RLAI was presented as well, including possible pharmacokinetic advantages, convenience, and monitoring aspects that might favor long-acting medication over the oral route. To be considered an RLAI acceptor, subjects randomly assigned to RLAI had to receive their first injection within 6 weeks of randomization. Clinicians were instructed to respect the decision of patients who declined RLAI after the second follow-up psychoeducation session. For RLAI refusers, clinicians continued to prescribe and encourage adherence with the ongoing oral antipsychotic regimen.
Setting and Provision of Care
The treatment service setting was a specialty program for treatment of first-episode schizophrenia patients located at the outpatient service of Kings County Hospital Center, a busy, inner-city public psychiatry outpatient clinic, and patients were seen by their treating clinicians (postgraduate year [PGY]-3 and PGY-4 psychiatry residents who were supervised by P.J.W. and A.E.) at routine biweekly medication monitoring visits regardless of whether they were in the RLAI or ORAL group. Risperidone long-acting injection was administered on-site. Oral medications were provided via written prescription and filled at a central pharmacy located nearby in the hospital complex. There was no direct out-of-pocket cost for medication in either condition.
Pharmacologic Intervention During the Initial Assessment Phase
At initial study entry, the selection of the oral antipsychotic to treat the acute psychotic episode had already been made by the patient’s inpatient or emergency room treatment team (doctors’ choice). Once the patient was enrolled in the assessment phase, the research clinicians reviewed the medication regimen, including medication choice, dose, and adjunctive therapy. In the assessment phase, study clinicians could prescribe any second-generation antipsychotic except clozapine within acceptable FDA-approved dose ranges. Patients receiving conventional or combination antipsychotics at study entry had their regimen adjusted with the goal of being on oral antipsychotic monotherapy before being invited to the RCT phase of the study. When the patients were switched between oral antipsychotic agents (eg, changing from haloperidol to a second-generation oral), oral risperidone was the first-line choice. The antipsychotic dosing philosophy was consistent with the first-episode psychopharmacology literature, ie, to dose at the lower end of the therapeutic dosage range13 (eg, a target dose of 3 mg/d of oral risperidone). Other commonly used psychiatric medications (eg, valproate sodium, lithium, lorazepam) were allowed with the exception of psychotropic agents that had the potential for major pharmacokinetic drug-drug interactions (eg, carbamazepine and paroxetine were not allowed).
Pharmacologic Intervention After Randomization
The treatment philosophy during the RCT followed an effectiveness orientation, with certain parameters and restrictions. Adjunctive therapies for affective or anxiety symptoms were allowed, as was switching oral antipsychotics for persistent efficacy or tolerability problems. Conventional antipsychotics and combination antipsychotics were not permitted except during antipsychotic crossovers. Subjects assigned to the oral recommendation were not allowed to receive any long-acting antipsychotic at any time during follow-up.
Patients assigned to the ORAL antipsychotic arm continued with their regimen. Subjects assigned to the RLAI recommendation could go on to receive oral antipsychotics if the subjects refused RLAI or if they were switched for clinical reasons to another antipsychotic not available as a long-acting injection.
Subjects who accepted RLAI received an initial injection of 25 mg of RLAI with initial overlap with oral risperidone for at least 3 weeks. The target maintenance dose for RLAI was always 25 mg every 2 weeks, with an allowable dose range between 25 and 50 mg intramuscularly every 2 weeks. After the crossover, oral supplementation was permitted for acute exacerbations of positive symptoms, but long-term use (> 4 weeks) of oral antipsychotic with RLAI was not permitted in maintenance phase treatment.
Treating clinicians were not involved in prospective adherence assessments, but clinical notes were used as one of the sources of information to track adherence behavior. All RCT subjects were followed for up to 2 years regardless of their adherence status. Every effort was made to locate and track patients who had dropped out of treatment and to restart treatment in the original randomized group in case of subsequent relapse and return to outpatient care.
Medication Management Visits
The study had an effectiveness orientation; we standardized the delivery of outpatient pharmacologic treatment in a way that attempted to reflect "real world" services. The prescribing psychiatrists were PGY-3 residents who were rotating through the Schizophrenia Research Service as part of their overall outpatient clinical service commitments. The PGY-3 psychiatrists made medication management decisions within the constraints of the protocol. Supervision was provided through a weekly meeting led by P.J.W. in which each case was reviewed individually. The duration of visits ranged from 20 to 30 minutes unless there were complications. Psychiatric residents were expected to be supportive and interested but were not allowed to conduct formal psychotherapy. Patients could be referred for additional psychosocial services. For patients receiving oral antipsychotics, the PGY-3 resident wrote a prescription that the patient could fill at the hospital pharmacy located at a different building within the medical complex. Patients receiving injections were given their injection at a treatment room on-site. Injections were administered by either the patient’s primary psychiatrist or a staff nurse. Patients did not receive any additional payment to attend clinical appointments, and there was no active outreach (eg, reminder calls) prior to scheduled visits. Patients were paid for research assessments.
Baseline and Outcome Assessments
In addition to assessments completed during the evaluation phase, research evaluations in the RCT included demographic and psychiatric history information and a premorbid adjustment scale, completed at RCT randomization. All symptom and tolerability rating scales described in the assessment phase were repeated at randomization to the RCT and at weeks 12, 36, 52, 78, and 104 of follow-up.
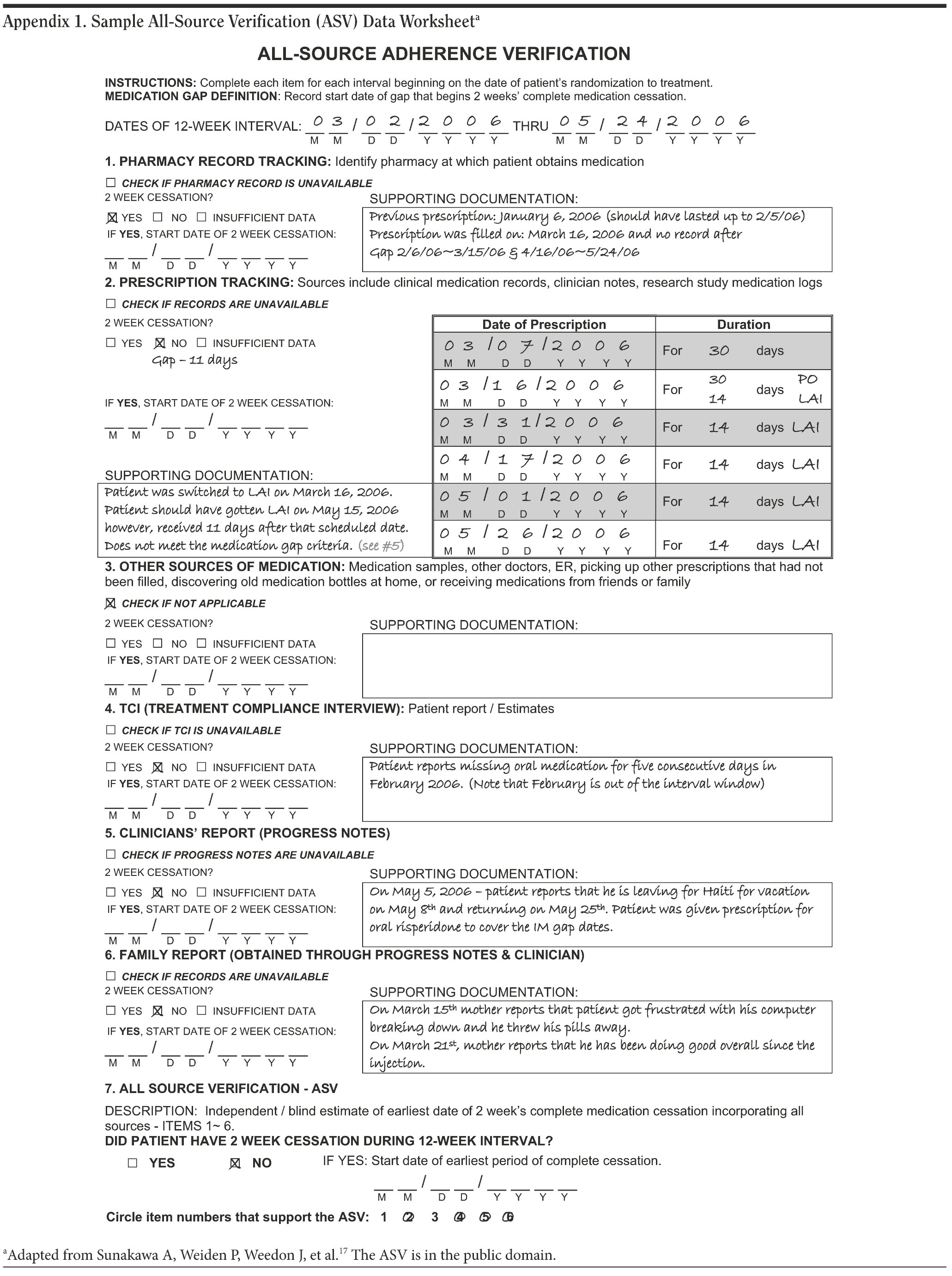
Assessment of adherence behavior. The major outcome criterion for nonadherent behavior was the time between randomization and a medication cessation episode (the GAP), defined as ≥ 14 consecutive days of not taking antipsychotic medication. The measure used was a multisource assessment measure known as All-Source Verification (ASV).17 The ASV approach collects information from various sources on adherence behavior in parallel and integrates these information sources into a single summary. The principle followed in medication gap assessment is to use the specific source that reveals the longest period of medication cessation. Key components of the ASV included (1) prescription refill data for oral medication (pharmacy records), (2) other sources of oral medication, (3) RLAI medication administration date (clinical records), (4) patient report, (5) clinician judgment (clinical notes), and (6) family report (clinical notes and family psychoeducation session reports). The ASV produces a running record of adherence behavior at a day-to-day interval level, with each day defined by either adherence or nonadherence. Subjects who take some but not all of their medication on any given day are categorized as adherent for that day, and a nonadherent day represents complete nonadherence for that day. When data sources yield conflicting results, the ASV takes a hierarchical approach that differentially ranks sources. In our study, pharmacy records and injection visits generally were the primary sources of adherence behavior outcome. In addition, each medical monitoring visit and family psychoeducation visit generated clinical notes that were used as source documents for the ASV. Not all sources were available for all subjects at each assessment point. Appendix 1 presents an example of a completed ASV form for the 12-week follow-up interval for a study subject.
For patients assigned to ORAL, the major outcome criterion for GAP was not taking any oral antipsychotic for ≥ 14 consecutive days. The same criterion for GAP was used for any patients randomly assigned to RLAI who were prescribed only oral antipsychotics. For patients receiving RLAI, the GAP criterion was met when patients were ≥ 14 days late for their scheduled injection (ie, patients had to miss an entire injection cycle). For the ORAL group, a missed prescription refill represented the initial definition of the start of a gap, but, using ASV, this start date could be modified on the basis of information from other sources that the gap had begun earlier. This article reports on the adherence behavior between initial randomization and 12-week follow-up in relation to (1) time until initial GAP and (2) proportion having at least 1 GAP within the initial 12-week follow-up period.
Assessment of adherence attitude. Adherence attitude was ascertained by an independent, blinded assessor using the Rating of Medication Influences (ROMI)18 scale at the 12-week follow-up point. The ROMI is a reliable and valid scale developed to measure salient attitudes and influences for schizophrenia patients taking antipsychotic medications.19,20 The ROMI is divided into 2 subscales: reasons for adherence (ROMI-A; 9 items) and reasons for nonadherence (ROMI-NA; 10 items). Each item covers a specific aspect known to influence medication adherence. For example, specific ROMI-A items include perceived benefit from medication, positive influence of family members, and positive influence of a clinician. Likewise, examples of ROMI-NA items include the perception that medication has no benefit, feeling stigmatized, or distress from side effects. Scaling of individual items ranges from 0 (no influence) to 2 (strong influence). The initial ROMI assessment was done at week 12 to ensure that all subjects had a significant period of medication exposure prior to being asked about medication attitudes. The interview was conducted in another area of the medical center at a separate time from clinical appointments.
Statistical Analyses
We report on (1) initial acceptance of RLAI treatment for the subgroup randomly assigned to the RLAI recommendation, (2) comparisons between RLAI and ORAL groups in adherence behavior during the first 12 weeks after randomization, and (3) comparisons in adherence attitudes reported at 12 weeks. The a priori analysis plan categorized RLAI groups in 2 ways. One grouping used initial randomization status, keeping all randomly assigned RLAI patients in the group regardless of whether the patient accepted RLAI (intent to treat [ITT]). The second grouping was to compare the subgroup of RLAI patients who accepted the RLAI recommendation with the group who stayed on oral antipsychotic (as actually treated [AAT]). In the AAT categorization, subjects randomly assigned to but declining the RLAI recommendation were included in the ORAL group along with those randomly assigned to oral medication. Data for adherence behavior were analyzed as 2 ×— 2 contingency tables with the Fisher exact test for proportions having a 14-day medication gap by week 12 and using Kaplan-Meier product limit survival methods for time until initial gap; between-group analyses were stratified by ITT and AAT, using the log-rank test of differences between groups. Adherence attitudes were assessed by comparing individual item scores between groups and total adherence and nonadherence scale scores between groups at 12 weeks using the Wilcoxon signed rank test for nonnormally distributed data.
RESULTS
This article reports only on results of the evaluation phase and the first 12 weeks of the RCT, which is ongoing at the time of this report.
Evaluation Phase
Seventy-four subjects entered this phase; 69% (51 of 74) were male, 34% (24 of 70) were African American, and 57% (40 of 70) were of Afro-Caribbean origin. The median age was 23 years, and 73% (45 of 62) were living with their family, predominantly parents or other lineal relatives. Median length of education was 12 years. Not all variables were available for all 74 assessment-phase subjects because of dropouts before the full evaluation was completed. Twenty-eight subjects (38%) were not eligible to continue to the RCT; failure to engage in outpatient treatment was the most common reason (n = 16), followed by medical reasons (n = 5), poor clinical response (n = 3), administrative reasons (n = 3), and not meeting SCID diagnostic criteria for the RCT (n = 1). Of the 46 subjects eligible to participate in the RCT, 38 (83%) consented to participate. Reasons for not consenting were refusal of maintenance treatment (n = 6) and refusal to consider RLAI randomization (n = 2). One patient was lost to follow-up after consenting and before being randomly assigned; 37 patients were included in randomization.
Medication status at study entry. At study entry, the most common antipsychotic was risperidone (n = 30; 81%), followed by haloperidol (n = 4; 11%), olanzapine (n = 2; 5%), and quetiapine (n = 1; 3%). The mean and median dose of oral risperidone was 4 mg/d (SD = 1.62 mg/d).
Randomized Controlled Trial
As shown in the CONSORT flowchart (Figure 1), 26 subjects were randomly assigned to a recommendation of changing to RLAI and 11 to a recommendation of staying on their current oral regimen. Seventy-six percent of subjects (28 of 37) were male, 32% (12 of 37) were African American, 62% (23 of 37) were of Afro-Caribbean origin, the median age at first hospitalization for psychosis and at the time of recruitment was 23 years, and 92% (34 of 37) were single. Virtually all lived with family, predominantly parents or other lineal relatives (73% [27 of 37]), and median length of education was 12 years.
Medication status at randomization and at 12-week follow-up. At the time of randomization, 34 of 37 patients were receiving oral risperidone monotherapy (mean and median doses remaining at 4 mg/d). At randomization, 2 subjects remained on combination antipsychotic regimens (risperidone/quetiapine and risperidone/haloperidol) and 1 subject was receiving olanzapine monotherapy. At the 12-week assessment, 19 of the 26 patients randomly assigned to RLAI received an injection. During the 12-week period, most patients (13 of 19; 68%) remained on their initial RLAI dose of 25 mg, but 6 of these 19 patients (32%) had been raised to the 37.5-mg dose. Most of the RLAI patients (88%) had finished the oral cross-taper by this time. The crossover was well tolerated and no adverse events occurred. All of the 11 patients in the ORAL group continued on the same antipsychotic; 3 of the 11 had their dose lowered, and 1 had the dose increased. No new adverse events occurred for the ORAL group during this time.
Acceptance of the initial RLAI recommendation. Seventy-three percent of subjects (19 of 26) randomly assigned to RLAI accepted the recommendation and received their first injection within 6 weeks after randomization and after completing their 2 psychoeducation sessions.
Adherence behavior at 12 weeks. By week 12 after randomization, 9 of the 37 subjects (24%) experienced at least 1 GAP. The effect of RLAI on initial adherence depended on the specific comparison method. Using ITT, there were no differences between RLAI and ORAL conditions on either end-point categorical analysis (RLAI, 6/26 = 23%; ORAL, 3/11 = 27%; P = 1.0) or Kaplan-Meier survival (Figure 2A; χ21 = 0.076, P = .783). Using AAT, the end-point categorical analysis approached significance (RLAI, 2/19 = 11%; ORAL, 7/18 = 39%; P = .063). The Kaplan-Meier analysis showed significant differences between groups, with RLAI acceptors being significantly more likely to remain adherent than the remaining ORAL group (Figure 2B; RLAI, 89% adherent [95% CI, 64%-97%]; ORAL, 59% adherent [95% CI, 32%-78%]; log-rank χ21 = 4.43; P = .035).
Adherence attitudes at 12 weeks. There were no statistically significant differences in medication attitudes between groups in total and individual item scores of the ROMI-A and ROMI-NA subscales for either the ITT or the AAT comparison. The mean (SD) total scores for ROMI-A and ROMI-NA subscales at 12 weeks were as follows (ITT comparison): ROMI-A, 0.76 (0.38) for RLAI and 0.74 (0.30) for ORAL, not significant; ROMI-NA, 0.50 (0.42) for RLAI and 0.58 (0.39) for ORAL, not significant. Both ITT groups reported clinician authority as the strongest adherence influence. (Data not presented but available from the corresponding author.)
CONCLUSIONS
Acceptability of RLAI in First-Episode Schizophrenia
This study addressed the question of whether it is feasible to recommend RLAI to first-episode patients who have recently been stabilized on oral antipsychotic after their initial psychotic episode. Put another way, the first step to addressing whether a long-acting approach is effective in first-episode patients is to establish whether this option is accepted in initial phases of treatment. We found that almost three-quarters of subjects accepted the recommendation of converting from their oral antipsychotic to risperidone given by long-acting injection. Almost all of the eventual RLAI acceptors initially stated a preference to stay on their oral regimen. Almost all of the subjects initially voiced reluctance to accept antipsychotic medication by long-acting injection but usually came to accept the recommendation when it was given in the context of a 2-session psychoeducation program tailored for first-episode patients and their families. These sessions presented the RLAI recommendation within the framework of a life-goals approach, with an emphasis on voluntary acceptance. Both the inclusion criteria and consent procedures emphasized the voluntary nature of maintenance treatment, and there was no sign of added coercion, stigma, or side-effect distress in either group at the 12-week follow-up. Therefore, we conclude that introducing a long-acting second-generation antipsychotic—risperidone long-acting injection—is feasible at any time after the diagnosis is established. It seems that the worst thing that happens is that some patients will decline this recommendation, but, even then, there is no sign that the recommendation of RLAI leads to any subsequent problems with stigma, therapeutic alliance, or overall attitudes toward medication. The commonly held clinical belief that first-episode patients would never accept a long-acting injection is untrue.
Effect of RLAI on Adherence Behavior
The follow-up results on short-term impact of the RLAI treatment on adherence behavior depends on how the RLAI condition is defined. In comparisons of initial randomization groups of those who did and did not receive RLAI recommendation, no differences were found. However, RLAI acceptors were significantly more likely to remain adherent at 12 weeks compared to those who stayed on treatment with ORAL. We believe there are 2 possible explanations for the better adherence in the RLAI acceptors. First, starting treatment with RLAI may have direct adherence benefits in preventing or delaying nonadherence in some individuals who would have otherwise stopped their oral medication. Second, refusal of the RLAI recommendation is a sign that the patient plans to stop oral therapy in the very near future. For these individuals, the real issue is not so much the long-acting route as it is the notion of staying on treatment with any antipsychotic.
Effect of RLAI on Adherence Attitudes
From the perspective of impact of RLAI on medication attitudes, there was no effect. Neither the recommendation nor the acceptance of injections was related to adherence attitudes at 12 weeks. We did not see any effect of RLAI on subsequent attitudes toward medication or adherence. There was no adverse effect on stigma, perceived coercion, or the doctor-patient relationship, all of which have been cited as potential concerns with using a long-acting route.21,22 This lack of effect of route of delivery on adherence attitude was found in both ITT and AAT analyses. From an attitude point of view, offering RLAI did not seem to "turn off" patients who declined the RLAI recommendation. We feel that this is reassuring since some clinicians are concerned that recommending a long-acting injection in first-episode patients might add to the stigma burden or harm the therapeutic alliance. There was no sign of dissatisfaction or of problems with a long-acting medication route as shown by the 12-week ROMI-A and ROMI-NA assessments. Review of individual ROMI items showed no sign of any adverse subjective effects on the therapeutic alliance or experience of pressure/coercion nor any sign of additional adverse events that might exacerbate adherence concerns. Therefore, there seems to be no subjective "cost" to initiating a long-acting medication route early in the course of maintenance therapy for first-episode schizophrenia patients. Whatever factors influence medication attitudes in first-episode schizophrenia, the route of medication does not seem to matter.
Study limitations include a relatively small sample size and the fact that although the treatment condition was randomized, actual treatment was provided by clinicians who were not blind to treatment. However, adherence attitudes were assessed by blinded clinical raters, and the approach to measuring adherence behavior has differential biases that would tend to show better adherence in the oral group, in that nonadherence to RLAI is always known. In addition, this study did not consider direct cost of medication. At the time the study was done, none of the second-generation medications were available in generic formulations. Now, there is a large cost differential between RLAI and oral risperidone. In "real world" clinical situations, there may be reluctance among payers to approve RLAI for first-episode patients.
A secondary finding is the rapidity with which stabilized first-episode patients stop their maintenance antipsychotic medication. Our cohort had gone through an initial evaluation phase in which patients who did not engage or who did not accept medication did not participate in the RCT. Even so, 27% of these first-episode patients who were adherent at the time of randomization had stopped their medication entirely for at least 2 weeks by the time they reached their 12-week follow-up assessment. The clinical implication is that nonadherence comes quickly among first-episode patients entering maintenance treatment even when it is a subgroup of first-episode patients who initially accept treatment. The differences between our findings and that of the Emsley report of excellent long-term adherence with RLAI in first-episode patients may be attributed to cultural and service differences between South African and US mental health treatment settings.23 It also seems that when US first-episode studies are compared to European first-episode studies,4,24 the high rates of nonadherence in US first-episode samples may be related, in part, to the lack of enriched treatment services in the United States relative to other countries.25-27
Most of the literature on effectiveness of long-acting formulations was done in the era of conventional antipsychotics. Oral versus depot conventional antipsychotics were compared in patients who had histories of persistent symptoms and/or patterns of frequent relapse.28 A more recent meta-analysis of long-acting injectable medication suggests that there is an advantage of a long-acting route of drug delivery in reducing relapse and rehospitalization, especially over longer periods of time.29 Additional benefits of long-acting injections are that they allow the clinician to disentangle nonresponse from nonadherence and to identify nonadherence as soon as an injection is missed. Rapid identification of nonadherence allows initiation of psychosocial interventions to address the adherence problem for a patient before symptom exacerbation and relapse. We believe that these same benefits apply at least as well to schizophrenia patients early in the course of their illness. While this current report did not assess for clinical benefits of RLAI during the 12-week follow-up interval, it is our belief that the clinical benefits of the long-acting route should not be limited to chronic patients who have clear histories of nonadherence. What this study shows is that most stabilized first-episode patients will voluntarily accept a long-acting second-generation antipsychotic when recommended as part of an integrated treatment plan. The caveat is that the high acceptance of RLAI occurred in a subgroup of stabilized first-episode patients who were able to engage in an outpatient treatment program. We would not expect that the high rate of RLAI acceptance would apply to those first-episode patients who fail to engage in outpatient treatment. These results support the feasibility and acceptability of using long-acting versions of a second-generation antipsychotic as a possible treatment strategy for the early phases of maintenance treatment of schizophrenia. Our findings show that there is no adverse effect on medication attitudes when RLAI is recommended. However, it is important to note that acceptance of a long-acting recommendation does not automatically translate to actual changes in adherence behavior. Data from the longer-term follow-up of subjects in this trial and larger long-term studies are needed to address these important questions.
Drug names: carbamazepine (Carbatrol, Equetro, and others), clozapine (FazaClo, Clozaril, and others), haloperidol (Haldol and others), lithium (Eskalith, Lithobid, and others), lorazepam (Ativan and others), olanzapine (Zyprexa), paroxetine (Paxil, Pexeva, and others), quetiapine (Seroquel), risperidone (Risperdal Consta, Risperdal, and others), valproate sodium (Depacon and others).
Author affiliations: Center for Cognitive Medicine, University of Illinois at Chicago (Dr Weiden); and Department of Psychiatry and Behavioral Sciences (Drs Weiden, Schooler, Elmouchtari, and Goldfinger and Ms Sunakawa) and Scientific Computing Center (Dr Weedon), SUNY Downstate Medical Center, Brooklyn, New York.
Financial disclosure: Dr Weiden is a consultant for and has received honoraria from AstraZeneca, Bristol-Myers Squibb/Otsuka America, Eli Lilly, Ortho-McNeil Janssen, Organon, Pfizer, Schering-Plough, Shire, Vanda, and Wyeth and has received grant/research support from AstraZeneca, Bristol-Myers Squibb/Otsuka America, and Ortho-McNeil Janssen. A family member of Dr Weiden’s has consulted for Pfizer within the last 3 years. Dr Schooler is a consultant for and has received honoraria from Organon, Ortho-McNeil Janssen, Lundbeck, and Schering Plough and has received grant/research support from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Ortho-McNeil Janssen, and Pfizer. Dr Goldfinger is a consultant for and has received honoraria from Teva; has received grant/research support from Ortho-McNeil Janssen; and is a stock shareholder of Eli Lilly, Johnson & Johnson, Ortho-McNeil Janssen, Pfizer, and Teva. Drs Weedon and Elmouchtari and Ms Sunakawa have no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
Funding/support: Supported by an investigator-initiated research grant from Ortho-McNeil Janssen Scientific Affairs, LLC, Titusville, New Jersey, to Dr Weiden, who as principal investigator was responsible for the design, conduct, analysis, and reporting of this clinical trial.
Previous presentation: Presented as 2 poster sessions at the 46th Annual Meeting of the American College of Neuropsychopharmacology, December 9-13, 2007, Boca Raton, Florida.
Acknowledgments: We gratefully acknowledge Ortho-McNeil Janssen for their grant support and the Kings County Hospital Center outpatient psychiatry service and the patients and their families who participated in this study. The following residents, attending psychiatrists, and psychologists provided clinical care to patients and families: Dinara Amanbekova, MD; Badari Birur, MD; Page Burkholder, MD; Alyse DiBenedetto, PsyD; Shilpa Diwan, MD; Nicole Elden, PsyD; Juan A. Gallego, MD; Diana Grigoreva, MD; Amjad Hindi, MD, Igor Kirzhner, MD; Nabil Kotbi, MD; Nikhil Palekar, MD; Mamta Sapra, MD; and Rajvee Vora, MD. The above-named individuals report no financial conflicts of interest in relation to this study.
REFERENCES
1. Robinson DG, Woerner MG, McMeniman M, et al. Symptomatic and functional recovery from a first episode of schizophrenia or schizoaffective disorder. Am J Psychiatry. 2004;161(3):473-479. PubMed doi:10.1176/appi.ajp.161.3.473
2. McEvoy JP, Lieberman JA, Perkins DO, et al. Efficacy and tolerability of olanzapine, quetiapine, and risperidone in the treatment of early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry. 2007;164(7):1050-1060. PubMed doi:10.1176/appi.ajp.164.7.1050
3. Lieberman JA, Phillips M, Gu H, et al. Atypical and conventional antipsychotic drugs in treatment-naive first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003;28(5):995-1003. PubMed
4. Kahn RS, Fleischhacker WW, Boter H, et al. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371(9618):1085-1097. PubMed doi:10.1016/S0140-6736(08)60486-9
5. Velligan DI, Lam F, Ereshefsky L, et al. Psychopharmacology: perspectives on medication adherence and atypical antipsychotic medications. Psychiatr Serv. 2003;54(5):665-667. PubMed doi:10.1176/appi.ps.54.5.665
6. American Psychiatric Association. Practice Guideline for the Treatment of Patients With Schizophrenia, Second Edition. Am J Psychiatry. 2004;161(suppl 2):1-56. PubMed
7. Lehman AF, Kreyenbuhl J, Buchanan RW, et al. The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2003. Schizophr Bull. 2004;30(2):193-217. PubMed
8. Kane JM, Leucht S, Carpenter D, et al. The Expert Consensus Guideline Series: Optimizing Pharmacologic Treatment of Psychotic Disorders. Introduction: methods, commentary, and summary. J Clin Psychiatry. 2003;64(suppl 12):5-19. PubMed
9. Weiden PJ, Preskorn SH, Fahnestock PA, et al. Translating the psychopharmacology of antipsychotics to individualized treatment for severe mental illness: a roadmap. J Clin Psychiatry. 2007;68(suppl 7):1-48. PubMed
10. Miller AL, Chiles JA, Chiles JK, et al. The Texas Medication Algorithm Project (TMAP) schizophrenia algorithms. J Clin Psychiatry. 1999;60(10):649-657. PubMed
11. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276. PubMed
12. Addington D, Addington J, Maticka-Tyndale E, et al. Reliability and validity of a depression rating scale for schizophrenics. Schizophr Res. 1992;6(3):201-208. PubMed doi:10.1016/0920-9964(92)90003-N
13. Guy W. ECDEU Assessment Manual for Psychopharmacology, Revised. US Dept Health, Education, and Welfare publication (ADM)76-338. Rockville, MD: National Institute of Mental Health; 1976:217-222.
14. Schooler NR, Chengappa KNR. Adverse effects measures. In: American Psychiatric Association Task Force for the Handbook of Psychiatric Measures, eds. Handbook of Psychiatric Measures. Washington, DC: American Psychiatric Association; 2000:151-168.
15. Barnes TR. The Barnes Akathisia Rating Scale-revisited. J Psychopharmacol. 2003;17(4):365-370. PubMed doi:10.1177/0269881103174013
16. Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;45(suppl 212):11-19. doi:10.1111/j.1600-0447.1970.tb02066.x
17. Sunakawa A, Weiden P, Weedon J, et al. Adherence outcomes in schizophrenia trials: advances in measuring time until discontinuation [presented at the 48th Annual NCDEU Meeting: New Research Approaches for Mental Health Interventions]. In: Poster Abstracts of the 48th Annual NCDEU Meeting: The Art and Science of Personalizing Treatments for Mental Disorders; May 27-30, 2008; Phoenix, AZ. Session II-65:147.
18. Weiden P, Rapkin B, Mott T, et al. Rating of Medication Influences (ROMI) scale in schizophrenia. Schizophr Bull. 1994;20(2):297-310. PubMed
19. Tunis SL, Faries DE, Stensland MD, et al. An examination of factors affecting persistence with initial antipsychotic treatment in patients with schizophrenia. Curr Med Res Opin. 2007;23(1):97-104. PubMed doi:10.1185/030079907X162665
20. Patel MX, De Zoysa N, Bernadt M, et al. A cross-sectional study of patients’ perspectives on adherence to antipsychotic medication: depot versus oral. J Clin Psychiatry. 2008;69(10):1548-1556. PubMed
21. Patel MX. De Zoysa N, Baker D, et al. Antipsychotic depot medication and attitudes of community psychiatric nurses. J Psychiatr Ment Health Nurs. 2005;12(2):237-244. PubMed doi:10.1111/j.1365-2850.2004.00826.x
22. Patel MX, Nikolaou V, David AS. Psychiatrists’ attitudes to maintenance medication for patients with schizophrenia. Psychol Med. 2003;33(1):83-89. PubMed doi:10.1017/S0033291702006797
23. Emsley R, Medori R, Koen L, et al. Long-acting injectable risperidone in the treatment of subjects with recent-onset psychosis: a preliminary study. J Clin Psychopharmacol. 2008;28(2):210-213. PubMed doi:10.1097/JCP.0b013e318167269d
24. Craig TK, Garety P, Power P, et al. The Lambeth Early Onset (LEO) Team: randomised controlled trial of the effectiveness of specialised care for early psychosis. BMJ. 2004;329(7474):1067. doi:10.1136/bmj.38246.594873.7C
25. Addington J, Leriger E, Addington D. Symptom outcome 1 year after admission to an early psychosis program. Can J Psychiatry. 2003;48(3):204-207. PubMed
26. Malla A, Norman R, Bechard-Evans L, et al. Factors influencing relapse during a 2-year follow-up of first-episode psychosis in a specialized early intervention service. Psychol Med. 2008;38(11):1585-1593. PubMed doi:10.1017/S0033291707002656
27. Petersen L, Thorup A, טqhlenschlaeger J, et al. Predictors of remission and recovery in a first-episode schizophrenia spectrum disorder sample: 2-year follow-up of the OPUS trial. Can J Psychiatry. 2008;53(10):660-670. PubMed
28. Schooler NR, Levine J, Severe JB, et al. Prevention of relapse in schizophrenia: an evaluation of fluphenazine decanoate. Arch Gen Psychiatry. 1980;37(1):16-24. PubMed
29. Mentschel CC, Leucht SM, Kane JM. Depot-drugs may reduce relapses in schizophrenic outpatients: a meta-analysis [presented at the 156th Annual Meeting of the American Psychiatric Association]. In: New Research Abstracts of the 156th Annual Meeting of the American Psychiatric Association; May 17-22, 2003; San Francisco, CA. Abstract NR191:71.