Abstract
Being able to recognize inadequate response to antidepressant treatment and distinguish it from treatment-resistant depression is key in order for clinicians to provide appropriate therapies. Although definitions vary, nonresponse is often defined as less than 25% improvement on a standardized rating scale, and partial response, as more than 25% but less than 50% improvement. Residual symptoms characteristic of inadequate response (less than 50% improvement) include low mood, anxiety, irritability, guilt, and somatic symptoms. Various factors that may contribute to inadequate response to an antidepressant include inadequate dose or duration, poor adherence, and misdiagnosis.
This activity is the first of four included in the series “Inadequate Response to Antidepressant Treatment in Major Depressive Disorder”:
- Implementing Measurement-Based Care to Determine and Treat Inadequate Response
- Inadequate Response to Treatment in Major Depressive Disorder: Augmentation and Adjunctive Strategies
- Overcoming Challenges to Treat Inadequate Response in Major Depressive Disorder
Financial support was provided by Otsuka Pharmaceutical Development & Commercialization, Inc., and Lundbeck, Inc.
Departments of Psychiatry and Family Medicine, University of Tennessee College of Medicine, Memphis, Tennessee (Dr Jackson); Massachusetts General Hospital Clinical Trials Network and Institute, Boston, Massachusetts (Dr Papakostas); Department of Psychiatry, Northwestern University, Feinberg School of Medicine, Chicago, Illinois (Dr Rafeyan); and University of Texas Southwestern Medical Center, Dallas, Texas (Dr Trivedi)
Financial disclosure
Dr Papakostas has been a consultant for AlphaSigma, Cala Health, Alkermes, Johnson & Johnson, and Otsuka and has received honoraria from Acadia, AlphaSigma, Alkermes, and Hypera S.A. Dr Jackson has been a consultant and speakers/advisory board member for and received honoraria from Allergan, Genentech, Otsuka, and Sunovion. Dr Rafeyan has received grant/research support from Eli Lilly, Sepracor, and Janssen and has been a speakers/advisory board member for AstraZeneca, Allergan, Alkermes, Eli Lilly, Janssen, Intra Cellular Therapies, Pfizer, Abbott, Otsuka, Lundbeck, Takeda, Teva, and Sunovion. Dr Trivedi has been a consultant or advisory board member for Allergan, Alto Neuroscience, Applied Clinical Intelligence, Axsome Therapeutics, Boehringer Ingelheim, Engage Health Media, GreenLight VitalSign6, Janssen, Lundbeck Research USA, Navitor, Otsuka, Perception Neuroscience, Pharmerit International, SAGE Therapeutics, and Signant Health; has received grant/research support from the National Institute of Mental Health, National Institute on Drug Abuse, Patient-Centered Outcomes Research Institute, and Cancer Prevention Research Institute of Texas; and has received editorial compensation from the American Psychiatric Association (Deputy Editor, American Journal of Psychiatry) and Oxford University Press.
This eReport is derived from the expert roundtable meeting “Inadequate Response to Antidepressant Treatment in Major Depressive Disorder,” which was held October 5, 2019.
This evidence-based peer-reviewed eReport was prepared by Healthcare Global Village, Inc. Financial support for preparation and dissemination of this eReport was provided by Otsuka Pharmaceutical Development & Commercialization, Inc., and Lundbeck, Inc., for educational purposes. The faculty acknowledges Evonne Krell for editorial assistance in developing the eReport. The opinions expressed herein are those of the faculty and do not necessarily reflect the views of Healthcare Global Village, Inc., the publisher, or the commercial supporters.
To cite: Jackson WC, Papakostas GI, Rafeyan R, et al. Recognizing inadequate response in patients with major depressive disorder. J Clin Psychiatry. 2020;81(3):OT19037BR2.
To share: https://doi.org/10.4088/JCP.OT19037BR2
Meet the faculty
Introduction
At a roundtable meeting, an expert panel discussed how to determine where major depressive disorder patients are on the response continuum and how to proceed with treatment when a patient experiences inadequate antidepressant response. Their discussion is interspersed in video clips throughout this activity, which reviews defining features and characteristic residual symptoms seen in IR, as well as how it can be distinguished from treatment-resistant depression.
More than one-third of patients with major depressive disorder (MDD) will have an inadequate or partial response to initial antidepressant treatment and require multiple treatment steps to achieve remission.1,2 It is therefore key that clinicians be able to recognize inadequate response (IR) and distinguish it from treatment-resistant depression (TRD) so that they can provide appropriate alternative therapies.
How Is Response Defined? When Is It Inadequate?
Definitions related to antidepressant response matter because they affect treatment decisions. Nonresponse is often defined as less than 25% improvement on a standardized rating scale, and partial response, as more than 25% but less than 50% improvement.10 At least 50% reduction in symptomatology would indicate response,10 and remission would be defined as a cutoff score on a rating scale, such as ≤7 on the Hamilton Depression Rating Scale or <5 on the PHQ-9, for at least 2 consecutive weeks.3,4
Remission is the treatment goal in MDD because it is associated with significant improvement in functioning as well as structural and metabolic changes that are not seen with only response.5,6 Failure to achieve remission typically results in residual symptoms, which are associated with increased risk of relapse, poorer outcomes, and decreased functioning.7
Defining Inadequate/Partial Response
In an online poll of visitors to Psychiatrist.com conducted in September 2019, health care professionals were asked which factor most clearly defines inadequate/partial response in patients with MDD who have been taking an antidepressant for an adequate trial (Figure 1).
Figure 1. What Factor Do You Think Most Clearly Defines Inadequate/Partial Response in Your Patients With Major Depressive Disorder Who Have Been Taking an Antidepressant for an Adequate Trial?
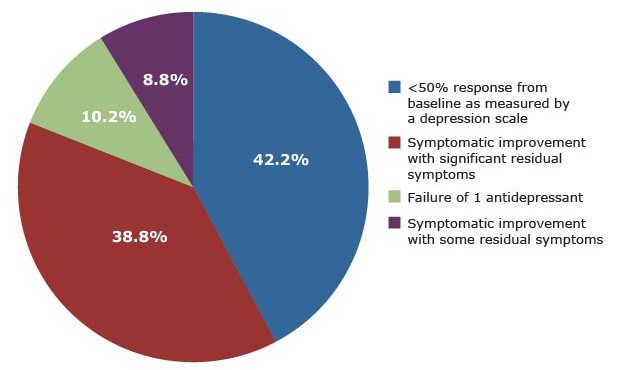
The meeting participants noted that the respondents’ top choice for defining inadequate/partial response—less than 50% response from baseline as measured by a rating scale—is significant because it indicates that clinicians are using measurement-based care (MBC) to track symptoms. (The second most popular definition, symptomatic improvement with significant or some residual symptoms, would certainly be a relevant clinical observation but does not suggest the use of a rating scale.)
Treatment-Resistant Depression
Dr Jackson cited a common definition of TRD as failure of 2 or more treatment trials of adequate dose and duration.7–9 A recent Agency for Healthcare Research and Quality report8 identified a lack of a standard definition of TRD and pointed to needs in 3 areas: clarifying failure (ie, lack of response or remission and after what length of time), defining adequate dose and duration, and determining whether TRD requires the prior use of different classes of antidepressants. Two caveats Dr Jackson noted regarding TRD definitions are that the defining criteria for treatment “failure” are inconsistent and that they are almost exclusively focused on pharmacologic therapies rather than on nonpharmacologic therapies or exercises, which can be helpful for patients.8
Treatment-Resistant Depression vs Inadequate Response
Although TRD definitions vary, Dr Trivedi observed that it’s easier to pinpoint complete treatment failure than IR because there is no improvement in symptoms or functioning, and patients recognize that nothing is changing. In contrast, patients with IR may show some symptomatic improvement (ie, sleep and appetite are better) while their functioning at work or home remains impaired. Dr Papakostas concluded that TRD patients tend to have longer treatment histories and more discouragement over their lack of improvement than patients with IR.
 Patient Perspective
Patient Perspective
In an interview conducted by the Depression and Bipolar Support Alliance (oral communication, December 2019), a patient described her growing frustration with each failed treatment trial:
“I have struggled with depression and anxiety for many years, and I have tried multiple, various prescriptions in order to help try to reduce that. It’s frustrating not being able to find something to help me feel normal.”
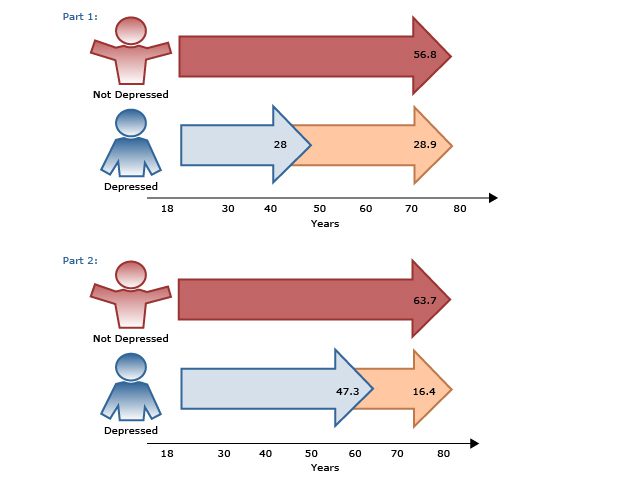
Other differences and similarities between TRD and IR are summarized in AV 1.
AV 1. Journey Along the Depression Road: Treatment-Resistant Depression and Inadequate Response
References: Fava,5 Gaynes et al,8 Al-Harbi,9 Nierenberg and DeCecco,10 Trivedi et al.11
Abbreviation: CBT=cognitive-behavioral therapy, MBCT=measurement-based cognitive therapy.
Video 1. Dr Trivedi Describes the Presentation of a Patient With IR Compared to a Patient With TRD
References: Jha et al,12 IsHak et al.13
Abbreviations: CO-MED=Combining Medications to Enhance Depression Outcomes, STAR*D=Sequenced Treatment Alternatives to Relieve Depression.
What Residual Symptoms Might Patients With IR Have?
What symptoms might clinicians expect to see in patients who are getting better but still have IR? Common residual symptoms include low mood, anxiety, irritability, guilt, and somatic symptoms.14 While irritability is especially problematic for patients psychosocially, anxiety tends to make remission even more challenging.15 Further, a STAR*D trial16 looked at the types and frequency of residual symptoms in patients who met criteria for remission (N=943). Among remitters, over 90% had at least 1 residual symptom; the most common symptoms were weight gain and insomnia (AV 2).
AV 2. Residual Symptoms in Patients With Depression
Data from Nierenberg et al.16
Abbreviation: QIDS-SR16=16-item Quick Inventory of Depressive Symptomatology, Self-Report.
Common Residual Symptoms
Common somatic symptoms include reduced libido, insomnia, fatigue, and pain.14 In primary care, over half of the medical outpatient visits are for somatic symptoms, and these are usually associated with depression and anxiety.17 Dr Rafeyan noted that anxiety and lack of motivation tend to linger as residual symptoms. Dr Papakostas concluded that irritability, sleep problems, lack of energy, and cognitive impairment are often associated with IR in patients with MDD.
 Patient Perspective
Patient Perspective
In an interview, one patient noted how lack of energy made her feel:
“There’s times where you don’t even want to get out bed. You just feel like you just don’t want to do anything. You just you know you have negativity about yourself. You can’t do things you used to be able to do.”
Video 2. The Experts Discuss Common Residual Symptoms
Abbreviation: TSH=thyroid-stimulating hormone.
Impact of Residual Symptoms
The expert panel also discussed the impact of residual symptoms in terms of function. Even if a patient is showing partial response, demotivation and impaired cognition and memory can cause problems at work and in personal relationships.14 Dr Jackson noted that patients may not only struggle with remembering important details in daily life and at work but also display an overly emotional response to external stimuli, which can cause interpersonal and professional problems. Clinicians should regularly assess patients’ symptoms so they know what response, if any, patients are experiencing and what may be hindering it. Rating scales, such as the 9-item Patient Health Questionnaire (PHQ-9) and Quick Inventory of Depressive Symptomatology (QIDS), provide clinicians with the rate of improvement from baseline and can pinpoint residual symptoms that remain even in patients who respond or remit during acute-phase treatment.16 For more on assessment tools in MBC, see the activity “Implementing Measurement-Based Care to Determine and Treat Inadequate Response.”
What Factors Might Contribute to Inadequate Response?
The number of antidepressant trials and the number of medication classes tried are certainly key variables when thinking about IR or TRD. But before counting an antidepressant trial as a failure, clinicians have a number of questions to consider (AV 3), including whether the dose and duration were adequate, whether the patient was adherent with treatment, and whether the patient’s diagnosis is correct.
AV 3. Factors That Can Affect the Success of Initial Antidepressant Monotherapy
Adapted with permission from Nemeroff.1
Dose. Dr Jackson commented that persistent use of an antidepressant dose that is too low is like trying to drive a car in second gear. With an inadequate dose, such as sertraline 25 mg/d or fluoxetine 20 mg/d,18,19 the treatment’s potential will most likely not be reached, as these are not the doses that are going to work for the majority of patients. Generally, an adequate dose is considered the maximum manufacturer-recommended dose, but the dose may be smaller for elderly patients.9
Duration. In terms of trial duration, MBC can guide treatment decisions more quickly than traditionally thought, such as waiting the typical 6 weeks or longer to measure response.1 For example, a study20 showed that if patients with MDD do not respond by 20% in 2 weeks, that may predict a lack of positive response at 4–8 weeks. Thus, a 2-week assessment may be used as a decision point at which to reassess and see if patients have achieved a 20% response to treatment.
Video 3. The Panel Discusses IR in Primary Care Patients and Compares Patient-Report Assessments With Global Clinical Improvement
Reference: Busner and Targum.21
Adherence. Poor adherence to prescribed treatment along with poor tolerability are factors that contribute to over half of treatment nonresponse in MDD.1 In a study22 of primary care patients with depression (N=155) newly prescribed an antidepressant, about 28% of patients stopped taking antidepressants during the first month, and 44% had stopped taking them by the third month of therapy, meaning that only about half of patients were still taking medication at 90 days. Adherence is just as poor among psychiatric patients, with approximately 50% nonadherent when assessed at 6 months after treatment initiation.23
Patient factors involved in nonadherence include concerns about side effects, fear of addiction, and belief that medications will not help.23 Clinicians can improve adherence by educating patients on their medication and the time course of anticipated improvement and by providing consistent follow-up.23
What patients think about treatment matters because preference predicts both therapeutic alliance and adherence in treatment. When patients with a treatment preference are matched to the treatment they prefer, their outcomes are better than if they receive a mismatch.24 In addition, what patients believe about treatment strategies will affect their preferences. Those who endorse psychosocial strategies indicate a preference for psychotherapy, while those who believe a biomedical explanation of depression tend to prefer antidepressant medication.24
Misdiagnosis. Clinicians must be aware that a response problem is not always the fault of pharmacologic agents or TRD but may result from misdiagnosis of patients with MDD with mixed features or bipolar disorder. MDD with mixed features often requires augmentation with an atypical antipsychotic rather than antidepressant monotherapy to help patients achieve remission.25
In a study26 that examined 61 patients referred to a mood disorder clinic with unipolar TRD, the patients were followed for at least 12 months and then interviewed with the Structured Clinical Interview. At intake, 35% of patients were diagnosed with bipolar disorder. At follow-up, the incidence of bipolar disorder was 59%. Of the patients with MDD, 52% were later classified as having bipolar spectrum disorder, and 80% showed evidence of bipolarity.
Conclusion
With initial treatment, many patients with MDD will experience nonresponse (less than 25% improvement in symptoms) or partial response (25%–49% reduction of symptoms). Even if a patient achieves partial response, though, he or she may have significant residual symptoms that require addressing. Clinicians must ensure that they are using adequate doses of medication and educating patients on the typical time to see improvement. Using MBC to regularly assess symptoms, adherence, and adverse effects, clinicians can address residual symptoms and other barriers that may be hindering response and remission. Clinicians should check the diagnosis of MDD, assess comorbid psychiatric and medical conditions, and consider therapies based on individual patient preference.
 Case Practice Question
Case Practice Question
Robert is a 43-year-old man with a history of MDD. He has tried pharmacologic treatment with fluoxetine and venlafaxine, but both were unsuccessful. His primary care physician utilized the 7-item Generalized Anxiety Disorder scale to assess his anxiety and found he has moderate symptoms (his score was 11/21). His Mood Disorder Questionnaire screen for bipolar disorder was negative. When questioned about his experience with the medications, Robert reveals that he typically took about half of the scheduled doses and was not on either agent for over 6 weeks.
Which statement best applies to Robert’s case?
a. He has TRD because he has failed 2 different antidepressants from different classes.
b. Exploring ways to improve adherence to the next therapy might significantly improve response.
c. Due to the presence of comorbid anxiety symptoms, psychotherapy should not be considered at this time.
 Discussion of Case Practice Questions
Discussion of Case Practice Questions
Preferred response: B. Exploring ways to improve adherence to the next therapy might significantly improve repsonse.
Explanation: Robert doesn’t have a proven diagnosis of TRD because his trials were not of adequate dose and duration due to nonadherence. Further, there is no evidence that comorbid anxiety should preclude psychotherapy as a treatment option. Exploring ways to improve adherence to the next therapy either pharmacologic or nonpharmacologic, is the best choice because it will provide the best opportunity to assess his response.
 Clinical Points
Clinical Points
- Ensure that patients receive an adequate dose and duration of each treatment trial before declaring it a failure.
- Perform regular assessments, as early as 2 weeks, to monitor response, residual symptoms, and adherence to treatment.
- Review the diagnosis and address comorbid medical and psychiatric conditions.
© Copyright 2020 Physicians Postgraduate Press, Inc.
References (26)

- Nemeroff CB. Prevalence and management of treatment-resistant depression. J Clin Psychiatry. 2007;68(suppl 8):17–25. PubMed
- Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. PubMed CrossRef
- McIntyre R, Kennedy S, Bagby RM, et al. Assessing full remission. J Psychiatry Neurosci. 2002;27(4):235–239. PubMed
- Schueller SM, Kwasny MJ, Dear BF, et al. Cut points on the Patient Health Questionnaire (PHQ-9) that predict response to cognitive-behavioral treatments for depression. Gen Hosp Psychiatry. 2015;37(5):470–475. PubMed CrossRef
- Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53(8):649–659. PubMed CrossRef
- Oluboka OJ, Katzman MA, Habert J, et al. Functional recovery in major depressive disorder: providing early optimal treatment for the individual patient. Int J Neuropsychopharmacol. 2018;21(2):128–144. PubMed CrossRef
- Berlim MT, Turecki G. Definition, assessment, and staging of treatment-resistant refractory major depression: a review of current concepts and methods. Can J Psychiatry. 2007;52(1):46–54. PubMed CrossRef
- Gaynes BN, Asher G, Hoffman V, et al. Definition of treatment-resistant depression in the Medicare population. Proj ID PSYT0816 Prep RTI–UNC Evid-Based Pract Cent Contract No HHSA290201500011IHHSA29032006T. Rockville, MD. Agency Healthc Res Qual;2018:140.
- Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence. 2012;6:369–388. PubMed CrossRef
- Nierenberg AA, DeCecco LM. Definitions of antidepressant treatment response, remission, nonresponse, partial response, and other relevant outcomes: a focus on treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16):5–9. PubMed
- Trivedi MH, Hollander E, Nutt D, et al. Clinical evidence and potential neurobiological underpinnings of unresolved symptoms of depression. J Clin Psychiatry. 2008;69(2):246–258. PubMed CrossRef
- Jha MK, Minhajuddin A, Greer TL, et al. Early improvement in work productivity predicts future clinical course in depressed outpatients: findings from the CO-MED Trial. Am J Psychiatry. 2016;173(12):1196–1204. PubMed CrossRef
- IsHak WW, James DM, Mirocha J, et al. Patient-reported functioning in major depressive disorder. Ther Adv Chronic Dis. 2016;7(3):160–169. PubMed CrossRef
- Israel JA. The impact of residual symptoms in major depression. Pharmaceuticals (Basel). 2010;3(8):2426–2440. PubMed CrossRef
- McIntyre RS, Konarski JZ, Soczynska JK, et al. Residual anxiety symptoms in depressed primary care patients. J Psychiatr Pract. 2007;13(2):125–128. PubMed CrossRef
- Nierenberg AA, Husain MM, Trivedi MH, et al. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol Med. 2010;40(1):41–50. PubMed CrossRef
- Culpepper L, Clayton A, Lieberman JA, et al. Treating depression and anxiety in primary care. Prim Care Companion J Clin Psychiatry. 2008;10(2):145–152. PubMed CrossRef
- Zoloft [package insert]. Reference ID: 4032692. New York, NY: Pfizer; 1991. Revised 2016.
- Prozac [package insert]. Reference ID: 2927282. Indianapolis, IN: Eli Lilly; 1987.
- Szegedi A, Jansen WT, van Willigenburg AP, et al. Early improvement in the first 2 weeks as a predictor of treatment outcome in patients with major depressive disorder: a meta-analysis including 6562 patients. J Clin Psychiatry. 2009;70(3):344–353. PubMed CrossRef
- Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4(7):28–37. PubMed
- Lin EH, Von Korff M, Katon W, et al. The role of the primary care physician in patients’ adherence to antidepressant therapy. Med Care. 1995;33(1):67–74. PubMed CrossRef
- Sansone RA, Sansone LA. Antidepressant adherence: are patients taking their medications? Innov Clin Neurosci. 2012;9(5-6):41–46. PubMed
- Steidtmann D, Manber R, Arnow BA, et al. Patient treatment preference as a predictor of response and attrition in treatment for chronic depression: research article: patient treatment preference. Depress Anxiety. 2012;29(10):896–905. PubMed CrossRef
- Hu J, Mansur R, McIntyre RS. Mixed specifier for bipolar mania and depression: highlights of DSM-5 changes and implications for diagnosis and treatment in primary care. Prim Care Companion CNS Disord. 2014;16(2):PCC.13r01599. PubMed CrossRef
- Sharma V, Khan M, Smith A. A closer look at treatment resistant depression: is it due to a bipolar diathesis? J Affect Disord. 2005;84(2–3):251–257. PubMed CrossRef
Save
Cite