Many studies have shown that educating patients about the potential adverse effects of a drug can increase the chances that those adverse effects will be experienced. Studies have further shown that how such information is communicated can also impact this nocebo risk. Additionally, information provided through patient education can influence treatment efficacy, perhaps by moderating the placebo response. There is therefore a need to optimize the manner in which patients are educated about their medications so that placebo-related benefits are enhanced and nocebo-related harm is minimized. This article provides suggestions on the subject for clinical practice as well as research. Nonspecific factors in psychopharmacology are important and should not be neglected.


ABSTRACT
Many studies have shown that educating patients about the potential adverse effects of a drug can increase the chances that those adverse effects will be experienced. Studies have further shown that how such information is communicated can also impact this nocebo risk. Additionally, information provided through patient education can influence treatment efficacy, perhaps by moderating the placebo response. There is therefore a need to optimize the manner in which patients are educated about their medications so that placebo-related benefits are enhanced and nocebo-related harm is minimized. This article provides suggestions on the subject for clinical practice as well as research. Nonspecific factors in psychopharmacology are important and should not be neglected.
J Clin Psychiatry 2017;78(9):e1310-e1312
https://doi.org/10.4088/JCP.17f12016
© Copyright 2017 Physicians Postgraduate Press, Inc.
Psychotropic drugs are associated with benefits but also with adverse effects (AEs); none will contest this point. Psychotherapies are also associated with AEs, a matter that is neither well recognized nor well studied.1 Curiously, the mere provision of information about treatment may also be associated with AEs, even when the information is offered with the best of intentions, in accordance with sound ethical principles, and with a view to improve treatment outcomes.
Educating Patients About Medications May Increase Their Experience of Adverse Effects
In a small, double-blind, randomized controlled trial (RCT), John et al2 assigned 39 antidepressant-naive depressed outpatients to education or control interventions just before the initiation of individualized antidepressant monotherapy. Patients in the education arm were exposed to a 10-min, personalized, face-to-face, interactively educational session on the nature of depression, antidepressant treatment, benefits and adverse effects of the prescribed drug, and plan of management. Control patients received treatment as usual (TAU). The study sought to identify how much patients retain about the education provided and how the education affects treatment adherence at a 6-week follow-up.
The educational intervention (relative to TAU) was found to have no effect on knowledge about and attitudes toward treatment at the 6-week study endpoint. The groups did not differ in treatment adherence, either. However, patients in the intervention group were discovered to have experienced a doubled AE burden relative to patients in the control group. The authors concluded, “For ethical reasons, patients need to be educated about their illness and its treatment. However, such education may be a two-edged sword, with an increased nocebo effect as the most salient consequence.”2(p425)
Treatment information and Its Relationship toTreatment Adverse Effects
The study by John et al2 was small, and the nocebo effect was identified in secondary analyses that carried an inflated type I error risk. Yet, the finding has ample precedence in the medical literature. More specifically, knowledge of the AEs of a drug increases the frequency with which the AEs are experienced and reported.3 This may explain why the profile of AEs reported by placebo-treated patients depends on the active drug being studied in the RCT.4
In a short review, Barsky5 discussed the iatrogenic harm potential of the physician’s words. Several examples were provided. One was of an RCT of 114 patients who received metoprolol for newly diagnosed hypertension. These patients were randomized into 3 groups: patients who were told about the drug and the possibility that it may cause erectile dysfunction (ED), patients who were told about the drug but not about ED as a possible AE, and patients who were not told about either the drug or the risk of ED. After 2 months, the incidence of ED was found to be 32%, 13%, and 8% in the 3 groups, respectively.6 Clearly, patients who knew about ED as an AE were more likely to suffer ED with metoprolol.
In a similar RCT of 120 patients treated with finasteride for a year, 43.6% of patients who were told about possible sexual AEs of the drug reported 1 or more sexual AEs; this number was just 15.3% in patients who were not told about sexual AEs.7
In an RCT of local anesthetic injection for an obstetric indication, 140 women were randomized to a reassuring explanation (“We are going to give you a local anesthetic that will numb the area and you will be comfortable during the procedure”) or an anxiety-provoking explanation (“You are going to feel a big bee sting; this is the worst part of the procedure”) prior to the injection. Visual analog scale pain ratings of discomfort related to the injection were 3 vs 5 with reassurance vs anxiety provocation, respectively; the difference was statistically significant.8
In an RCT of atorvastatin (10 mg/d; n = 5,101) vs placebo (n = 5,079), the frequency of muscle-related AEs was 2.03% per annum with atorvastatin vs 2.00% per annum with placebo across a median follow-up of 3.3 years; the difference was not statistically significant (hazard ratio [HR], 1.03; 95% confidence interval [CI], 0.88-1.21). During a nonrandomized, nonblind extension phase of the study, the frequency of muscle-related AEs was 1.26% vs 1.00% per annum in atorvastatin users (n = 6,409) and nonusers (n = 3,490), respectively, across a median follow-up of 2.3 years; the difference was statistically significant (HR, 1.41; 95% CI, 1.10-1.79).9 Again, knowledge of the drug and its associated AEs predisposed to the experience of the AEs.
Treatment Information and Reduced Efficacy
Favorable beliefs about a treatment can contribute to its efficacy; this is the well-known placebo effect.10 It is also well known that unfavorable beliefs about a treatment can diminish treatment efficacy.11 In this context, information provided to patients can shape patient beliefs. For example, information that an antimigraine drug is or may be a placebo diminishes the efficacy of the drug in patients experiencing an acute migraine episode.12 More subtly, information that a treatment is inexpensive may carry the connotation that the treatment is less effective, resulting in decreased treatment efficacy.13-15
Optimizing Communications About Treatment Adverse Effects
From the previous sections, it is clear that providing information about treatment can influence what patients actually experience in terms of efficacy and AEs. Health care providers can therefore potentially manipulate treatment outcomes while being perfectly truthful.
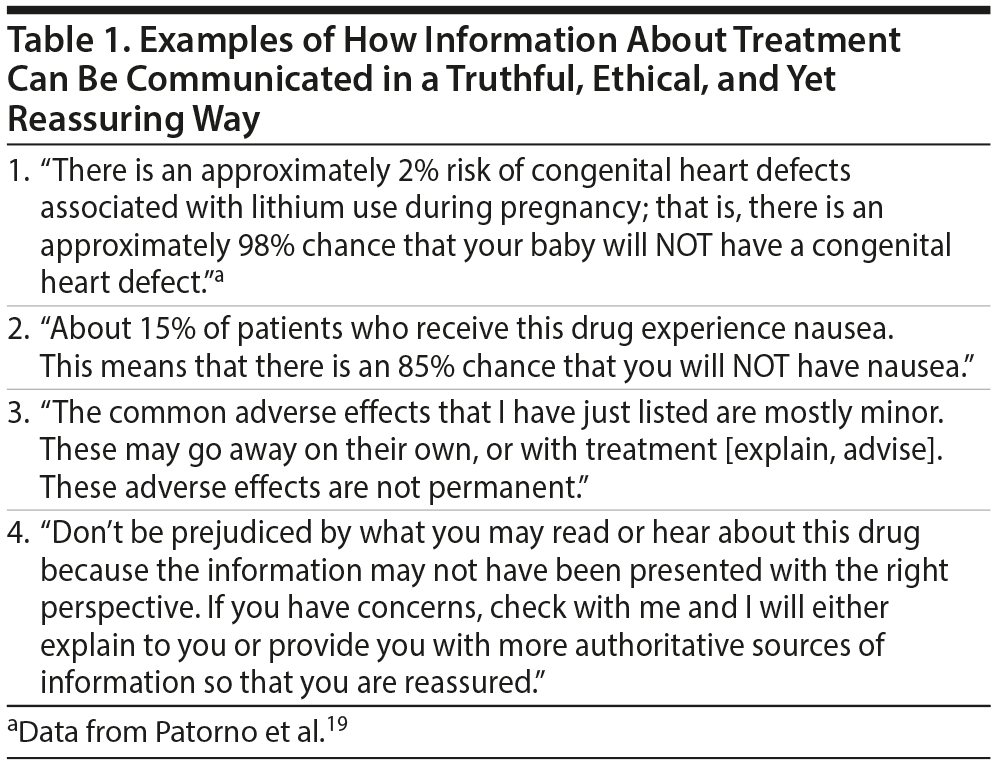
How may this be done within an ethical framework? Bingel11 presented a lucid discussion on avoidance of nocebo effects to optimize treatment outcomes. Suggestions included the presentation of efficacy and adverse effects in a balanced manner, teaching and training patients about strategies to cope with adverse effects, provision of references to web-based and other information systems that offer evidence-based information, and so on. However, practical examples were not provided. Examples of possible communications are therefore suggested in Table 1.
Particular effort should be made to identify and address myths, misconceptions, and fears related to treatment-emergent AEs; these could vary with age, gender, and culture. Particular effort should also be made to ensure that the patient does not misinterpret the information provided because of a mental state that is colored by anxiety, depression, or other psychiatric disturbance.
Optimizing Communications About Treatment Efficacy
Evidence-based education about treatment efficacy would require physicians to provide information about response and remission rates for drug vs placebo, and associated information about numbers needed to treat (NNTs). This information would also be available to patients through online resources. However, RCT data are not the right data for patients to apply to their individual contexts if only because patients entering RCTs know that they may be assigned to placebo, and this knowledge may diminish treatment response.16,17 In contrast, patients in clinical practice know that they will receive an active treatment; there is no risk of receiving placebo. It could therefore be more appropriate to communicate to patients information about response and remission rates (related to the advised treatment) obtained from open-label, nonrandomized clinical trials.
It is particularly inappropriate to communicate to patients information about NNTs because NNTs are obtained after subtracting the effect of placebo from the outcome with active drug.18 In everyday clinical practice, the placebo effect is included in the therapeutic effect and is not subtracted from it.
In sum, RCT data and metrics derived therefrom provide more pessimistic information than is usually applicable to everyday clinical practice; therefore, such data may compromise patient expectations and reduce treatment response.
A Suggestion for Study
It goes without saying that it is the ethical duty of a physician to provide information about the efficacy and AEs of the medicines that are to be prescribed; but how much information should a patient receive? It is clearly impractical and unnecessary to list every possible AE, from common to rare.
Whereas it is the right of the patient to receive information about the prescribed medication, could it not also be the right of the patient to waive this privilege? There is a need to debate the ethics of this idea and to formally study outcomes when patients waive their right to receive information about AEs after an inquiry such as “Research has shown that describing possible adverse effects of medications increases the risk that these adverse effects will be actually be experienced. This is because listing adverse effects may cause some patients to worry about them, and worry can affect the way the mind and body function, resulting in the adverse effects occurring. Therefore, would you let us know, please, how much you wish to be told about the possible adverse effects that your medicines may cause?”
Such studies would be particularly important for AEs, such as sexual dysfunction, that are be more likely to be induced by suggestion.6,7
A Short Diversion
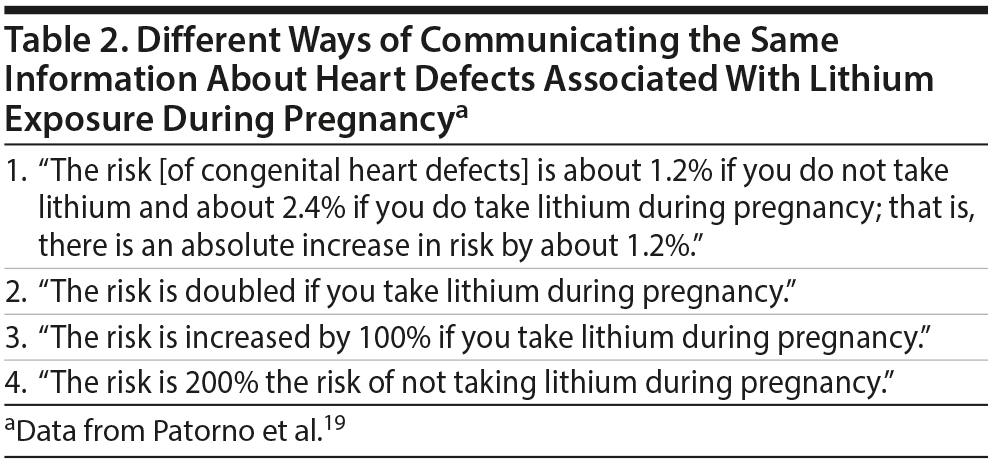
A large retrospective study19 found cardiac malformations in 16 (2.41%) of 663 infants who had been exposed to lithium during pregnancy and in 15,251 (1.15%) of 1,322,955 unexposed infants. This can be communicated in several ways to pregnant women who are considering lithium treatment (Table 2). As is obvious, the alarm in the minds of listening patients would progressively increase from options 1 to 4, presented in Table 2. Thus, the way in which information is communicated can even influence the patient’s decision to accept or refuse the medication that is suggested, and if the treatment is accepted, adherence to treatment could be compromised by worries about inefficacy and AEs, triggered by inappropriately worded communications.
Parting Notes
Besides optimizing the content of the education that is provided to patients about their treatment, it is also necessary for prescribers to cultivate nonverbal skills that communicate calm, confidence, professional concern, reassurance, and optimism. Warmth in the tone of voice, a cheerful expression, an empathetic attitude, a willingness to listen and address concerns, and, above all, a personal approach will all go a long way in diminishing nocebo responses. In this regard, Chaput de Saintonge and Herxheimer20 provided a detailed, instructive review on harnessing placebo effects in health care.
Psychopharmacology is a science, and the principles of evidence-based medicine must drive its practice. However, psychopharmacology is also an art, and strategies that benefit patients must be utilized to the fullest extent possible within the limits of ethical practice. Nonspecific factors in psychopharmacology are important to physicians and their patients and should not be neglected.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
REFERENCES
1. Linden M, Schermuly-Haupt M-L. Definition, assessment and rate of psychotherapy side effects. World Psychiatry. 2014;13(3):306-309. PubMed CrossRef
2. John AP, Singh NM, Nagarajaiaj, et al. Impact of an educational module in antidepressant-naive patients prescribed antidepressants for depression: pilot, proof-of-concept, randomized controlled trial. Indian J Psychiatry. 2016;58(4):425-431. PubMed CrossRef
3. Barsky AJ, Saintfort R, Rogers MP, et al. Nonspecific medication side effects and the nocebo phenomenon. JAMA. 2002;287(5):622-627. PubMed CrossRef
4. Kaptchuk TJ, Miller FG. Placebo effects in medicine. N Engl J Med. 2015;373(1):8-9. PubMed CrossRef
5. Barsky AJ. The iatrogenic potential of the physician’s words [published online ahead of print October 31, 2017]. JAMA. PubMed CrossRef
6. Cocco G. Erectile dysfunction after therapy with metoprolol: the Hawthorne effect. Cardiology. 2009;112(3):174-177. PubMed CrossRef
7. Mondaini N, Gontero P, Giubilei G, et al. Finasteride 5 mg and sexual side effects: how many of these are related to a nocebo phenomenon? J Sex Med. 2007;4(6):1708-1712. PubMed CrossRef
8. Varelmann D, Pancaro C, Cappiello EC, et al. Nocebo-induced hyperalgesia during local anesthetic injection. Anesth Analg. 2010;110(3):868-870. PubMed CrossRef
9. Gupta A, Thompson D, Whitehouse A, et al; ASCOT Investigators. Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid-Lowering Arm (ASCOT-LLA): a randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase. Lancet. 2017;389(10088):2473-2481. PubMed CrossRef
10. J Clin Psychiatry. 2012;73(10):1322-1325. PubMed CrossRef
11. Bingel U; Placebo Competence Team. Avoiding nocebo effects to optimize treatment outcome. JAMA. 2014;312(7):693-694. PubMed CrossRef
12. Kam-Hansen S, Jakubowski M, Kelley JM, et al. Altered placebo and drug labeling changes the outcome of episodic migraine attacks. Sci Transl Med. 2014;6(218):218ra5. PubMed CrossRef
13. Waber RL, Shiv B, Carmon Z, et al. Commercial features of placebo and therapeutic efficacy. JAMA. 2008;299(9):1016-1017. PubMed CrossRef
14. Espay AJ, Norris MM, Eliassen JC, et al. Placebo effect of medication cost in Parkinson disease: a randomized double-blind study. Neurology. 2015;84(8):794-802. PubMed CrossRef
15. Andrade C. Cost of treatment as a placebo effect in psychopharmacology: importance in the context of generic drugs. J Clin Psychiatry. 2015;76(4):e534-e536. PubMed CrossRef
16. Sneed JR, Rutherford BR, Rindskopf D, et al. Design makes a difference: a meta-analysis of antidepressant response rates in placebo-controlled versus comparator trials in late-life depression. Am J Geriatr Psychiatry. 2008;16(1):65-73. PubMed CrossRef
17. Rutherford BR, Sneed JR, Roose SP. Does study design influence outcome? the effects of placebo control and treatment duration in antidepressant trials. Psychother Psychosom. 2009;78(3):172-181. PubMed CrossRef
18. Andrade C. The numbers needed to treat and harm (NNT, NNH) statistics: what they tell us and what they do not. J Clin Psychiatry. 2015;76(3):e330-e333. PubMed CrossRef
19. Patorno E, Huybrechts KF, Bateman BT, et al. Lithium use in pregnancy and the risk of cardiac malformations. N Engl J Med. 2017;376(23):2245-2254. PubMed CrossRef
20. Chaput de Saintonge DM, Herxheimer A. Harnessing placebo effects in health care. Lancet. 1994;344(8928):995-998. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top