Residual symptoms are experienced by most patients being treated for depression, including those who have achieved remission. These symptoms prevent individuals from fully recovering and feeling truly “well.” Often, symptoms that are related to deficits in positive affect (such as anhedonia, pessimism, and lack of motivation) persist after the other symptoms of depression (such as irritability, distress, and low mood) have resolved. Clinicians must assess patients for residual symptoms using rating scales (eg, the Patient Health Questionnaire, Clinical Positive Affect Scale) and adjust treatment to improve patients’ sense of well-being and normalcy.
From the Department of Psychiatry and the Depression Clinical Research Program, Harvard Medical School and Massachusetts General Hospital, Boston.
Supported by an educational grant from Forest Laboratories, Inc.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
Many patients being treated for depression experience residual symptoms, and the presence of residual symptoms increases the likelihood of a depressive relapse.1 Residual symptoms also lead to functional impairment2 and reductions in quality of life.3 Although most people would assume that remission from depression indicates a complete resolution of symptoms and a return to full functioning, this assumption is false. Definitions of remission vary, but, according to the APA Practice Guideline for the Treatment of Patients With Major Depressive Disorder,4 remission from depression means that a patient has experienced 3 or more consecutive weeks without sad mood or loss of pleasure/interest in activities and is experiencing no more than 3 of the other symptoms of a major depressive episode. Thus, the very definition of remission allows for residual symptoms, and an individual can achieve remission but still not feel completely well. As a common hindrance to full recovery, residual symptoms must be included in assessment and treatment of patients with depression.
Residual Symptoms Are Common
Successful treatment of depression should lead to increases in motivation and positive affect and reductions in distress and negative affect, and should also ameliorate other symptoms of depression such as anxiety or insomnia. However, for many patients, this is not the case. In the STAR*D study,1 more than 90% of patients in remission had one or more residual symptom. One study5 found that only 18% of patients who reached remission with fluoxetine treatment had no threshold or subthreshold symptoms of depression. Also in the fluoxetine study,5 anhedonia, defined as diminished interest or pleasure, was the third most prevalent residual symptom (27%) after sleep disturbance (44%) and fatigue (38%). Other common residual symptoms in patients with remitted depression include anxiety, guilt, impaired concentration, changes in appetite/weight, and depressed mood.1,2,5 Residual core mood symptoms (eg, depressed mood, anhedonia) have been found to have a greater negative impact on a patient’s functioning than residual anxiety, sleep disturbance, or physical symptoms.2 Residual symptoms of depression can also affect functioning through an association with demoralization and lack of motivation.
Mood Is Linked to Motivation
Positive and negative affect. Depression is a mood disorder, and mood includes dimensions of both positive and negative affect.6 The dimension of negative affect refers to the extent to which an individual experiences unpleasant feelings such as dysphoria, irritability, distress, hostility, or guilt, among others. The dimension of positive affect refers to an individual’s joie de vivre and ability to experience pleasure, motivation, excitement, and enthusiasm.6 An untreated individual with depression typically experiences high negative affect and low positive affect, or low motivation and high distress (AV 1).6,7 This combination can lead to a general state of demoralization in which the person feels helpless, hopeless, and unable to meet expectations or cope with life’s difficulties.8
AV 1. Positive and Negative Affect
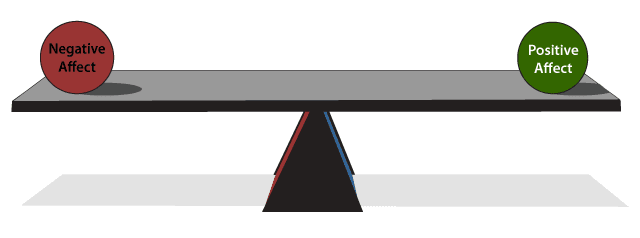
Mood can be conceived of as a balance between positive and negative affect. Positive affect refers to motivation and the ability to feel excitement, enthusiasm, and joy. Negative affect involves distress, anxiety, irritability, and anhedonia. Healthy individuals experience high positive and low negative affect, but those with depression have high negative affect and low positive affect.
Based on Watson and Tellegen6 and Shelton and Tomarken7
The complexity of motivation. Anhedonia, a core symptom of major depressive disorder, is defined as a loss of pleasure in response to stimuli that were previously considered pleasurable. However, anhedonia is deeply intertwined with motivation, and both are complex constructs.9 Motivation can be conceived of as a cycle in which an individual perceives the possibility of a future reward and decides whether or not to expend the effort to attain the reward. This is typically done by gauging the costs and benefits of obtaining the reward. After the effort is expended and the reward attained, the individual will evaluate if the benefit of the reward justified the effort needed to get it (AV 2).
AV 2. The Cycle of Motivation
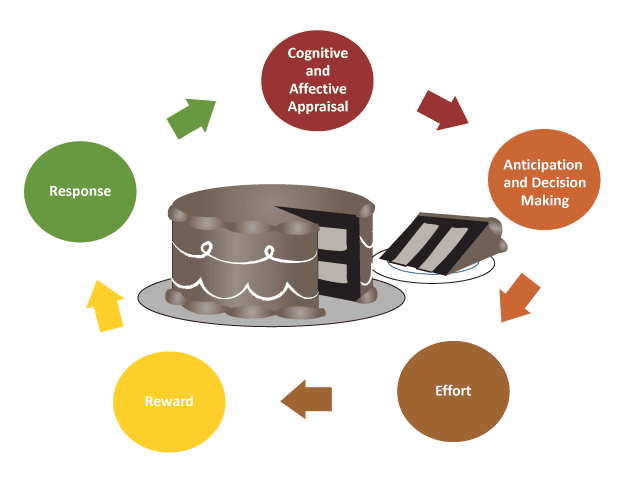
Normally, when presented with the opportunity for a reward, an individual will appraise it, anticipate pleasure and decide to try to obtain it, and then expend the necessary effort. After obtaining the reward, the person responds to it, and the cycle restarts with a new reward. Depressed individuals may be unable to anticipate pleasure from having the reward, have difficulty making decisions, or lack the motivation to expend effort to obtain the reward.
Individuals with depression have been found to have a dysfunctional system of motivation, which often manifests as anhedonia. Treadway and Zald10 differentiated consummatory anhedonia, which is the inability to derive enjoyment from pleasurable stimuli, from motivational anhedonia, which is the reduced motivation to pursue pleasurable stimuli. Motivational anhedonia may result from anticipatory anhedonia, in which individuals predict that an upcoming event will not be enjoyable (ie, they cannot perceive that pleasure might result from the stimulus). Another type of anhedonia is decisional anhedonia, in which individuals are unable to realistically assess the costs and benefits of actions, causing difficulty in making decisions, and therefore they do nothing.
Depression appears to be associated more with anticipatory and motivational anhedonia than with consummatory anhedonia. Pizzagalli and colleagues11 found that individuals with depression responded positively to rewards, but, when confronted with a situation in which they had to expend effort to attain the reward, they were less likely than healthy controls to modify their behavior to earn the reward. Sherdell and colleagues12 also found that individuals with and without depression had similar positive responses to reward, but those with depression were less willing to work for the reward. Motivation to expend effort for rewards in the depressed group was predicted by participants’ levels of anticipatory anhedonia. In individuals with depression, therefore, dissociation exists between liking something and being willing to work to get it.
Unfortunately, depressed individuals experiencing anhedonia are difficult to treat. In the STAR*D study,13 individuals experiencing diminished interest, enjoyment, activity, and decisiveness at baseline were significantly less likely to benefit from citalopram treatment than individuals experiencing other depressive symptoms such as mood/anxiety disturbance, pessimism, or sleep/appetite problems.
Residual Symptoms Should Be Assessed and Treated
Clearly, if an individual with depression is to fully recover and resume a sense of well-being, residual symptoms must be addressed. Unresolved depressive symptoms related to distress and anxiety often receive more attention during treatment, but improving residual symptoms related to positive affect is just as important for achieving full recovery.7 To help assess patients’ mood and anxiety symptoms, clinicians may use a tool such as the PHQ-9, QIDS-SR, or GAD-7 at follow-up visits, but these scales may not capture patients’ sense of well-being or lack of positive affect. For these areas, the WHO-5 or the Clinical Positive Affect Scale (CPAS) may be used. The WHO-5 is a patient-rated instrument that measures 5 quality-of life factors over the previous 2 weeks. Patients rate each statement on a scale from 0 (at no time) to 5 (all of the time). The CPAS14 is a simple, 16-item self-report scale used to assess consummatory, anticipatory, and motivational aspects of positive affect. It measures components of positive affect not typically measured in clinical trials or clinical practice. The scale asks respondents to rate their ability to look forward to activities, enjoy them, and get things done over the previous week.14
Because individuals with anhedonia tend to experience poorer antidepressant outcomes, these patients should be identified so that clinicians can try different treatment strategies such as cognitive behavioral therapy, other antidepressants or pharmacotherapies, or even exercise.13 Only by considering the whole patient, including all of the intricate forces and qualities that affect mood and motivation, will a clinician be able to provide treatment that not only rids the patient of depression but also returns him or her to wellness.
Conclusion
Residual symptoms are a common problem in patients with depression, even for those who have achieved a level of remission. Among the common residual symptoms of sleep/concentration problems, fatigue, and guilt, anhedonia is important to recognize because of its association with motivation. Patients with depression who lack motivation will not expend effort to return to their usual activities and may respond poorly to antidepressant treatment. Untreated patients with depression tend to have increased negative affect and decreased positive affect, which leads to pessimism and reduced quality of life. Clinicians should assess patients not only for common residual symptoms but also for their sense of well-being and level of positive affect using rating scales such as the WHO-5 and CPAS. Treatment can then be adjusted to alleviate all symptoms that are hindering patients from feeling well.
Clinical Points
- Remember that reduced positive affect may persist once other symptoms of depression have resolved
- Use an instrument such as the Clinical Positive Affect Scale to assess patients’ positive affect
- Continue to target residual symptoms once a patient has achieved remission to improve the patient’s overall well-being
Drug Names
citalopram (Celexa and others), fluoxetine (Prozac and others)
Abbreviations
APA = American Psychiatric Association
CPAS = Clinical Positive Affect Scale
GAD-7 = Generalized Anxiety Disorder 7-item scale
PHQ-9 = 9-item Patient Health Questionnaire
QIDS-SR = Quick Inventory of Depressive Symptomatology-Self-Report
STAR*D = Sequenced Treatment Alternatives to Relieve Depression
WHO-5 = World Health Organization 5-item Well-being Index
References
- Nierenberg AA, Husain MM, Trivedi MH, et al. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol Med. 2010;40(1):41–50. PubMed
- Romera I, Pérez V, Ciudad A, et al. Residual symptoms and functioning in depression, does the type of residual symptom matter? a post-hoc analysis. BMC Psychiatry. 2013;13:51. PubMed
- Woo JM, Jeon HJ, Noh E, et al. Importance of remission and residual somatic symptoms in health-related quality of life among outpatients with major depressive disorder: a cross-sectional study. Health Qual Life Outcomes. 2014;12:188. PubMed
- Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice Guideline for the Treatment of Patients With Major Depressive Disorder. Arlington, VA: American Psychiatric Association; 2010.
- Nierenberg AA, Keefe BR, Leslie VC, et al. Residual symptoms in depressed patients who respond acutely to fluoxetine. J Clin Psychiatry. 1999;60(4):221–225. Full Text
- Watson D, Tellegen A. Toward a consensual structure of mood. Psychol Bull. 1985;98(2):219–235. PubMed
- Shelton RC, Tomarken AJ. Can recovery from depression be achieved? Psychiatr Serv. 2001;52(11):1469–1478. PubMed
- Fava GA, Mangelli L, Ruini C. Assessment of psychological distress in the setting of medical disease. Psychother Psychosom. 2001;70(4):171–175. PubMed
- Treadway MT, Bossaller NA, Shelton RC, et al. Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J Abnorm Psychol. 2012;121(3):553–558. PubMed
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35(3):537–555. PubMed
- Pizzagalli DA, Iosifescu D, Hallett LA, et al. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 2008;43(1):76–87. PubMed
- Sherdell L, Waugh CE, Gotlib IH. Anticipatory pleasure predicts motivation for reward in major depression. J Abnorm Psychol. 2012;121(1):51–60. PubMed
- Uher R, Perlis RH, Henigsberg N, et al. Depression symptom dimensions as predictors of antidepressant treatment outcome: replicable evidence for interest-activity symptoms. Psychol Med. 2012;42(5):967–980. PubMed
- Nierenberg AA, Bentley KH, Farabaugh AH, et al. The absence of depressive symptoms is not the presence of wellness: validation of the Clinical Positive Affect Scale. Aust N Z J Psychiatry. 2012;46(12):1165–1172. PubMed
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
CME Background Information
Supported by an educational grant from Forest Laboratories, Inc.
Participants may receive credit by reading the activity, correctly answering the posttest question, and completing the evaluation.
Objective
After completing this educational activity, you should be able to:
Assess residual symptoms in patients with depression to help patients achieve remission
Financial Disclosure
The faculty for this CME activity and the CME Institute staff were asked to complete a statement regarding all relevant personal and financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure is as follows:
Dr Nierenberg has been a consultant for Abbott, American Psychiatric Association, Appliance Computing (Mindsite), Basilea, BrainCells, Brandeis University, Bristol-Myers Squibb, Clintara, Corcept, Dey, Dainippon Sumitomo, Eli Lilly, EpiQ/Mylan, Forest, Genaissance, Genentech, GlaxoSmithKline, Hoffman LaRoche, Infomedic, Lundbeck, Janssen, Jazz, MedAvante, Merck, Methylation Sciences, Naurex, Novartis, PamLabs, Pfizer, PGx Health, Ridge Diagnostics, Shire, Schering-Plough, Somerset, Sunovion, Takeda, Targacept, and Teva; has consulted through the MGH Clinical Trials Network and Institute (CTNI) for AstraZeneca, BrainCells, Dainippon Sumitomo/Sepracor, Johnson & Johnson, Labopharm, Merck, Methylation Sciences, Novartis, PGx Health, Shire, Schering-Plough, Targacept, and Takeda/Lundbeck; has received grant/research support from American Foundation for Suicide Prevention, AHRQ, Brain and Behavior Research Foundation, Bristol-Myers Squibb, Cederroth, Cephalon, Cyberonics, Elan, Eli Lilly, Forest, GlaxoSmithKline, Janssen, Lichtwer, Marriott Foundation, Mylan, NIMH, PamLabs, PCORI, Pfizer, Shire, Stanley Foundation, Takeda, and Wyeth-Ayerst; has received honoraria from Belvoir Publishing, University of Texas Southwestern Dallas, Brandeis University, Bristol-Myers Squibb, Hillside Hospital, American Drug Utilization Review Society, American Society for Clinical Psychopharmacology, Baystate Medical Center, Columbia University, CRICO, Dartmouth Medical School, Health New England, Harold Grinspoon Charitable Foundation, IMEDEX, International Society for Bipolar Disorders, Israel Society for Biological Psychiatry, Johns Hopkins University, MJ Consulting, New York State, Medscape, MBL Publishing, MGH Psychiatry Academy, National Association for Continuing Education, SUNY Buffalo, University of Wisconsin, University of Pisa, University of Michigan, University of Miami, APSARD, SciMed, Slack Publishing, and Wolters Klower Publishing; is a stock/shareholder of Appliance Computing (MindSite), BrainCells, and MedAvante; and holds copyrights for the Clinical Positive Affect Scale and the MGH Structured Clinical Interview for the Montgomery Asberg Depression Scale exclusively licensed to the MGH CTNI.
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this enduring material for a maximum of 0.5 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
The American Academy of Physician Assistants (AAPA) accepts certificates of participation for educational activities certified for AMA PRA Category 1 Credit™ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 0.5 hours of Category I credit for completing this program.
To obtain credit for this activity, study the material and complete the CME Posttest and Evaluation.
Application for CME credit has been filed with the American Academy of Family Physicians. Determination of credit is pending.
Release, Review, and Expiration Dates
This Psychlopedia activity was published in July 2015 and is eligible for AMA PRA Category 1 Credit™ through July 31, 2017. The latest review of this material was June 2015.
Statement of Need and Purpose
Depression causes functional impairments that do not always improve with antidepressant treatment. One factor complicating depression treatment is that both serotonin and norepinephrine are implicated in depression, but the most-used antidepressants, the selective serotonin reuptake inhibitors (SSRIs), target serotonin. Function is one area of unmet need in depression treatment, but residual symptoms also cause a negative impact on patient outcomes. One way to target these other factors may be to investigate medications with different mechanisms of action than current medications. This activity was designed to meet the needs of participants in CME activities provided by the CME Institute of Physicians Postgraduate Press, Inc., who have requested information on depression.
Disclosure of Off-Label Usage
Dr Nierenberg has determined that, to the best of his knowledge, no investigational information about pharmaceutical agents that is outside US Food and Drug Administration–approved labeling has been presented in this activity.
Review Process
The entire faculty of the series discussed the content at a peer-reviewed planning session, the Chair reviewed the activity for accuracy and fair balance, and a member of the External Advisory CME Board who is without conflict of interest reviewed the activity to determine whether the material is evidence-based and objective.
Acknowledgment
This Psychlopedia activity is derived from the planning teleconference series “Barriers to Remission in Major Depressive Disorder: Residual Symptoms, Functional Impairment, and Medication Mechanism of Action,” which was held in December 2014 and supported by an educational grant from Forest Laboratories, Inc. The opinions expressed herein are those of the faculty and do not necessarily reflect the opinions of the CME provider and publisher or the commercial supporter.
Save
Cite
