ABSTRACT
Objective: Second-generation antipsychotics (SGAs) are prescribed for a wide range of indications in women of reproductive age. The National Pregnancy Registry for Atypical Antipsychotics (NPRAA) was established to determine the risk of major malformations among infants exposed to these medications during the first trimester relative to a comparison group of unexposed infants of mothers with histories of psychiatric morbidity.
Methods: Women, aged 18–45 years, with histories of psychiatric illness were prospectively followed through pregnancy and during the postpartum period. Pediatric and maternal medical records were obtained and screened for evidence of major malformations. Potential cases were adjudicated by a dysmorphologist who was blinded to drug exposure. Recruitment to the Registry, which is based at the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH), includes nationwide provider referral, self-referral, and advertisement through the MGH Center for Women’s Mental Health website.
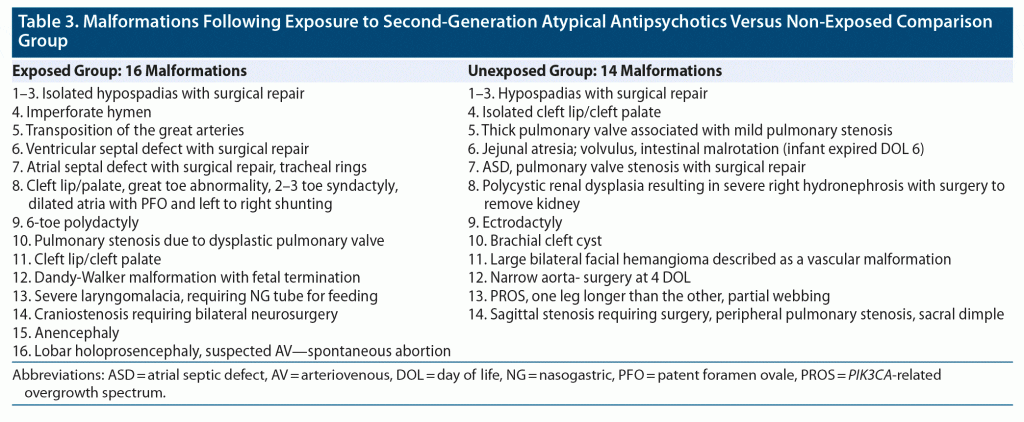
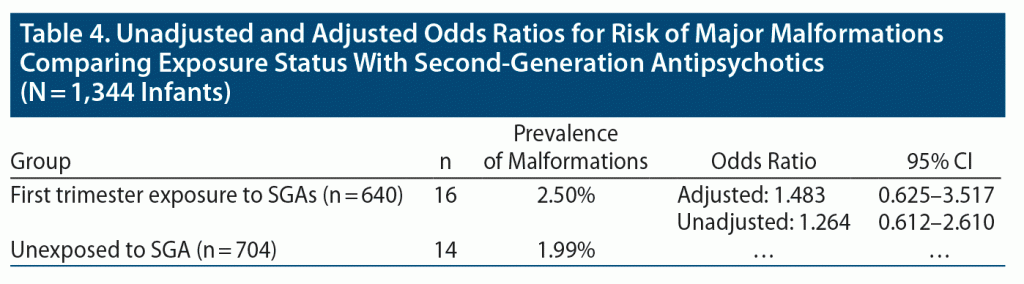
Results: As of April 9, 2020, 1,906 women had enrolled, including 889 in the exposure group and 1,017 controls. A total of 1,311 women completed the study and were eligible for inclusion in the analysis. Medical records were obtained for 81.3% of participants. Among 640 live births in the exposure group, 16 (2.50%) had confirmed major malformations reported, and among 704 live births in the control group, 14 (1.99%) had confirmed major malformations reported. The estimated odds ratio for major malformations comparing exposed and unexposed infants was 1.48 (95% CI, 0.625–3.517).
Conclusions: Data from the Registry assessing SGAs as a class indicate that they are unlikely to have a major teratogenic effect. These findings provide pertinent information for women and their health care providers regarding decisions about atypical antipsychotic use during pregnancy.
Trial Registration: ClinicalTrials.gov identifier: NCT01246765
See commentary by Burt and RasminskyBurt and Rasminsky
J Clin Psychiatry 2021;82(4):20m13745
To cite: Viguera AC, Freeman MP, Góez-Mogollón L, et al. Reproductive safety of second-generation antipsychotics: updated data from the Massachusetts General Hospital National Pregnancy Registry for Atypical Antipsychotics. J Clin Psychiatry. 2021;82(4):20m13745.
To share: https://doi.org/10.4088/JCP.20m13745
© Copyright 2021 Physicians Postgraduate Press, Inc.
aMassachusetts General Hospital, Ammon-Pinizzotto Center for Women’s Mental Health, Boston, Massachusetts
bHarvard Medical School, Boston, Massachusetts
cCleveland Clinic, Cleveland Clinic Neurological Institute, Cleveland, Ohio
dHarvard T.H. Chan School of Public Health, Boston, Massachusetts
eMount Sinai Hospital, Prenatal Diagnosis and Medical Genetics Program, Toronto, Ontario, Canada
*Corresponding author: Adele C. Viguera, MD, Cleveland Clinic, Cleveland Clinic Neurological Institute, 9500 Euclid Ave, Desk P 58, Cleveland, OH 44195 ([email protected]).
Notice of correction 9/15/2021: Table 1 has been corrected to reflect that the fifth psychotropic category listed below “First Trimester Psychotropic Medication Use” is atypical antidepressants.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation. This activity is free.
CME Objective
After studying this article, you should be able to:
- Use current evidence to weigh risks of atypical antipsychotic prescription in pregnant women
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Nurses Credentialing Center (ANCC) and the American Academy of Physician Assistants (AAPA) accept certificates of participation for educational activities certified for AMA PRA Category 1 Credit™ from organizations accredited by the ACCME.
Release, Expiration, and Review Dates
This educational activity was published in August 2021 and is eligible for AMA PRA Category 1 Credit™ through August 31, 2023. The latest review of this material was July 2021.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Marlene P. Freeman, MD, Editor in Chief, has received research funding from JayMac and Sage; has been a member of the advisory boards for Otsuka, Alkermes, and Sunovion; and has been a member of the Independent Data Safety and Monitoring Committee for Janssen. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
Over the past decade, national and international pregnancy registries have emerged as an effective and efficient means of collecting robust reproductive safety data on a variety of medications.1–4 While these registries may vary with respect to methods of data collection and definitions of major malformations, they are uniformly prospective in study design and allow for the assessment of potential confounding variables and the selection of controls (ie, either healthy or subjects with similar illnesses to those exposed), which are critical to the interpretation of reproductive safety outcomes.
Since atypical antipsychotics are increasingly being used by women of reproductive age as primary or adjunctive therapy across a wide range of psychiatric disorders (including bipolar disorder, schizophrenia, unipolar depression, and anxiety disorders), the need for accurate reproductive safety information across these medications is increasing.5–7 With the advent of the Pregnancy and Lactation Labeling Rule (PLLR) of the US Food and Drug Administration (FDA), a more descriptive summary of outcomes of exposure to medications during pregnancy and lactation is now required to be listed on drug labels instead of the previously used category labeling system of A, B, C, and X.8 Manufacturers are also required to state whether a pregnancy registry exists for their particular medication (https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/Labeling/ucm093307.htm). Therefore, the National Pregnancy Registry for Atypical Antipsychotics (NPRAA) is a timely and ideal mechanism for collecting important reproductive safety data on second-generation antipsychotics (SGAs). Modeled after the North American Antiepileptic Drug Registry and based at Massachusetts General Hospital, the National Pregnancy Registry for Atypical Antipsychotics), was established in 2008.9 The Registry is the first hospital-based pregnancy registry in North America to systematically and prospectively examine the risk of major malformations among infants exposed in utero to SGAs. In addition, data for other important secondary outcomes including neonatal, obstetrical, and neurobehavioral outcomes are also collected.
The objective of this report is to present updated results from the NPRAA since the last publication describing reproductive safety data for this class of psychotropics.10 Due to the increase in the Registry’s sample size over time, the absolute and relative risk of major malformations observed in the study population are now more precise. Given the removal of the pregnancy category labeling system and the renewed emphasis from regulatory agencies on data collected from well-designed pregnancy registries, these current data are particularly timely.
METHODS
Methods and procedures used by the NPRAA are described in detail elsewhere.9,10 Briefly, this ongoing prospective cohort study follows pregnant women with psychiatric illness, aged 18–45 years, who are exposed and unexposed to SGAs during pregnancy. Second-generation antipsychotics in the Registry are any medication and formulation in the class that is approved by the FDA, whether brand or generic versions, if available. These medications include aripiprazole (Abilify, Abilify Maintena), aripiprazole lauroxil (Aristada), asenapine (Saphris), clozapine (Clozaril), iloperidone (Fanapt), lurasidone (Latuda), olanzapine (Zyprexa), olanzapine pamoate (Zyprexa Relprevv), paliperidone (Invega), quetiapine (Seroquel), risperidone (Risperdal, Risperdal Consta), and ziprasidone (Geodon). Additional SGAs will be included in the Registry as these medications become available.
Since our last publication in 2016, the following medications have been incorporated in the Registry: brexpiprazole (Rexulti), cariprazine (Vraylar), and paliperidone palmitate (Invega Trinza, Invega Sustenna). Participants are recruited primarily through health care provider referrals, consultations at the Massachusetts General Hospital Center for Women’s Mental Health, and the Center’s website (http://womensmentalhealth.org).10 The exposed group consists of women who have used at least one SGA during the first trimester of pregnancy. The comparison group consists of women with a history of psychiatric illness who are being treated with a variety of psychotropic medications other than SGAs. The NPRAA is registered with clinicaltrials.gov (NCT01246765).
Participants are interviewed over the phone at 3 time points across pregnancy: at enrollment, at 7 months, and at 3 months postpartum. The initial interview ascertains information regarding demographic characteristics, medication use and dosage changes (if any, before and during pregnancy), social habits (ie, smoking, alcohol consumption, and illicit drug use), medical and psychiatric history, and family history of birth defects. The 7-month interview collects data on changes in medication or dosage (if any) and intervening medical problems across pregnancy. During the final, postpartum interview, information is gathered from maternal reports regarding pharmacotherapy, labor, delivery, and neonatal health outcomes.
Outcome data are also obtained through systematic review of obstetric, labor and delivery, and pediatric medical records. Information regarding primary and secondary outcomes is abstracted from medical records by a trained research coordinator and a senior study physician-investigator (A.C.V.). If a major malformation is noted, pediatric medical records are redacted and sent to a trained dysmorphologist (D.C.) blinded to medication exposure to confirm presence of a malformation. Final adjudication of the records is ultimately the responsibility of the dysmorphologist.
All participants in the Registry provide verbal informed consent, and all study procedures were approved by the Massachusetts General Hospital Institutional Review Board.
Release of findings and other major policy decisions are governed by a Scientific Advisory Board made up of experts in the fields of teratology, epidemiology, pediatrics, and pharmacology.9 The NPRAA is financially supported by multiple manufacturers of SGAs who voluntarily agree to support the research initiative with a fixed proportion of Registry operating costs. This mechanism of support for a pregnancy registry is endorsed by the FDA in respective guidance documents.8 Since the Registry’s inception, manufacturers have chosen to either participate or decline, and several companies have renewed participation while others have deferred. A full listing of current and past sponsors is included on the Registry’s website https://womensmentalhealth.org/research/pregnancyregistry/). Study sponsors have no role in research design, data collection, analysis, interpretation, or manuscript preparation and review. All medications in the class are studied regardless of manufacturer support per the scientific mission of the NPRAA.
The primary outcome is the presence of a major malformation identified within 6 months of birth. A major malformation is defined as a structural abnormality with surgical, clinical, or cosmetic importance.11 Clear chromosomal and single gene abnormalities were excluded. Other exclusions included minor malformations, deformations, birthmarks, physiologic features due to prematurity, and any findings by prenatal sonography or at surgery (or autopsy) that were not identified by an examining pediatrician (or other medical professional). Secondary outcomes include neonatal, obstetrical, and neurobehavioral outcomes, which are not included in this analysis.
Statistical Analyses
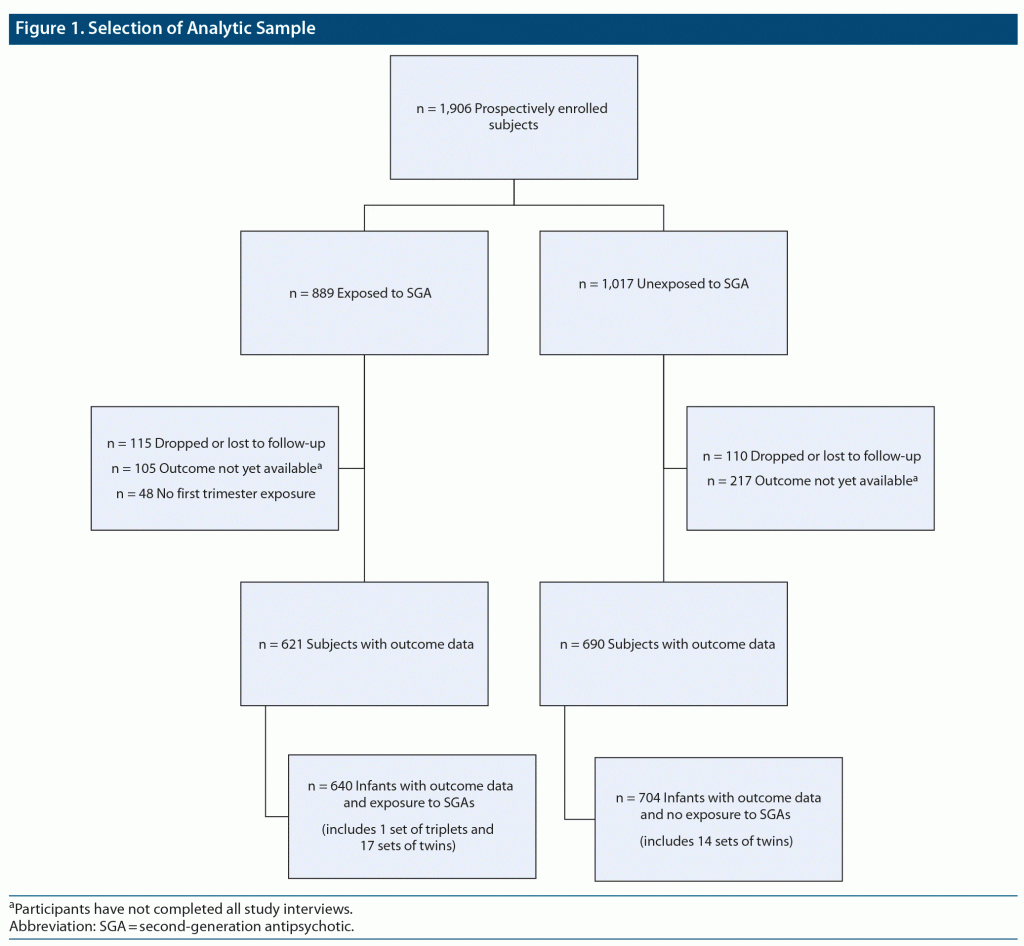
The primary exposure in this study was SGA use during the first trimester of pregnancy (< 13 weeks’ gestational age). This exposure was operationalized into a binary variable: use of an atypical during first trimester (exposed) or no use during the entire pregnancy (unexposed). Women who used SGAs only during the second and/or third trimester of pregnancy were excluded from the analysis (Figure 1).
A number of binary and continuous predictors were also examined. All predictors were provided by maternal report and were measured before or concurrently with SGA use and before the outcome of interest occurred (Table 1). Chronicity of illness was calculated as the difference between the participant’s current age and age at onset of first symptoms divided by the participant’s current age.
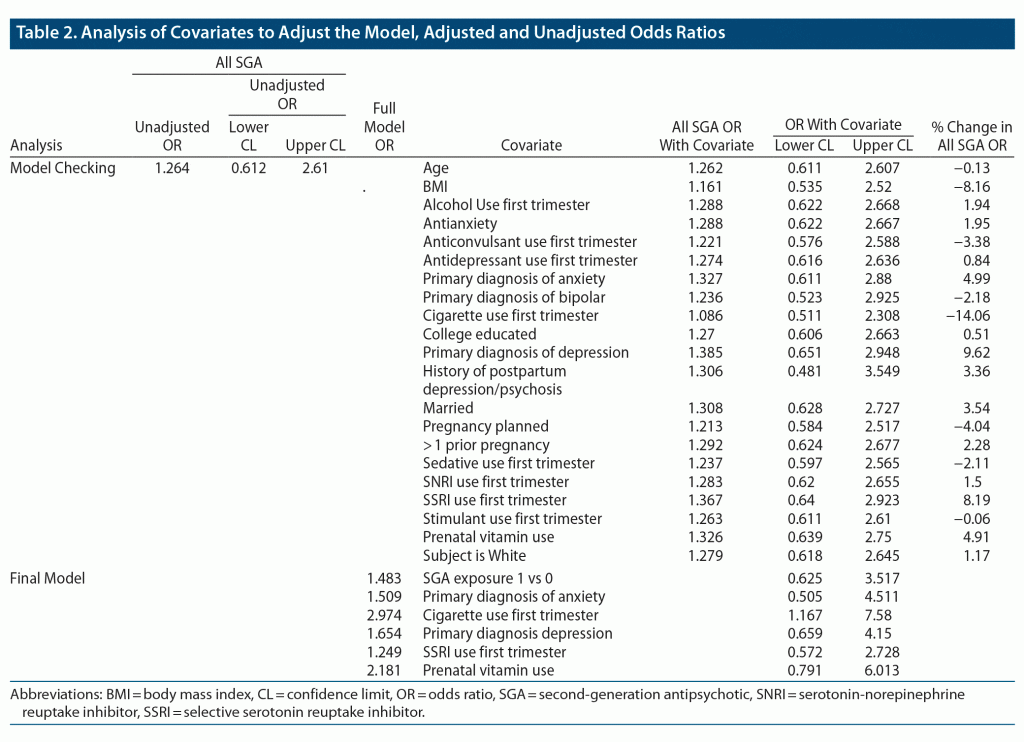
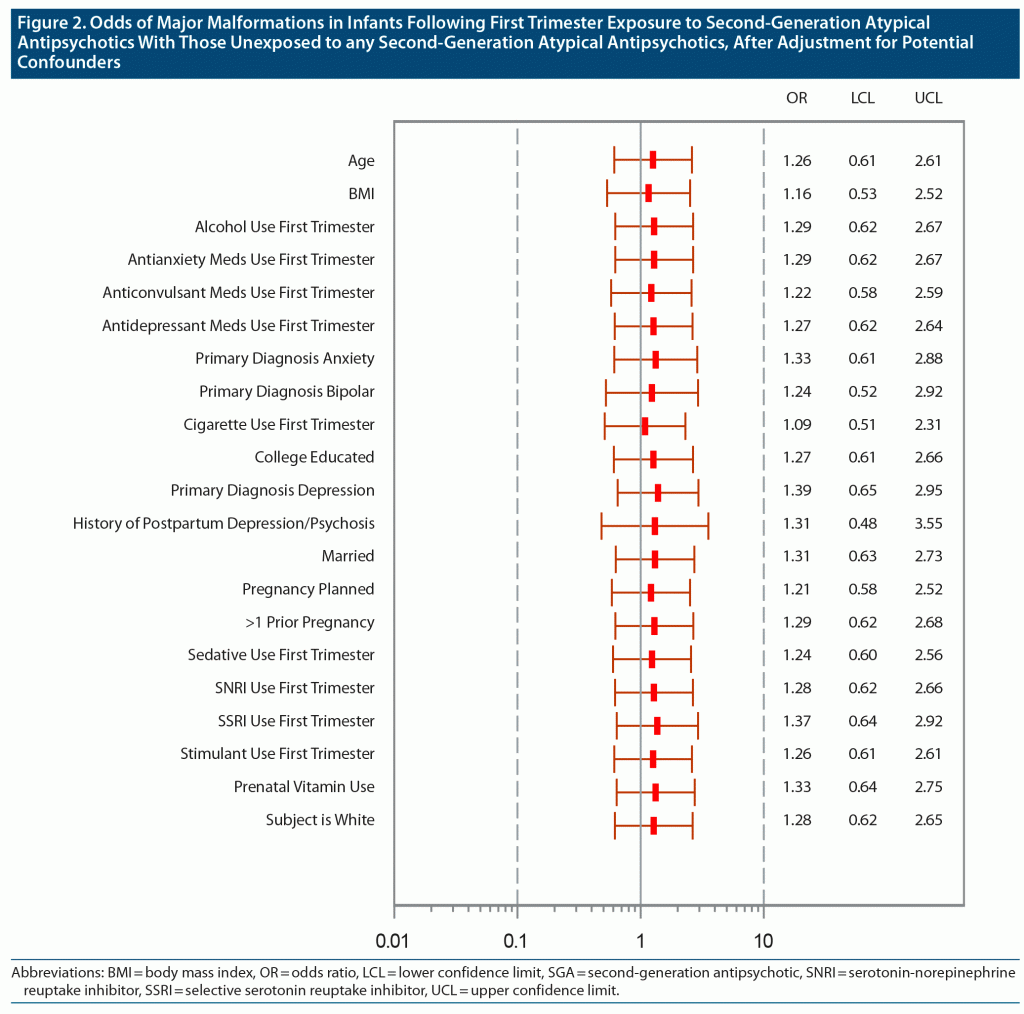
We compared the unadjusted odds of a major malformation among infants exposed and unexposed to atypical antipsychotics. We also examined possible confounding by a number of factors, listed in Table 2. Because the majority of women included in the comparison group also had psychiatric conditions and used psychotropic medications, confounding by factors associated with both psychiatric illness and risk of malformations was reduced. Each potential confounding factor was added individually to the crude logistic regression model, and the change in the odds ratio was examined to assess the magnitude and direction of the bias. When the confounders changed the odds ratio of the comparison by 5%, they were included in the final multivariate logistic regression model (Figure 2). Confounders included cigarette use in the first trimester, a primary diagnosis of depression or anxiety, selective serotonin reuptake inhibitor (SSRI) use in the first trimester, and prenatal vitamin use. Each odds ratio estimate for the effect of atypical exposure on malformations after adjustment is displayed in Table 2.
All analyses were completed using SAS, version 9.4 (2013; SAS Institute; Cary, North Carolina).
RESULTS
Between the NPRAA’s inception in November 2008 and data analysis cutoff point of April 9, 2020, which was determined by our Scientific Advisory Board, a total of 1,906 women had been enrolled. For this analysis, 1,311 subjects were eligible based on completion of the postpartum interview at time of data extraction; 621 had first trimester exposure to an atypical antipsychotic, and 690 had no exposure to an atypical antipsychotic during pregnancy. The remaining women in the sample were ineligible because they either had not completed their postpartum interview (n = 322, 16.89%) or else dropped out of the study, were lost to follow-up, or had a spontaneous or therapeutic abortion without a known major malformation (n = 225, 11.80%). An additional 48 women were not included because they were exposed to an atypical antipsychotic during their second or third trimester, but not during their first trimester (Figure 1). Medical records were obtained for 81.3% of participants.
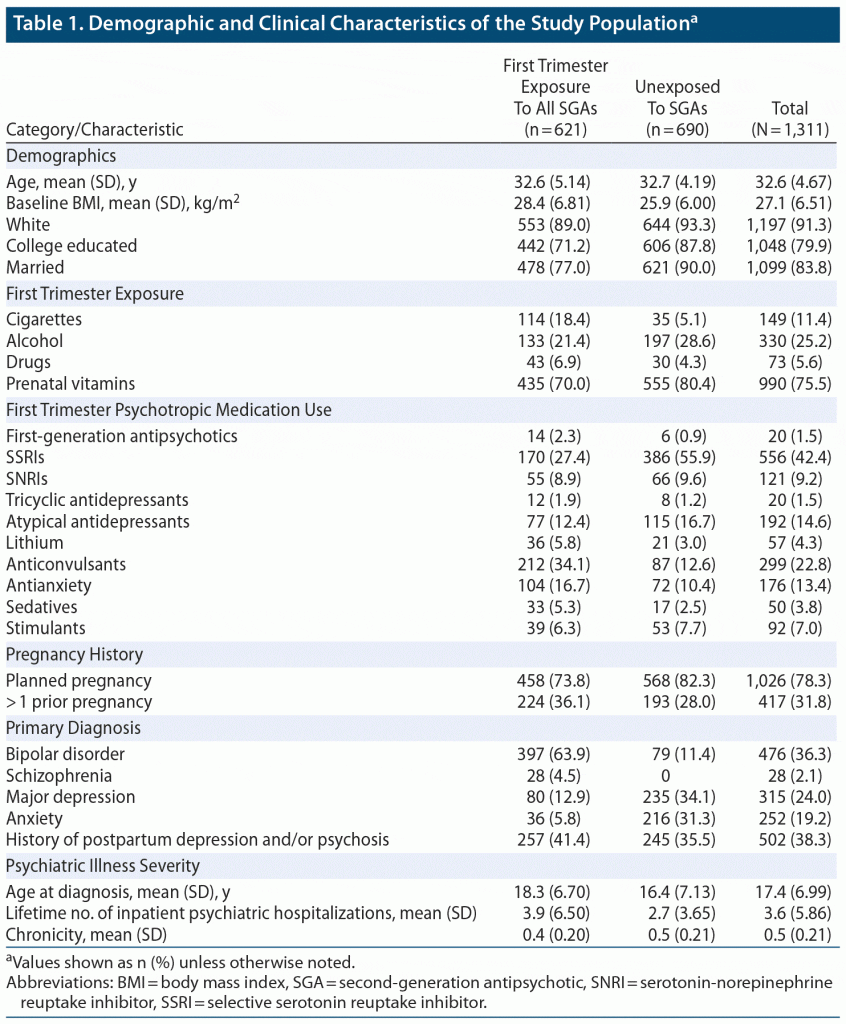
The participants’ demographic and clinical characteristics are summarized in Table 1. There were notable differences between the participants in the exposed and unexposed groups. Women exposed to an atypical antipsychotic were less likely to have a college education (71.2% vs 87.8%) and less likely to be married (77% vs 90%). Exposed women also had a higher rate of first trimester cigarette usage (18.4% vs 5.1%) than unexposed women.
Regarding psychiatric history, exposed participants were found to have a higher age at initial onset of their primary psychiatric diagnosis and lower proportion of lifetime illness than those unexposed to an atypical antipsychotic. Women in the exposed group were more likely to have a diagnosis of bipolar disorder (63.9% vs 11.4%) than those in the unexposed group; those in the unexposed group were more likely to have a primary diagnosis of major depression (34.1% vs 12.9%) or anxiety (31.3% vs 5.8%).
In order of prevalence, the most frequently used atypical antipsychotics in the exposed group were quetiapine, aripiprazole, and lurasidone. Separate analysis of major malformations among participants taking quetiapine has been discussed in detail elsewhere.12
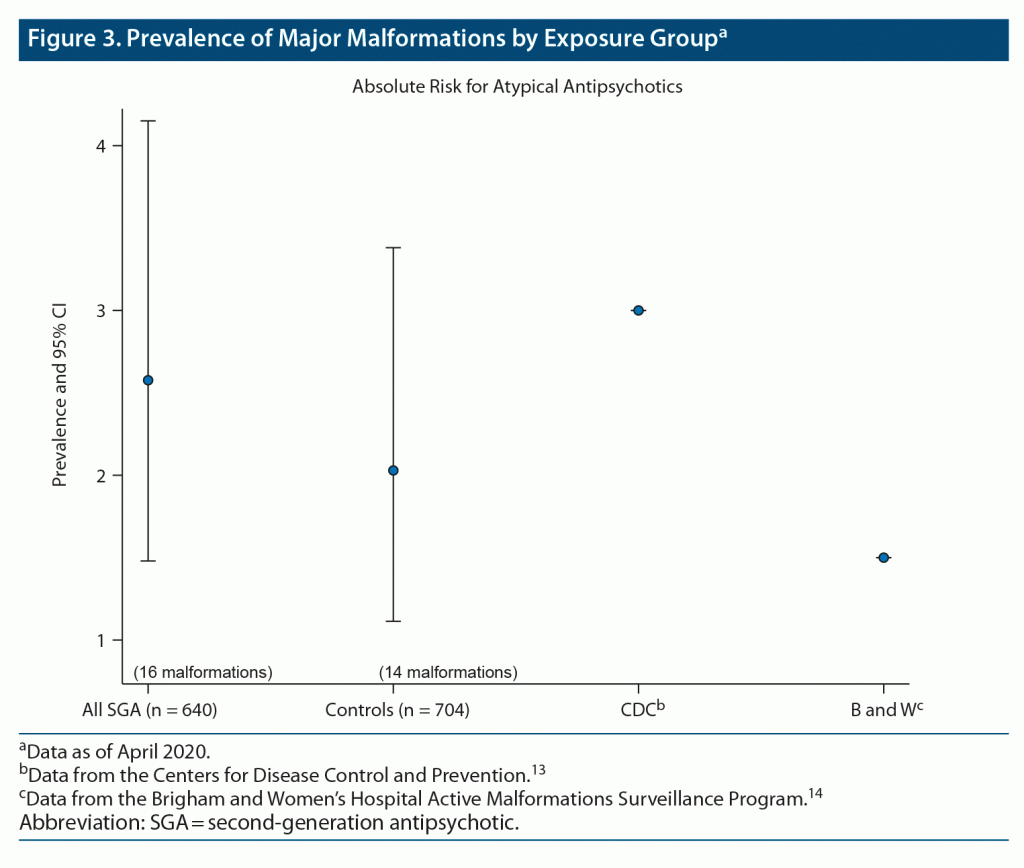
Among the 640 SGA-exposed infants, including 17 twin pregnancies and 1 triplet pregnancy, evaluated for this analysis, 16 major malformations were identified. In the comparison group of 704 unexposed infants, including 14 twin pregnancies, 14 major malformations were identified. Further description of the noted malformations is seen in Table 3. The prevalence in the exposed group was estimated to be 2.50% as compared to 1.99% in the unexposed group (Table 4). Figure 3 depicts the prevalence of major malformations by exposure group compared to Centers for Disease Control (CDC) surveillance data13 as well as data from the Brigham and Women’s Hospital Active Malformations Surveillance Program.14
DISCUSSION
Release of reproductive safety data from the National Pregnancy Registry for Atypical Antipsychotics is governed by our Scientific Advisory Board.9 These data present a more recent update from our last report regarding risk estimates for major malformations following first trimester exposure to atypical antipsychotics as a class.10 Previously, we published aggregate data on 303 subjects and reported an odds ratio for major malformations comparing exposed to unexposed infants of 1.25 (95% CI, 0.13–12.19).10 Based on our current data, the estimated odds ratio for major malformations was 1.48 (95% CI, 0.625–3.517). Therefore, it is reassuring that our odds ratio estimate appears likely to be less than that of other major teratogens, although more modest effects cannot be ruled out either.15,16
Our current and past findings are consistent with some, but not all studies.17–20 Two large epidemiologic studies19,20 reported a 1.5 to 2-fold increase in the risk of major malformations. In addition, they also found a significant increase in cardiac defects, mostly atrial and ventricular septal defects, among SGA-exposed infants. While cardiac septal defects are among the most common congenital malformations in the general population, it is also very likely that detection bias may play a major role in the preponderance of cardiac defects.21 Pregnant women on treatment with atypical antipsychotic medications are more likely to be offered fetal echocardiography and further surveillance as compared to women not taking such medications. Such a detection bias has been previously noted with antidepressants such as SSRIs.22 In one study,22 women using antidepressants during pregnancy had a 30% higher rate of ultrasound diagnostics, and exposed infants were twice as likely to receive an echocardiogram compared to unexposed infants. Interestingly, we did not observe a significant increase in cardiac defects in this analysis or in our earlier findings.10
It is also reassuring that our findings are consistent with one of the largest studies to date18 involving a nationwide sample of over one million women enrolled in Medicaid. In that study, the estimated risk ratio, after adjusting for psychiatric conditions, was 1.05 (95% CI, 0.96–1.16) among infants exposed versus not exposed to SGAs, demonstrating no increased risk for major malformations for the medication as a class.18
Furthermore, a hallmark of a teratogen is that it tends to cause a specific type or pattern of malformations.8,11 We found no preponderance of one single type of major malformation or specific pattern of malformations among the exposed and unexposed groups. In addition, it is important to emphasize that the most clinically relevant measure in communicating teratogenic risk is the absolute risk. We report an absolute risk of 2.5%, which is consistent with the CDC’s national rate of major malformations in the general population.13 However, the absolute risk for a major malformation in our unexposed group was 1.99%, which was lower than expected and may be due to random error or higher rates of healthy behavior in women who choose to enroll in a pregnancy registry.
In previous publications on the topic of SGA exposure and pregnancy, potential confounders were variably controlled for in the analyses. These potential confounders relate to both atypical antipsychotic use and other factors known to impact risk for malformations and include diagnosis and severity of illness, having a planned pregnancy, maternal age, health and lifestyle indicators such as body mass index, and first trimester use of other psychotherapeutic drugs, prenatal vitamins, alcohol, and/or cigarettes.23–27 We observed that some confounders, such as cigarette use, attenuated the odds ratio, while others, such as a diagnosis of depression or anxiety and SSRI use in the first trimester, increased the unadjusted odds.
This study has a number of key strengths. The precise determination of the primary outcome, major malformations, is more rigorous than in most studies. Because these outcome data are obtained prospectively, the Registry allows for evaluation of the relationship between atypical antipsychotic use during the first trimester of pregnancy and major malformations while minimizing the potential for recall bias. The rigorous record review of infant outcomes followed by adjudication of our malformations by a trained dysmorphologist blinded to medication exposure is another major strength of this study design. Moreover, the utilization of phone interviews with participants along with medical record review allows the research team to obtain sound information about the presence of the primary outcome, but also facilitates gathering more in-depth contextual information on lifestyle and demographic factors as well as detailed weekly medication use patterns, information that typically unavailable from the medical record alone. This method also allows for an accurate picture of pharmacotherapy throughout pregnancy and information on factors that could confound the relationship between first trimester atypical antipsychotic exposure and major malformations. Another major strength of this study is the inclusion of a comparable reference group of women with psychiatric disorders using the same methods for recruitment, enrollment, and the ascertainment of outcomes as used for women in the exposure group.2 Instead of using a reference group of healthy controls, our comparison group of women with histories of psychiatric illness helps to limit confounding by indication that is so often a problem in other studies.17–20 Typically, the confounding effect of the severity of underlying clinical indication (ie, psychiatric illness) and its associated behaviors are unaccounted for in large claims databases or national birth registries.
With respect to limitations, the extent to which these results are generalizable to the larger population of women taking atypical antipsychotics is unknown. For instance, the demographics of our study population are overwhelmingly White, married, and well-educated women. Enrollment of women in pregnancy registries is also voluntary and tends to self-select for women who may be higher functioning, more motivated, and better informed perhaps than nonparticipants. Therefore, interpretation of findings based on women who participate in registries may differ fundamentally from interpretation of those of nonparticipants, thus modifying the effect of the drug.2 For example, the majority of the women in the exposure group have a bipolar disorder diagnosis and few have a diagnosis of schizophrenia, resulting in an underrepresentation of women with schizophrenia, who are often treated with atypical antipsychotic medications. Additionally, these results do not provide risk assessments for each individual medication in this class.
Another limitation to note is the lack of systematic genetic testing of infants presenting with major malformations following prenatal exposure to different medications.28 With the recent advent of genome sequencing and chromosome microarray analysis and whole exome/genome sequencing, some major malformations were found to be the result of microscopic and submicroscopic chromosome abnormalities as well as single gene disorders. Such malformations and those due to clear chromosomal abnormalities were excluded from the analysis.28 However, since not all infants in the study sample received genetic testing, there is the possibility that cases caused by an undiagnosed genetic mechanism rather than a drug exposure were included. If this were true, our current estimates would be artificially inflated compared to the true odds ratio. Thus, in the absence of systematic genetic testing of all infants (which would be cost-prohibitive), it is reassuring that cases due to an undiagnosed genetic cause would likely be equally distributed among the exposed and unexposed groups.
In conclusion, our results suggest that the use of atypical antipsychotics during the first trimester does not substantially increase the risk of major malformations. While the heterogeneity of studies examining reproductive safety of SGAs makes it difficult to compare the risk for malformations across studies, the accumulated evidence, thus far, suggests a low absolute risk and argues against a major teratogenic effect associated with SGAs. A major clinical implication of these findings is that for women with major mood and/or psychotic disorders, treatment with an atypical antipsychotic during pregnancy may be the most prudent clinical decision, much as continued treatment is recommended for pregnant women with other serious and chronic medical conditions, such as epilepsy.29 Given the flexibility and richness of the Registry’s infrastructure, future efforts of the NPRAA include examination of obstetrical and neonatal outcomes as well as the long-term neurobehavioral effects of in utero exposure to atypical antipsychotics in children. Furthermore, enrollment into the Registry is ongoing. Therefore, larger samples sizes will further narrow the confidence interval around the risk estimates and allow for adjustment of likely sources of confounding, thus providing critical data to help patients and clinicians weigh the benefits and risks of atypical antipsychotic use during pregnancy.
Submitted: November 4, 2020; accepted March 9, 2021.
Published online: August 3, 2021.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, no investigational information about pharmaceutical agents or device therapies that is outside US Food and Drug Administration–approved labeling has been presented in this article.
Financial disclosure: Dr Viguera: Research Support: National Pregnancy Registry for Atypical Antipsychotics (NRPAA): Alkermes Biopharmaceuticals; Aurobindo Pharma; Janssen Pharmaceutica; Otsuka Pharmaceuticals; Sunovion Pharmaceuticals, Inc.; Teva Pharmaceuticals; SAGE Therapeutics, Inc. Other Research Support: None; Advisory/Consulting: Up-to-Date; Speaking/Honoraria: None; Royalty/patent, other income: None. Dr Freeman: NRPAA: Alkermes, Aurobindo, Janssen, Otsuka, Sunovion, Teva, SAGE Therapeutics. Other research support: JayMac Pharmaceuticals, SAGE Therapeutics. As an employee of MGH, Dr Freeman works with the MGH CTNI, which has had research funding from multiple pharmaceutical companies and National Institute of Mental Health. Advisory/Consulting: Advisory Boards: Eliem, Sage. Independent Data Safety and Monitoring Committee: Janssen (Johnson & Johnson), Novartis. Steering Committee for Educational Activities: Medscape. Educational activities: WebMD. Dr Hernández-Díaz: Consulting fees (e.g. advisory boards): Roche and UCB, and her institution received funding from Takeda for unrelated project; epidemiologist for the North American Antiepileptic Drugs pregnancy registry and advisor for the Antipsychotics Pregnancy Registry, which are funded by multiple companies. The Pharmacoepidemiology Program at the Harvard T.H. Chan School of Public Health is partially supported by training grants from Pfizer, UCB, Bayer, and Asisa. Dr Cohen: NRPAA: Alkermes, Aurobindo, Janssen, Otsuka, Sunovion, Teva, SAGEage Therapeutics. Other Research Support: Brain & Behavior Research Foundation; JayMac Pharmaceuticals; National Institute on Aging; National Institutes of Health; National Institute of Mental Health; SAGE Therapeutics; Takeda/Lundbeck. Advisory/Consulting: Alkermes (through MGH Clinical Trials Network Initiative); JDS Therapeutics; Praxis Precision Medicines (through MGH Clinical Trials Network Initiative). Mss Góez-Mogollón, Sosinsky, McElheny, Church, Young, Caplin, and Mr Chitayat have nothing to disclose.
Funding/support: Current Sponsors of the NPRAA: Alkermes, Inc. (2016–Present); Johnson & Johnson/Jannsen Pharmaceuticals, Inc (2019–present); Otsuka America Pharmaceutical, Inc (2008–Present); Sunovion Pharmaceuticals, Inc (2011–Present), SAGE Therapeutics (2019–Present); Teva Pharmaceuticals (2018–Present); Aurobindo Pharma (2020–Present). Past Sponsors of the NPRAA: Forest/Actavis Pharmaceuticals (2016–2018); AstraZeneca Pharmaceuticals (2009–2014, declined to sponsor: 2014–Present); Ortho-McNeil-Janssen Pharmaceuticals, Inc (2009–2014, declined to sponsor: 2015–Present); Pfizer Inc (2009–2011, declined to sponsor: 2012–Present).
Role of the sponsor: The sponsors of the NPRAA are pharmaceutical manufactures of medications of interest. In exchange for their support, sponsors receive safety reporting information and draft versions of manuscripts for comment at a minimum of 30 days before submission for comment.
Previous presentation: The current submission includes information published in Reproductive Safety of Second-Generation Antipsychotics: Current Data From the Massachusetts General Hospital National Pregnancy Registry for Atypical Antipsychotics.10 Information encompassed in this submission was presented in a poster titled The National Pregnancy Registry for Psychiatric Medications: Effects of Fetal Exposure to Atypical Antipsychotics on Risk for Major Malformations at the American Society of Clinical Psychopharmacology annual meeting (virtual) May 29–30, 2020.
Acknowledgments: The authors thank and recognize Peter Gaccione, MA, for his contributions to data management and for his statistical programing for the National Pregnancy Registry. Mr Gaccione has nothing to disclose.
Clinical Points
- Incomplete reproductive safety data for atypical antipsychotics prompted the establishment of the National Pregnancy Registry for Atypical Antipsychotics (NPRAA).
- Findings from the NPRAA suggest that use of atypical antipsychotics during the first trimester does not substantially increase the risk of major malformations.
- For pregnant women with major mood and/or psychotic disorders, treatment with an atypical antipsychotic may be the most prudent clinical decision when weighing overall risks and benefits, including untreated illness.
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Women’s Mental Health section. Please contact Marlene P. Freeman, MD, at [email protected].
References (29)

- Gerard E, Pack AM. Pregnancy registries: what do they mean to clinical practice? Curr Neurol Neurosci Rep. 2008;8(4):325–332. PubMed CrossRef
- Gliklich RE, Dreyer NA, Leavy MB. Registries for Evaluating Patient Outcomes: A User’s Guide. Rockville, MD: Agency for Healthcare Research and Quality (US); 2014.
- Tomson T, Battino D, Craig J, et al; ILAE Commission on Therapeutic Strategies. Pregnancy registries: differences, similarities, and possible harmonization. Epilepsia. 2010;51(5):909–915. PubMed CrossRef
- Holmes LB, Wyszynski DF, Lieberman E. The AED (antiepileptic drug) pregnancy registry: a 6-year experience. Arch Neurol. 2004;61(5):673–678. PubMed CrossRef
- Toh S, Li Q, Cheetham TC, et al. Prevalence and trends in the use of antipsychotic medications during pregnancy in the US, 2001–2007: a population-based study of 585,615 deliveries. Arch Women Ment Health. 2013;16(2):149–157. PubMed CrossRef
- Çamsarı U, Viguera AC, Ralston L, et al. Prevalence of atypical antipsychotic use in psychiatric outpatients: comparison of women of childbearing age with men. Arch Women Ment Health. 2014;17(6):583–586. PubMed CrossRef
- Alexander GC, Gallagher SA, Mascola A, et al. Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Pharmacoepidemiol Drug Saf. 2011;20(2):177–184. PubMed CrossRef
- Freeman MP, Farchione T, Yao L, et al. Psychiatric medications and reproductive safety: scientific and clinical perspectives pertaining to the US FDA Pregnancy and Lactation Labeling Rule. J Clin Psychiatry. 2018;79(4):18ah38120. PubMed CrossRef
- Cohen LS, Viguera AC, McInerney KA, et al. Establishment of the National Pregnancy Registry for Atypical Antipsychotics. J Clin Psychiatry. 2015;76(7):986–989. PubMed CrossRef
- Cohen LS, Viguera AC, McInerney KA, et al. Reproductive safety of second-generation antipsychotics: current data from the Massachusetts General Hospital National Pregnancy Registry for Atypical Antipsychotics. Am J Psychiatry. 2016;173(3):263–270. PubMed CrossRef
- Holmes LB. Need for inclusion and exclusion criteria for the structural abnormalities recorded in children born from exposed pregnancies. Teratology. 1999;59(1):1–2. PubMed CrossRef
- Cohen LS, Góez-Mogollón L, Sosinsky AZ, et al. Risk of major malformations in infants following first-trimester exposure to quetiapine. Am J Psychiatry. 2018;175(12):1225–1231. PubMed CrossRef
- Update on Overall Prevalence of Major Birth Defects—Atlanta, Georgia, 1978–2005. CDC. 2008. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5701a2.htm ED: changed tags to eref and added URL; please review/verify. /rhb
- Holmes LB, Nasri H, Westgate MN, et al. The Active Malformations Surveillance Program, Boston in 1972–2012: methodology and demographic characteristics. Birth Defects Res. 2018;110(2):148–156. PubMed CrossRef
- McBride WG. Thalidomide and Congenital Abnormalities. Lancet. 1961;278(7216):1358. CrossRef
- Wyszynski DF, Nambisan M, Surve T, et al; Antiepileptic Drug Pregnancy Registry. Increased rate of major malformations in offspring exposed to valproate during pregnancy. Neurology. 2005;64(6):961–965. PubMed CrossRef
- McKenna K, Koren G, Tetelbaum M, et al. Pregnancy outcome of women using atypical antipsychotic drugs: a prospective comparative study. J Clin Psychiatry. 2005;66(4):444–449, quiz 546. PubMed CrossRef
- Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry. 2016;73(9):938–946. PubMed CrossRef
- Habermann F, Fritzsche J, Fuhlbrück F, et al. Atypical antipsychotic drugs and pregnancy outcome: a prospective, cohort study. J Clin Psychopharmacol. 2013;33(4):453–462. PubMed CrossRef
- Reis M, Källén B. Maternal use of antipsychotics in early pregnancy and delivery outcome. J Clin Psychopharmacol. 2008;28(3):279–288. PubMed CrossRef
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890–1900. PubMed CrossRef
- Bar-Oz B, Einarson T, Einarson A, et al. Paroxetine and congenital malformations: meta-analysis and consideration of potential confounding factors. Clin Ther. 2007;29(5):918–926. PubMed CrossRef
- Clarren SK, Smith DW. The fetal alcohol syndrome. N Engl J Med. 1978;298(19):1063–1067. PubMed CrossRef
- Shiono PH, Klebanoff MA, Berendes HW. Congenital malformations and maternal smoking during pregnancy. Teratology. 1986;34(1):65–71. PubMed CrossRef
- Naeye RL. Maternal age, obstetric complications, and the outcome of pregnancy. Obstet Gynecol. 1983;61(2):210–216. PubMed
- Werler MM, Shapiro S, Mitchell AA. Periconceptional folic acid exposure and risk of occurrent neural tube defects. JAMA. 1993;269(10):1257–1261. PubMed CrossRef
- Werler MM, Louik C, Shapiro S, et al. Prepregnant weight in relation to risk of neural tube defects. JAMA. 1996;275(14):1089–1092. PubMed CrossRef
- Toufaily MH, Westgate MN, Lin AE, et al. Causes of congenital malformations. Birth Defects Res. 2018;110(2):87–91. PubMed CrossRef
- Harden CL, Meador KJ, Pennell PB, et al; American Academy of Neurology; American Epilepsy Society. Practice parameter update: management issues for women with epilepsy—focus on pregnancy (an evidence-based review): teratogenesis and perinatal outcomes: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73(2):133–141. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite