To cite: Ballard C, Aarsland D. Relieving caregiver burden through evidence-based treatment for dementia-related psychosis. J Clin Psychiatry 2021;82(4):AD19038AH5C
To share: https://doi.org/10.4088/JCP.AD19038AH5C
© Copyright 2021 Physicians Postgraduate Press, Inc.
aUniversity of Exeter College of Medicine and Health, United Kingdom
bNational Institute for Health Research Biomedical Research Centre at South London and Maudsley NHS Foundation and Institute of Psychiatry, Psychology & Neuroscience at King’s College London, United Kingdom
An estimated 50 million people worldwide are living with dementia.1 The most common type of dementia is Alzheimer’s disease (AD), accounting for about 60%–70% of dementia cases,1 followed by vascular dementia (VaD) with a prevalence of about 20%.2 Other types of dementia include dementia with Lewy bodies (DLB), Parkinson disease (PD) dementia, and frontotemporal dementia (FTD).3 Along with cognitive decline, 90% of patients with dementia experience behavioral and psychological symptoms of dementia, such as psychosis, aggression, agitation, and depression.4 Dementia-related psychosis (DRP), which includes delusions and hallucinations, contributes to institutionalization, cognitive decline, and caregiver burden.5
In this Academic Highlights, Drs Ballard and Aarsland will address best practices for improving outcomes for people living with DRP and their care partners.
Overview of Dementia-Related Psychosis
A review6 of 55 studies showed that psychosis occurred in about 40% of patients with AD; delusions occurred more frequently (36%) than hallucinations (18%). Recurrent visual hallucinations are a core feature of DLB7 and are a common characteristic of PD dementia.8 Simple hallucinations are rare, with most patients experiencing complex hallucinations daily. The experiences often feature people or animals.8,9
Dr Aarsland stated that delusions and hallucinations may increase in frequency over time and be persistent in some individuals.10,11 According to a study12 of 83 patients with dementia and at least 1 psychotic symptom, most patients experienced psychotic symptoms at a frequency between daily and weekly. Thirty-seven patients (45%) were mildly distressed by their psychotic symptoms, and 14 (17%) were severely distressed.12
A complex interplay of factors can contribute to delusions and hallucinations in people with dementia (Figure 1).13–16
Figure 1. Factors Contributing to Psychotic Symptoms in Patients With Dementia
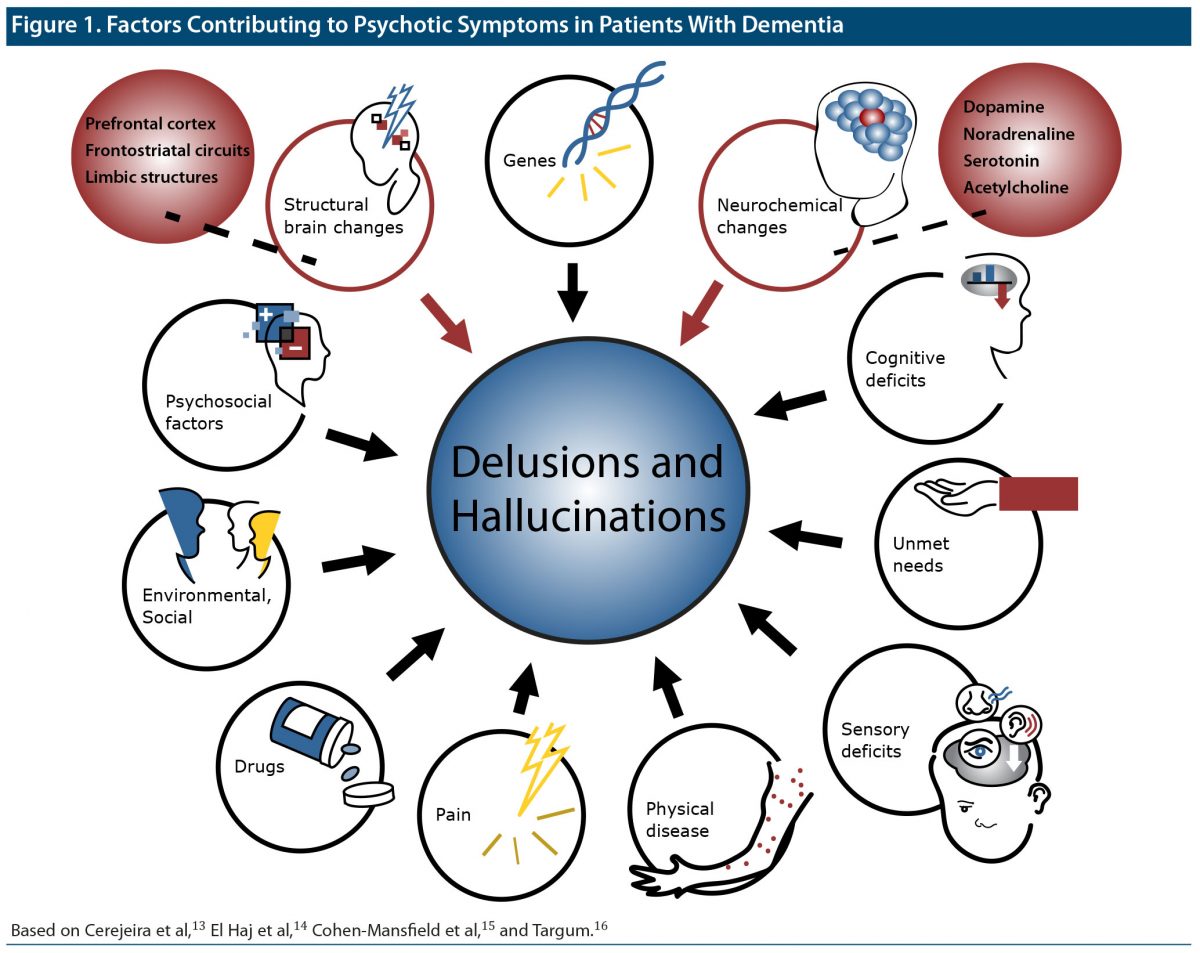
According to Dr Aarsland, clinicians must probe factors that contribute to DRP in discussions with the patient (if possible) and the care partner. When talking to patients and carers, it is important to always explore both whether apparent delusions might in fact be true and whether there are factors before or after the occurrence that might initiate or maintain the behaviors. Based on Dr Aarsland’s clinical experience, it is usually not helpful for clinicians or carers to argue with patients or try to convince them that these thoughts or sensory impressions are not real; it is more helpful to try to understand and acknowledge the patient’s emotional reaction.
Impact of Dementia-Related Psychosis on Patients and Caregivers
Delusions and hallucinations may directly impact the patient’s ability to perform cognitive and functional tasks,17 which can contribute to carer distress.18,19 Further adding to carer distress, Dr Aarsland explained, patients experiencing hallucinations, such as seeing a person in the house who should not be there, may run after “the person” or even call the police for help.
Additionally, although some psychotic symptoms might not be dramatic, they are often accompanied by troublesome affective and behavioral symptoms that can be stressful for the carer. For instance, sleep disturbances associated with psychotic symptoms may not only lead to increased agitation and reduced daytime functioning in patients, which are challenging for carers to address, but also may cause lack of sleep for carers.20 Psychotic symptoms can also adversely impact the emotional connection between the care partner and the patient.21
Family Perspectives
Here, a relative describes her ongoing stress:
“My mother was diagnosed with vascular dementia almost 2 years ago… She is very bitter towards me since she blames me for losing her independence. I’m her power of attorney and she barely understands what that is and thinks I just go shopping with her money. She is always asking about her money and where it is. She also wants to know how much there is. She has financial scammers that are still in regular contact with her. She tells them everything. I even found out that [a scammer] tried getting in a lawyer to see her to do a new will. The only reason they didn’t succeed is because of COVID. I’m in a constant state of panic and it’s affecting my health.”22
Dr Aarsland recommended several scales to measure the impact of DRP on care partners, including the Zarit Burden Interview,23 the Relatives’ Stress Scale (RSS),24 and the Neuropsychiatric Inventory Caregiver Distress Scale (NPI-D).25 Cognitive-behavioral therapy26,27 or emotion-based and problem-focused interventions26 may help care partners cope with DRP.
Although psychotic symptoms can have an immense negative impact on carers,28,29 the symptoms do not necessarily cause distress to patients.12 Patients’ degree of insight appears to affect whether they consider hallucinations to be threatening.30 One study31 found that 40% of delusions did not cause discomfort to the patients. Dr Ballard said that discussing this perspective with caregivers may help to relieve some of their own distress.
Another point that may help care partners is that psychotic symptoms are not persistent in every patient32–34; they can last for less than 3 months.35
Dr Ballard explained that he has found it important to talk with patients and caregivers about the impact of DRP on well-being, their level of distress (and patients’ insight), the potential course of DRP, and the potential risks of medications used to treat DRP. Then, it is possible for people to knowledgeably discuss treatment options.
Terminology is important when discussing DRP with patients and caregivers. Dr Ballard noted that the lay understanding of psychosis is usually associated with schizophrenia. Individuals may be more comfortable if providers avoid terms such as psychotic and instead describe symptoms according to their presentations.
Evidence-Based Treatment
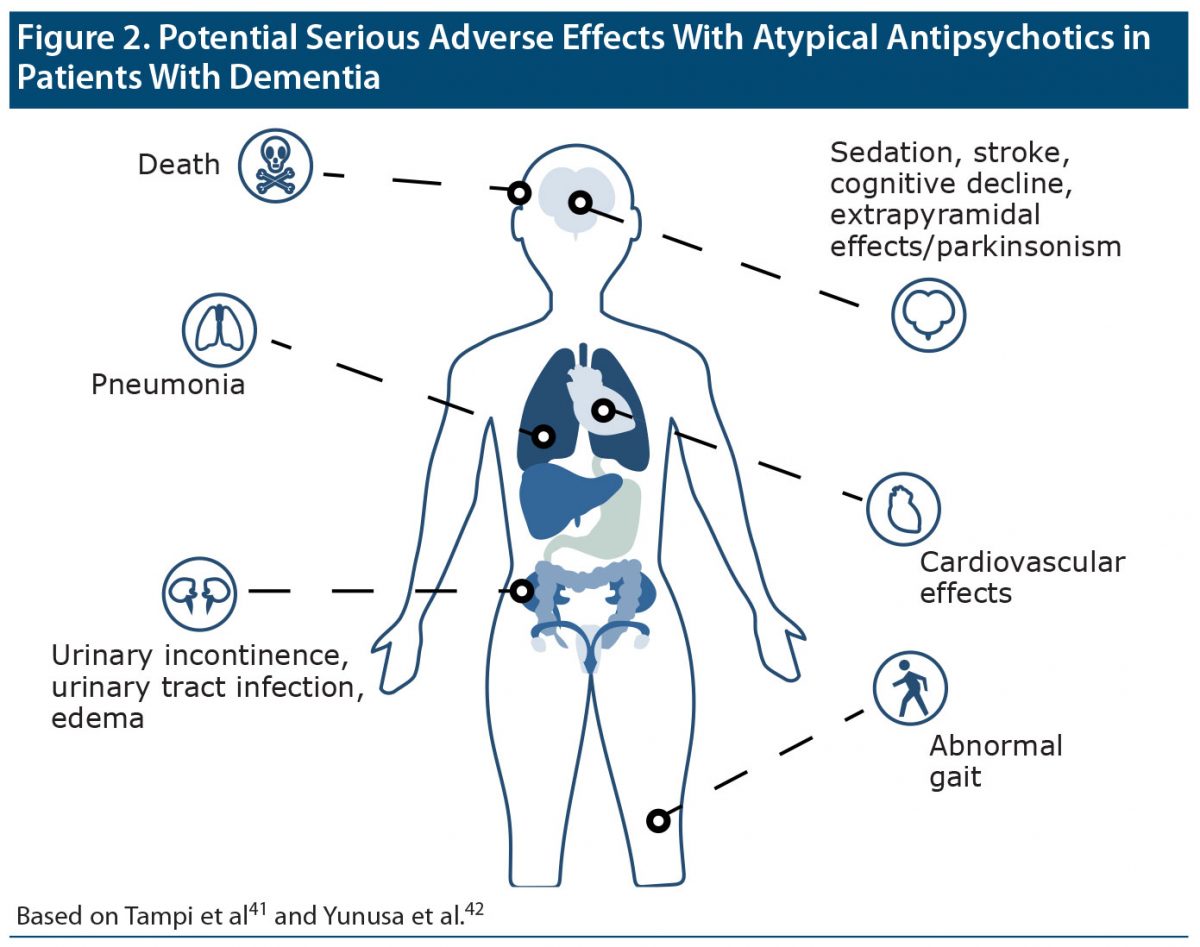
Dr Ballard explained that pharmacologic treatment is probably not necessary if symptoms are not severe and are not causing aggressive behavior that puts the patient and others in danger.36–38 Although atypical antipsychotics may play a limited role in the short-term management of psychosis in patients with dementia,39,40 any benefit has to be balanced against the risk of significant harms (Figure 241,42).38 Dr Ballard noted that the US Food and Drug Administration (FDA) requires a “black box” warning43 on atypical antipsychotic labels indicating an increased risk of mortality, other known harms, and limited efficacy in patients with dementia,4 and European agencies have similar warnings.44 Cholinesterase inhibitors may provide a pharmacologic treatment alternative for psychosis in people with DLB,45 but benefits are less clear in people with other dementias.
Figure 2. Potential Serious Adverse Effects With Atypical Antipsychotics in Patients With Dementia
Guidance on Atypical Antipsychotics in DRP
According to the American Psychiatric Association (APA) practice guideline46 on the use of antipsychotics for agitation or psychosis in individuals with dementia, antipsychotic medication should be used only when symptoms are severe, are dangerous, and/or cause significant distress to the patient. The APA recommends that, before nonemergency treatment with an antipsychotic is initiated, the potential risks and benefits must be assessed by the clinician and discussed with the patient (if feasible), family, and/or the surrogate decision maker. If the risk-benefit assessment favors the use of an antipsychotic, the treatment should be initiated at a low dose and titrated up to the minimum effective dose as tolerated.41,46 (The first-generation antipsychotic haloperidol should not be used in the absence of delirium.) Response should be assessed with a quantitative measure. Dr Ballard recommended the Neuropsychiatric Inventory (NPI)47–49 for assessment and monitoring.
If no response is evident after 4 weeks, the medication should be tapered and withdrawn.46 Among those who respond adequately, an attempt to taper and withdraw the medication should be made within 16 weeks unless previous attempts at tapering were associated with recurrence of symptoms. A long-acting injectable antipsychotic agent should not be used.
Dr Ballard explained that since the 2016 publication of the APA guidelines,46 evidence has become available on pimavanserin, a selective 5-HT2A inverse agonist, which is FDA-approved for the treatment of hallucinations and delusions associated with PD.50 A meta-analysis by Zhang et al51 concluded that pimavanserin is effective in the treatment of PD psychosis. Unlike other atypical antipsychotics, pimavanserin has no clinically significant antagonism of dopaminergic, muscarinic, or histaminergic receptors.52 Evidence53 indicates that pimavanserin may decrease hallucinations and delusions in patients with AD, PD dementia, VaD, DLB, and FTD (including among nursing home residents with severe psychotic symptoms54), but it is not FDA-approved for this indication.
Dr Ballard summarized that a patient treated with an antipsychotic for DRP may have marginal benefits in the context of many potential harms; however, for patients with severe symptoms, the risks may be worthwhile with short-term treatment. Atypical antipsychotics are not a widespread, long-term treatment solution.5
Family Perspective
Here, a family member describes the struggle to find therapy for a patient with PD-related psychosis:
“My father-in-law is very sensitive to certain medications. [One antipsychotic] made him hallucinate more. . . . He goes through phases. He’s generally happy, then has a few days of constant hallucinations and gets aggressive when the sun goes down.”56
Case Practice Question
Annette, aged 85 years, has Parkinson disease dementia of moderate severity. Annette lives at home with her husband. She has become increasingly suspicious over the last 5 days. According to guidelines provided by the American Psychiatric Association, which of the following statements is accurate?
a. This is a clear presentation of dementia-related psychosis.
b. Given the severity of Annette’s symptoms, an atypical antipsychotic should be started as soon as possible.
c. The first step should be investigation of possible causes of delirium.
d. A nonpharmacologic intervention is the top priority.
Discussion of the Case Practice Question
Preferred response: c. The first step should be investigation of possible causes of delirium.
Explanation: Given the acute presentation associated with increased confusion, investigation of delirium should be the first step. Guidelines provided by the American Psychiatric Association46 recommend that antipsychotic medication should be used only when symptoms are severe, are dangerous, and/or cause significant distress to the patient.
Alternatives to Antipsychotics
Nonpharmacologic interventions. Dr Ballard stated that, although practice guidelines38 for the treatment of BPSD recommend first-line use of non-pharmacologic interventions,38,57,58 these strategies are more effective for the treatment of agitation than psychosis.
Medications. Both delusions and hallucinations were reduced with citalopram compared with placebo59; however, adverse effects may limit its practical application at the 30 mg/d dose.60 Masupirdine is a potentially beneficial treatment for BPSD in patients with AD.61 Trials45,62,63 assessing cholinesterase inhibitors in people with LBD have shown significant efficacy in reducing psychotic symptoms, particularly visual hallucinations,64 but quetiapine65 has not demonstrated benefit. Finally, tau-directed therapies could be a potential treatment for DRP.66
Conclusion
Mechanisms behind DRP include neurobiological, environmental, social, and psychological factors. Clinicians must be prepared to offer strategies and educational support to relieve the burden of DRP on patients and their care partners. Depending on the levels of distress and danger to patients or others, short-term medication may be needed.
Clinical Points
- Consider environmental, social, and psychological factors behind DRP.
- Assess the distress and burden of DRP in both patients and care partners using appropriate rating scales.
- Talk with caregivers about whether the psychotic symptoms are causing distress to patients; if not, caregivers may be able to feel less distressed themselves.
- Explain to care partners that antipsychotics have a limited role as short-term treatment for DRP; long-term efficacy is marginal, and the risk of serious adverse effects is considerable.
References
- Dementia. World Health Organization. Published September 19, 2019. Accessed July 14, 2020. https://www.who.int/news-room/fact-sheets/detail/dementia
- Rizzi L, Rosset I, Roriz-Cruz M. Global epidemiology of dementia: Alzheimer’s and vascular types. BioMed Res Int. 2014;2014:908915. PubMed CrossRef
- Devanand DP, Grossberg GT, Khachaturian Z, et al. More Than Cognition: The Prevalence of Neuropsychiatric Symptoms in Dementia-Related Psychosis. More Than Cognition. https://morethancognition.neurologyreviews.com/newsletter/prevalence-neuropsychiatric-symptoms-dementia-related-psychosis/. Accessed July 14, 2020.
- Marcinkowska M, Śniecikowska J, Fajkis N, et al. Management of dementia-related psychosis, agitation and aggression: a review of the pharmacology and clinical effects of potential drug candidates. CNS Drugs. 2020;34(3):243–268. PubMed CrossRef
- Leroi I, Voulgari A, Breitner JCS, et al. The epidemiology of psychosis in dementia. Am J Geriatr Psychiatry. 2003;11(1):83–91. PubMed CrossRef
- Ropacki SA, Jeste DV. Epidemiology of and risk factors for psychosis of Alzheimer’s disease: a review of 55 studies published from 1990 to 2003. Am J Psychiatry. 2005;162(11):2022–2030. PubMed CrossRef
- McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88–100. PubMed CrossRef
- Mosimann UP, Rowan EN, Partington CE, et al. Characteristics of visual hallucinations in Parkinson disease dementia and dementia with lewy bodies. Am J Geriatr Psychiatry. 2006;14(2):153–160. PubMed CrossRef
- Ffytche DH, Creese B, Politis M, et al. The psychosis spectrum in Parkinson disease. Nat Rev Neurol. 2017;13(2):81–95. PubMed CrossRef
- Steinberg M, Shao H, Zandi P, et al; Cache County Investigators. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2008;23(2):170–177. PubMed CrossRef
- van der Linde RM, Dening T, Stephan BCM, et al. Longitudinal course of behavioural and psychological symptoms of dementia: systematic review. Br J Psychiatry. 2016;209(5):366–377. PubMed CrossRef
- Ballard CG, Saad K, Patel A, et al. The prevalence and phenomenology of psychotic symptoms in dementia sufferers. Int J Geriatr Psychiatry. 1995;10(6):477–485. CrossRef
- Cerejeira J, Lagarto L, Mukaetova-Ladinska EB. Behavioral and psychological symptoms of dementia. Front Neurol. 2012;3:73. PubMed CrossRef
- El Haj M, Roche J, Jardri R, et al. Clinical and neurocognitive aspects of hallucinations in Alzheimer’s disease. Neurosci Biobehav Rev. 2017;83:713–720. PubMed CrossRef
- Cohen-Mansfield J, Dakheel-Ali M, Marx MS, et al. Which unmet needs contribute to behavior problems in persons with advanced dementia? Psychiatry Res. 2015;228(1):59–64. PubMed CrossRef
- Targum SD. Treating psychotic symptoms in elderly patients. Prim Care Companion J Clin Psychiatry. 2001;3(4):156–163. PubMed CrossRef
- Murray PS, Kumar S, Demichele-Sweet MAA, et al. Psychosis in Alzheimer’s disease. Biol Psychiatry. 2014;75(7):542–552. PubMed CrossRef
- Terum TM, Testad I, Rongve A, et al. The association between specific neuropsychiatric disturbances in people with Alzheimer’s disease and dementia with Lewy bodies and carer distress. Int J Geriatr Psychiatry. 2019;34(10):1421–1428. PubMed CrossRef
- Jennings LA, Reuben DB, Evertson LC, et al. Unmet needs of caregivers of individuals referred to a dementia care program. J Am Geriatr Soc. 2015;63(2):282–289. PubMed CrossRef
- Waite F, Evans N, Myers E, et al. The patient experience of sleep problems and their treatment in the context of current delusions and hallucinations. Psychol Psychother. 2016;89(2):181–193. PubMed CrossRef
- Cheng S-T. Dementia caregiver burden: a research update and critical analysis. Curr Psychiatry Rep. 2017;19(9):64. PubMed CrossRef
- Need advice – what do I tell her every time? It’s a constant stress. reddit. www.reddit.com/r/dementia/comments/mldo4y/need_advice_what_do_i_tell_her_every_time_its_a/. Published April 6, 2021. Accessed April 14, 2021.
- Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20(6):649–655. PubMed CrossRef
- Greene JG, Smith R, Gardiner M, et al. Measuring behavioural disturbance of elderly demented patients in the community and its effects on relatives: a factor analytic study. Age Ageing. 1982;11(2):121–126. PubMed CrossRef
- Kaufer DI, Cummings JL, Christine D, et al. Assessing the impact of neuropsychiatric symptoms in Alzheimer’s disease: the Neuropsychiatric Inventory Caregiver Distress Scale. J Am Geriatr Soc. 1998;46(2):210–215. PubMed CrossRef
- Brodaty H, Donkin M. Family caregivers of people with dementia. Dialogues Clin Neurosci. 2009;11(2):217–228. PubMed CrossRef
- Cheng S-T, Au A, Losada A, et al. Psychological interventions for dementia caregivers: what we have achieved, what we have learned. Curr Psychiatry Rep. 2019;21(7):59. PubMed CrossRef
- Hallikainen I, Koivisto AM, Välimäki T. The influence of the individual neuropsychiatric symptoms of people with Alzheimer disease on family caregiver distress: a longitudinal ALSOVA study. Int J Geriatr Psychiatry. 2018;33(9):1207–1212. PubMed CrossRef
- Hiyoshi-Taniguchi K, Becker CB, Kinoshita A. What behavioral and psychological symptoms of dementia affect caregiver burnout? Clin Gerontol. 2018;41(3):249–254. PubMed CrossRef
- Renouf S, Ffytche D, Pinto R, et al. Visual hallucinations in dementia and Parkinson’s disease: a qualitative exploration of patient and caregiver experiences. Int J Geriatr Psychiatry. 2018;33(10):1327–1334. PubMed CrossRef
- Cohen-Mansfield J, Cohen R, Golander H, et al. The impact of psychotic symptoms on the persons with dementia experiencing them. Am J Geriatr Psychiatry. 2016;24(3):213–220. PubMed CrossRef
- Vilalta-Franch J, López-Pousa S, Calvó-Perxas L, et al. Psychosis of Alzheimer disease: prevalence, incidence, persistence, risk factors, and mortality. Am J Geriatr Psychiatry. 2013;21(11):1135–1143. PubMed CrossRef
- Ballard CG, O’Brien JT, Swann AG, et al. The natural history of psychosis and depression in dementia with Lewy bodies and Alzheimer’s disease: persistence and new cases over 1 year of follow-up. J Clin Psychiatry. 2001;62(1):46–49. PubMed CrossRef
- Ballard CG, Margallo-Lana M, Fossey J, et al. A 1-year follow-up study of behavioral and psychological symptoms in dementia among people in care environments. J Clin Psychiatry. 2001;62(8):631–636. PubMed CrossRef
- Ballard C, O’Brien J, Coope B, et al. A prospective study of psychotic symptoms in dementia sufferers: psychosis in dementia. Int Psychogeriatr. 1997;9(1):57–64. PubMed CrossRef
- Deardorff WJ, Grossberg GT. Behavioral and psychological symptoms in Alzheimer’s dementia and vascular dementia. Handb Clin Neurol. 2019;165:5–32. PubMed CrossRef
- Kales HC, Lyketsos CG, Miller EM, et al. Management of behavioral and psychological symptoms in people with Alzheimer’s disease: an international Delphi consensus. Int Psychogeriatr. 2019;31(1):83–90. PubMed CrossRef
- Reese TR, Thiel DJ, Cocker KE. Behavioral disorders in dementia: appropriate nondrug interventions and antipsychotic use. Am Fam Physician. 2016;94(4):276–282. PubMed
- Donegan K, Fox N, Black N, et al. Trends in diagnosis and treatment for people with dementia in the UK from 2005 to 2015: a longitudinal retrospective cohort study. Lancet Public Health. 2017;2(3):e149–e156. PubMed CrossRef
- Maust DT, Kim HM, Chiang C, et al. Association of the Centers for Medicare & Medicaid Services’ National Partnership to Improve Dementia Care With the Use of Antipsychotics and Other Psychotropics in Long-term Care in the United States From 2009 to 2014. JAMA Intern Med. 2018;178(5):640–647. PubMed CrossRef
- Tampi RR, Tampi DJ, Balachandran S, et al. Antipsychotic use in dementia: a systematic review of benefits and risks from meta-analyses. Ther Adv Chronic Dis. 2016;7(5):229–245. PubMed CrossRef
- Yunusa I, Alsumali A, Garba AE, et al. Assessment of reported comparative effectiveness and safety of atypical antipsychotics in the treatment of behavioral and psychological symptoms of dementia: a network meta-analysis. JAMA Netw Open. 2019;2(3):e190828. PubMed CrossRef
- Lenzer J. FDA warns about using antipsychotic drugs for dementia. BMJ. 2005;330(7497):922. PubMed CrossRef
- Stocks SJ, Kontopantelis E, Webb RT, et al. Antipsychotic prescribing to patients diagnosed with dementia without a diagnosis of psychosis in the context of national guidance and drug safety warnings: longitudinal study in UK general practice. Drug Saf. 2017;40(8):679–692. PubMed CrossRef
- Mori E, Ikeda M, Kosaka K; Donepezil-DLB Study Investigators. Donepezil for dementia with Lewy bodies: a randomized, placebo-controlled trial. Ann Neurol. 2012;72(1):41–52. PubMed CrossRef
- Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543–546. PubMed CrossRef
- Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. PubMed CrossRef
- Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48(suppl 6):S10–S16. PubMed CrossRef
- de Medeiros K, Robert P, Gauthier S, et al. The Neuropsychiatric Inventory-Clinician rating scale (NPI-C): reliability and validity of a revised assessment of neuropsychiatric symptoms in dementia. Int Psychogeriatr. 2010;22(6):984–994. PubMed CrossRef
- Cruz MP. Pimavanserin (Nuplazid): a treatment for hallucinations and delusions associated with Parkinson’s disease. P&T. 2017;42(6):368–371. PubMed
- Zhang H, Wang L, Fan Y, et al. Atypical antipsychotics for Parkinson’s disease psychosis: a systematic review and meta-analysis. Neuropsychiatr Dis Treat. 2019;15:2137–2149. PubMed CrossRef
- Yunusa I, El Helou ML, Alsahali S. Pimavanserin: a novel antipsychotic with potentials to address an unmet need of older adults with dementia-related psychosis. Front Pharmacol. 2020;11:87. PubMed CrossRef
- Tariot P, Foff EP, Cummings JL, et al. HARMONY study: pimavanserin significantly prolongs time to relapse of dementia-related psychosis. Innov Aging. 2020;4(suppl 1):163–164. PubMed CrossRef
- Ballard C, Youakim JM, Coate B, et al. Pimavanserin in Alzheimer’s disease psychosis: efficacy in patients with more pronounced psychotic symptoms. J Prev Alzheimers Dis. 2019;6(1):27–33. PubMed. 10.14283/jpad.2018.30
- Ballard C, Lana MM, Theodoulou M, et al; Investigators DART AD. A randomised, blinded, placebo-controlled trial in dementia patients continuing or stopping neuroleptics (the DART-AD trial). PLoS Med. 2008;5(4):e76. PubMed CrossRef
- Parkinson caused Psychosis and Personality Transformation. reddit. https://www.reddit.com/r/Parkinsons/comments/lkibxb/parkinson_caused_psychosis_and_personality/. Published March 2021. Accessed April 14, 2021.
- de Oliveira AM, Radanovic M, de Mello PCH, et al. Nonpharmacological interventions to reduce behavioral and psychological symptoms of dementia: a systematic review. BioMed Res Int. 2015;2015:218980. PubMed
- Arai A, Khaltar A, Ozaki T, Katsumata Y. Influence of social interaction on behavioral and psychological symptoms of dementia over 1 year among long-term care facility residents. Geriatr Nur (Lond). Published online October 7, 2020.
- Leonpacher AK, Peters ME, Drye LT, et al; CitAD Research Group. Effects of citalopram on neuropsychiatric symptoms in Alzheimer’s dementia: evidence from the CitAD Study. Am J Psychiatry. 2016;173(5):473–480. PubMed CrossRef
- Porsteinsson AP, Drye LT, Pollock BG, et al; CitAD Research Group. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682–691. PubMed CrossRef
- Nirogi R, Shinde A, Jayarajan P, et al. Potential benefits of masupirdine (SUVN-502) on behavioral and psychological symptoms in patients with moderate Alzheimer’s disease (5090). Neurology. 2020;94(15 suppl). https://n.neurology.org/content/94/15_Supplement/5090. Accessed January 22, 2021.
- McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031–2036. PubMed CrossRef
- Ikeda M, Mori E, Matsuo K, et al. Donepezil for dementia with Lewy bodies: a randomized, placebo-controlled, confirmatory phase III trial. Alzheimers Res Ther. 2015;7(1):4. PubMed CrossRef
- Matsunaga S, Kishi T, Yasue I, et al. Cholinesterase inhibitors for lewy body disorders: a meta-analysis. Int J Neuropsychopharmacol. 2015;19(2):pyv086. PubMed CrossRef
- Kurlan R, Cummings J, Raman R, et al; Alzheimer’s Disease Cooperative Study Group. Quetiapine for agitation or psychosis in patients with dementia and parkinsonism. Neurology. 2007;68(17):1356–1363. PubMed CrossRef
- Chong FP, Ng KY, Koh RY, et al. Tau proteins and tauopathies in Alzheimer’s disease. Cell Mol Neurobiol. 2018;38(5):965–980. PubMed CrossRef
CME Background Information
Overview
Dementia-related psychosis (DRP) occurs in all forms of dementia and at all stages. What tactics should be used when talking with patients and their care partners about DRP? How is it best treated?
Learning Objective
Provide evidence-based therapies to manage psychosis in patients with dementia
Target Audience
Neurologists, Geriatric Psychiatrists, and Nurse Practitioners and Physician Assistants who specialize in Neurology and Psychiatry
Support Statement
Supported by an educational grant from ACADIA Pharmaceuticals Inc.
Learning Objective
After completing this educational activity, you should be able to:
- Provide evidence-based therapies to manage psychosis in patients with dementia
Release, Review, and Expiration Dates
This brief report activity was published in June 2021 and is eligible for AMA PRA Category 1 Credit™ through August 31, 2023. The latest review of this material was May 2021.
Statement of Need and Purpose
Hallucinations and delusions are key behaviors contributing to behavioral crises in patients with dementia, and a lack of consistency in assessment of behaviors has been found. Clinicians may lack awareness of the incidence of psychotic symptoms in different forms of dementia, as dementia pathology has been incorrectly diagnosed on the basis of the presence or absence of psychosis. Some patients may not be able to describe their psychotic symptoms, requiring caregiver input to aid clinicians in recognition. Rating scales can be implemented to aid identification. Clinicians need education about assessing patients with dementia for hallucinations and delusions and about the incidence of psychotic symptoms in different forms of dementia. In addition, many patients with dementia-related psychosis (DRP) receive antipsychotics and are on treatment for over a year, although guidelines recommend tapering after 4 months. Clinicians need education to implement guideline-concordant care that is tailored to the individual patient, incorporating current information on the risks and benefits of nonpharmacologic and pharmacologic interventions for DRP.
Disclosure of Off-Label Usage
Dr Ballard has determined that, to the best of his knowledge, no therapies are approved by the US Food and Drug Administration for the treatment of dementia-related psychosis.
Review Process
The faculty members agreed to provide a balanced and evidence-based presentation and discussed the topics and CME objectives during the planning sessions. The faculty’s submitted content was validated by CME Institute staff, and the activity was evaluated for accuracy, use of evidence, and fair balance by the Chair and a peer reviewer who is without conflict of interest.
Acknowledgment
This Academic Highlights section of The Journal of Clinical Psychiatry presents the highlights of the teleconference series “Dementia-Related Psychosis: Recognition and Treatment,” which was held in May and June 2020. This report was prepared and independently developed by the CME Institute of Physicians Postgraduate Press, Inc., and was supported by an educational grant from ACADIA Pharmaceuticals Inc.
The opinions expressed herein are those of the faculty and do not necessarily reflect the opinions of the CME provider and publisher or the commercial supporter.
Support Statement
Supported by an educational grant from ACADIA Pharmaceuticals Inc.
Learning Objective
After completing this educational activity, you should be able to:
- Provide evidence-based therapies to manage psychosis in patients with dementia
Release, Review, and Expiration Dates
This brief report activity was published in June 2021 and is eligible for AMA PRA Category 1 Credit™ through August 31, 2023. The latest review of this material was May 2021.
Statement of Need and Purpose
Hallucinations and delusions are key behaviors contributing to behavioral crises in patients with dementia, and a lack of consistency in assessment of behaviors has been found. Clinicians may lack awareness of the incidence of psychotic symptoms in different forms of dementia, as dementia pathology has been incorrectly diagnosed on the basis of the presence or absence of psychosis. Some patients may not be able to describe their psychotic symptoms, requiring caregiver input to aid clinicians in recognition. Rating scales can be implemented to aid identification. Clinicians need education about assessing patients with dementia for hallucinations and delusions and about the incidence of psychotic symptoms in different forms of dementia. In addition, many patients with dementia-related psychosis (DRP) receive antipsychotics and are on treatment for over a year, although guidelines recommend tapering after 4 months. Clinicians need education to implement guideline-concordant care that is tailored to the individual patient, incorporating current information on the risks and benefits of nonpharmacologic and pharmacologic interventions for DRP.
Disclosure of Off-Label Usage
Dr Ballard has determined that, to the best of his knowledge, no therapies are approved by the US Food and Drug Administration for the treatment of dementia-related psychosis.
Review Process
The faculty members agreed to provide a balanced and evidence-based presentation and discussed the topics and CME objectives during the planning sessions. The faculty’s submitted content was validated by CME Institute staff, and the activity was evaluated for accuracy, use of evidence, and fair balance by the Chair and a peer reviewer who is without conflict of interest.
Acknowledgment
This Academic Highlights section of The Journal of Clinical Psychiatry presents the highlights of the teleconference series “Dementia-Related Psychosis: Recognition and Treatment,” which was held in May and June 2020. This report was prepared and independently developed by the CME Institute of Physicians Postgraduate Press, Inc., and was supported by an educational grant from ACADIA Pharmaceuticals Inc.
The opinions expressed herein are those of the faculty and do not necessarily reflect the opinions of the CME provider and publisher or the commercial supporter.
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this enduring material for a maximum of 1.00 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Nurses Credentialing Center (ANCC) and the American Academy of Physician Assistants (AAPA) accept certificates of participation for educational activities certified for AMA PRA Category 1 Credit™ from organizations accredited by the ACCME.






