Objective: To evaluate the influence of long-term zolpidem use on the incidence of developing Parkinson’s disease.
Method: 2,961 subjects who used zolpidem for the first time longer than 3 months between 1998 and 2000 were identified in the National Health Insurance system of Taiwan. Subjects without a history of zolpidem use were randomly selected as a comparison cohort and frequency matched to zolpidem users based on age, sex, and index date. The diagnosis of Parkinson’s disease was based on the criteria of the International Classification of Diseases, Ninth Revision, Clinical Modification. Its incidence until the end of 2009 was calculated and its hazard ratios (HRs) and 95% CIs were estimated using Cox proportional hazards regression models and Kaplan-Meier analysis.
Results: The overall incidence of Parkinson’s disease was greater among zolpidem users than in the comparison cohort (HR = 1.88; 95% CI, 1.45-2.45). However, there was no difference in Parkinson’s disease incidence between these 2 cohorts after 5 years of observation. The risk of Parkinson’s disease increased with increasing zolpidem dose, with an HR of 0.70 for low-dose users (< 400 mg/y) and 2.94 for high-dose users (≥ 1,600 mg/y). The incidence of Parkinson’s disease was greater in subjects using zolpidem only (HR = 2.35; 95% CI, 1.66-3.33) compared to those using benzodiazepines only (HR = 1.31; 95% CI, 0.91-1.90). By stratified analysis, zolpidem use with concurrent depression (HR = 4.79) increased the risk of Parkinson’s disease compared to that of zolpidem users without concurrent depression.
Conclusions: Zolpidem use might unmask preclinical Parkinson’s disease, especially in patients with depression. However, large population-based, unbiased, randomized trials are warranted to confirm this finding.
Risk of Parkinson’s Disease Following Zolpidem Use: A Retrospective, Population-Based Cohort Study

ABSTRACT
Objective: To evaluate the influence of long-term zolpidem use on the incidence of developing Parkinson’s disease.
Method: 2,961 subjects who used zolpidem for the first time longer than 3 months between 1998 and 2000 were identified in the National Health Insurance system of Taiwan. Subjects without a history of zolpidem use were randomly selected as a comparison cohort and frequency matched to zolpidem users based on age, sex, and index date. The diagnosis of Parkinson’s disease was based on the criteria of the International Classification of Diseases, Ninth Revision, Clinical Modification. Its incidence until the end of 2009 was calculated and its hazard ratios (HRs) and 95% CIs were estimated using Cox proportional hazards regression models and Kaplan-Meier analysis.
Results: The overall incidence of Parkinson’s disease was greater among zolpidem users than in the comparison cohort (HR = 1.88; 95% CI, 1.45-2.45). However, there was no difference in Parkinson’s disease incidence between these 2 cohorts after 5 years of observation. The risk of Parkinson’s disease increased with increasing zolpidem dose, with an HR of 0.70 for low-dose users (< 400 mg/y) and 2.94 for high-dose users (≥ 1,600 mg/y). The incidence of Parkinson’s disease was greater in subjects using zolpidem only (HR = 2.35; 95% CI, 1.66-3.33) compared to those using benzodiazepines only (HR = 1.31; 95% CI, 0.91-1.90). By stratified analysis, zolpidem use with concurrent depression (HR = 4.79) increased the risk of Parkinson’s disease compared to that of zolpidem users without concurrent depression.
Conclusions: Zolpidem use might unmask preclinical Parkinson’s disease, especially in patients with depression. However, large population-based, unbiased, randomized trials are warranted to confirm this finding.
J Clin Psychiatry 2015;76(1):e104-e110
© Copyright 2015 Physicians Postgraduate Press, Inc.
Submitted: September 11, 2013; accepted April 29, 2014 (doi:10.4088/JCP.13m08790).
Corresponding author: Chia-Hung Kao, MD, Graduate Institute of Clinical Medical Science and School of Medicine, College of Medicine, China Medical University. No. 2, Yuh-Der Rd, Taichung 404, Taiwan ([email protected]).
Parkinson’s disease is the second-most prevalent neurodegenerative disorder after Alzheimer’s disease.1 From the perspective of neurotransmitters, both dopaminergic and GABAergic neurons may play critical roles in the regulation of the cortical-basal ganglia circuitry. Pharmacologic modulation of these neurotransmitter cascades may affect the clinical manifestations of Parkinson’s disease.2
Zolpidem is a short-acting imidazopyridine hypnotic drug that is highly selective for GABAA receptors that are frequently used to treat insomnia. It has been known that the internal globus pallidus and substantia nigra, the main output structures of the basal ganglia, contain dense zolpidem-binding sites,3 and the long-term effect of zolpidem on the extrapyramidal system is intriguing but is currently unknown.
Despite its sedative effect, zolpidem has been shown to improve motor symptoms in patients with Parkinson’s disease.3-6 Daniele et al3 showed that zolpidem improved parkinsonian motor disturbance by 21%-59%. However, the latency of the effect was slightly longer (45-60 minutes) and the beneficial period shorter (2-4 hours) than those of levodopa.3 Evidente4 reported that zolpidem improved dystonia in X-linked dystonia-parkinsonism syndrome, but the duration of improvement declined from 4 hours to 2-3 hours during the first year of use. Chen et al5 reported that zolpidem improved akinesia, dystonia, and dyskinesia in patients with advanced-stage Parkinson’s disease, and the effect was retained during a 6-month follow-up. These findings suggest that the short-term use of zolpidem may improve motor function in some patients with Parkinson’s disease. However, such reports are scant, with small case numbers, or have short follow-up periods. Thus, the influence of zolpidem use on the development of Parkinson’s disease remains unclear. Because short-term use of zolpidem diminishes parkinsonian features, this epidemiologic study was conducted using a large Taiwan insurance database (2,961 zolpidem users and 11,844 controls), with a longer follow-up period (3 months to 10 years) to determine whether the use of zolpidem affects the risk of developing Parkinson’s disease.
METHOD
Data Source
In 1996, the National Health Insurance (NHI) program of Taiwan consolidated 13 insurance programs into a universal, single-payer system that provided reimbursement for the health care expenses of 99% of the 23 million residents of Taiwan. The NHI program contracted 97% of the hospitals and clinics in Taiwan. The National Health Research Institutes (NHRI) managed a subset of the NHI claims database, the National Health Insurance Research Database (NHIRD), that contained all the medical registries and the claims data of 1 million patients randomly selected from 1996 to 2009. The details of this database were published previously,7 and previous studies described the validity of the diagnoses in the NHI files.8,9 The diagnoses were coded according to the criteria of the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). All data were reidentified and analyzed anonymously. The Ethics Review Board at China Medical University approved the study (CMU-REC-101-012).

- There is a significant association between zolpidem use and increased incidence of Parkinson’s disease.
- Clinicians should monitor any signs of Parkinson’s disease when prescribing zolpidem, especially in younger patients.
- Zolpidem use might unmask preclinical Parkinson’s disease, especially in patients with depression.
Participants and Study Design
From the NHIRD, 3,053 subjects who used zolpidem for the first time for more than 3 months between 1998 and 2000 were identified. Ninety-two subjects with a history of Parkinson’s disease (ICD-9 code 332) before using zolpidem and those missing sex or age data were excluded. The index date was defined as the date at which zolpidem was first used and then maintained for 3 months. For each zolpidem user, 4 comparison subjects without a history of zolpidem use before or during the study period were randomly selected and frequency matched to the zolpidem users based on age and sex. The index date assigned for each comparison subject was the same as their matched zolpidem user. Each subject was followed up until a diagnosis of Parkinson’s disease was made, death, withdrawal from the NHI program, end of the study (December 31, 2009), or when the subject was censored from the NHI because of loss to follow-up.
The subjects’ comorbidities were identified on their first visit before the index date and included diabetes mellitus (ICD-9-CM code 250), hypertension (ICD-9-CM codes 401-405), hyperlipidemia (ICD-9-CM code 272), sleep disorder (ICD-9-CM codes 780.5 and 307.4), anxiety (ICD-9-CM codes 300.0, 300.2, 300.3, 308.3, and 309.81), stroke (ICD-9-CM codes 430-438 at discharge), depression (ICD-9-CM codes 296.2, 296.3, 300.4, and 311), ischemic heart disease (ICD-9-CM codes 410-414), and head injury (ICD-9-CM codes 850-854 and 959.01). The sleep disorders analyzed did not include sleep apnea syndrome (ICD-9-CM codes 780.51, 780.53, and 780.57).
To ensure the validity of diagnoses, only the consistent diagnoses coded for more than 3 times during the follow-up period before the end of 2010 were included in the analysis, except for head injury, which might be coded only once on the first visit after the event. Subjects who used benzodiazepines, haloperidol, metoclopramide, prochlorperazine, risperidone, or flunarizine for at least 3 months before the index date were also included in the analysis.
Statistical Analysis
The distributions of age, sex, comorbidity, and medication history of the zolpidem users were compared to those of the comparison subjects using χ2 tests. The incidence density rate (IR) per 1,000 person-years for each cohort and the incidence density rate ratio (IRR) were examined using Poisson regression. The hazard ratio (HR) and 95% CI were analyzed by Cox proportional hazards regression to estimate the effects of zolpidem use on the risk of Parkinson’s disease. Comorbidity and medication (ie, zolpidem, benzodiazepines, haloperidol, metoclopramide, prochlorperazine, risperidone, and flunarizine) effects on Parkinson’s disease were investigated by IR, HR, and 95% CI.
The relationship between zolpidem dosage and the risk of Parkinson’s disease for an annual intake of zolpidem stratified by tertile (ie, < 400 mg, 400 to < 1,600 mg, or ≥ 1,600 mg) was examined. Kaplan-Meier analysis was used for separate estimates of the cumulative incidence of Parkinson’s disease between subjects treated with or without zolpidem. The significance of the differences was assessed by log-rank test. All analyses were performed using SAS, Version 9.1 for Windows computer software (SAS Institute; Cary, North Carolina). Statistical significance was set at P < .05.
RESULTS
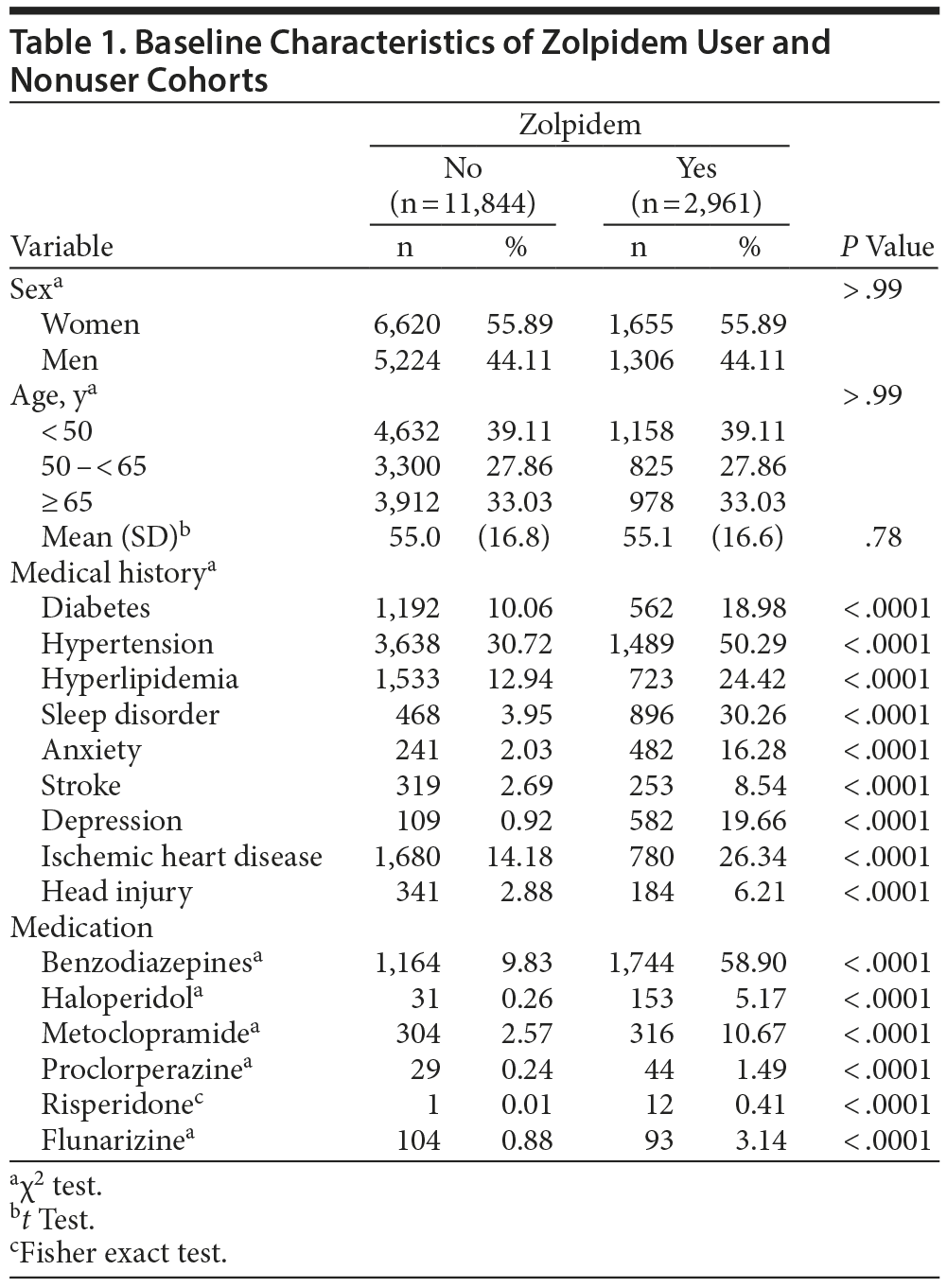
A total of 2,961 and 11,844 subjects were included in the zolpidem user and comparison cohorts, respectively. The distributions of the baseline characteristics of these 2 cohorts are listed in Table 1. Women accounted for 55.89% of the subjects in each cohort. The mean age range was 13.0 to 99.9 years (zolpidem users were 14.1 to 97.7 years old; nonusers were 13.0 to 99.7 years old). The zolpidem users had a higher prevalence of diabetes, hypertension, hyperlipidemia, sleep disorder, anxiety, stroke, depression, ischemic heart disease, and head injury than the comparison cohort. They also had higher prevalence of using benzodiazepines, haloperidol, metoclopramide, prochlorperazine, risperidone, and flunarizine (P < .0001, Table 1).
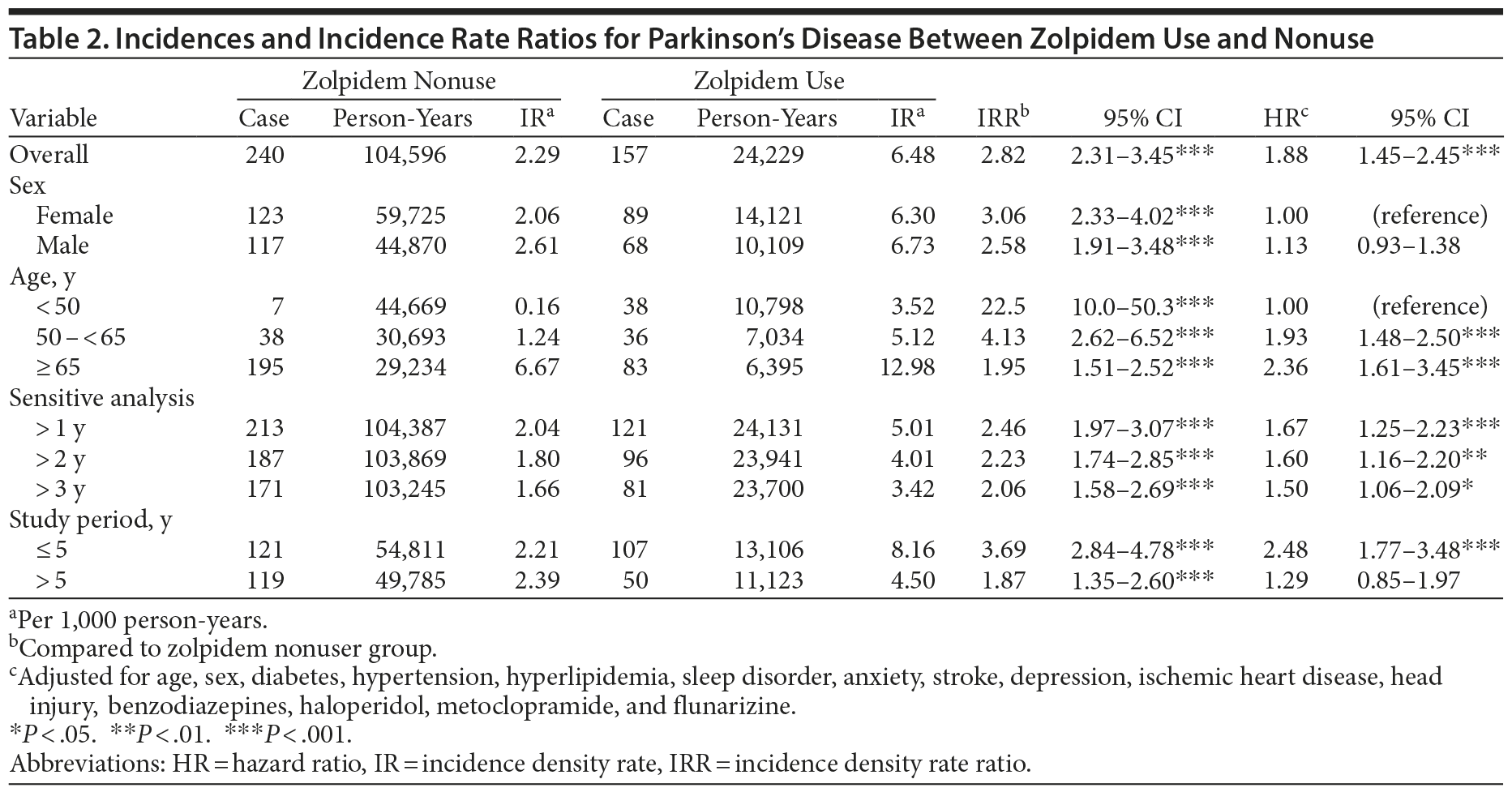
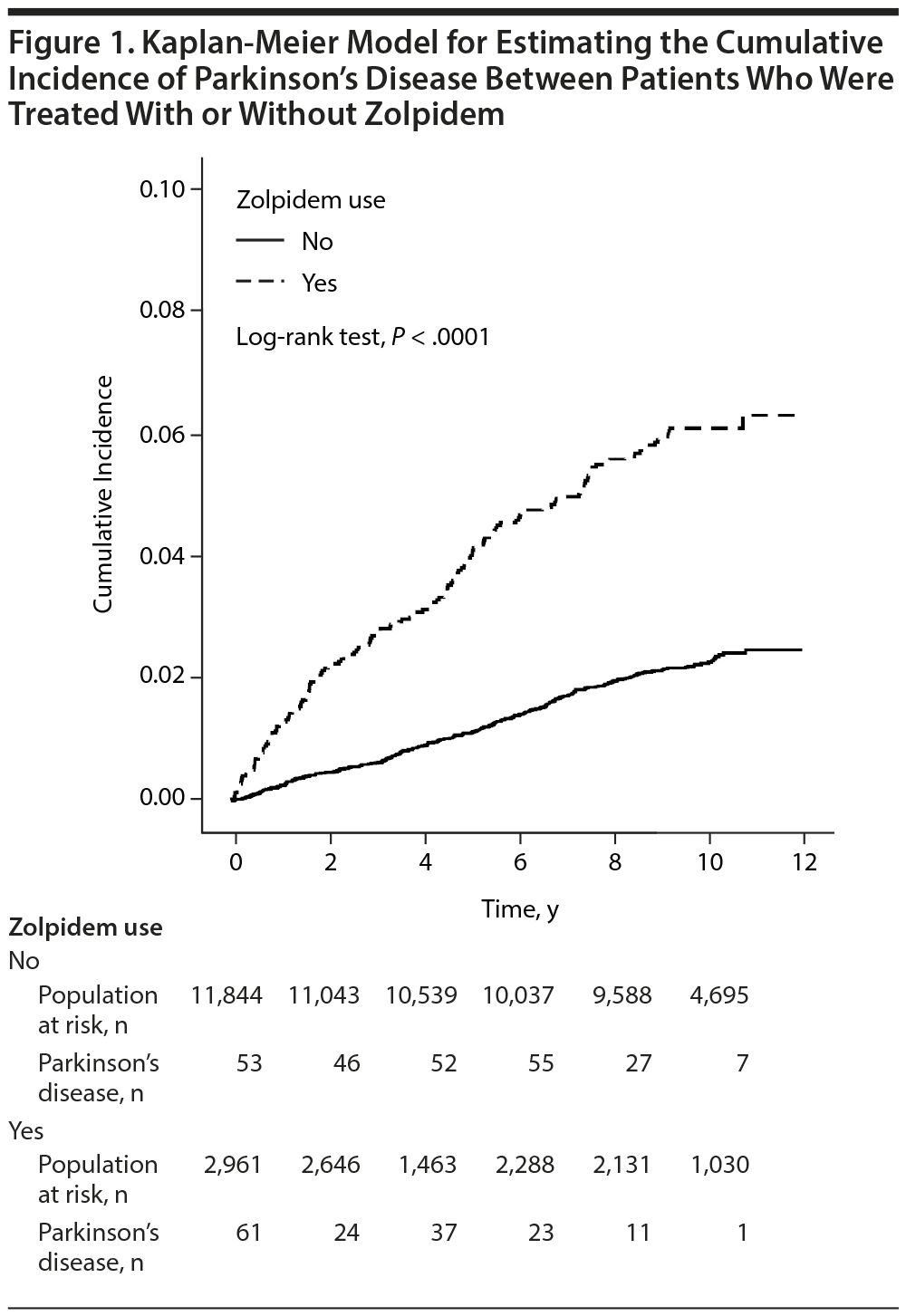
The incidences of Parkinson’s disease among the 2 cohorts are listed in Table 2. When the analysis was stratified by sex, age, or duration of follow-up, the zolpidem cohort had a higher incidence of Parkinson’s disease than the comparison cohort (6.48 vs 2.29 per 1,000 person-years; IRR, 2.82; 95% CI, 2.31-3.45) (Table 2). Kaplan-Meier analysis showed that the cumulative incidence for Parkinson’s disease was approximately 4% higher in the zolpidem users after a 12-year follow-up period (P < .0001, by log-rank test) (Figure 1). Compared to the IRR in control patients, the IRR of Parkinson’s disease among the zolpidem users decreased with increasing age (IRR: 22.5; 95% CI, 10.0-50.3, for subjects aged < 50 years; IRR: 1.95; 95% CI, 1.51-2.52, for subjects aged ≥ 65 years). Moreover, the incidence of Parkinson’s disease among zolpidem users increased by approximately 50% during follow-up for more than 3 years compared to the controls in the same follow-up duration. In duration-stratified analysis, however, follow-up > 5 years showed no significant difference in incidence of Parkinson’s disease between the 2 cohorts.
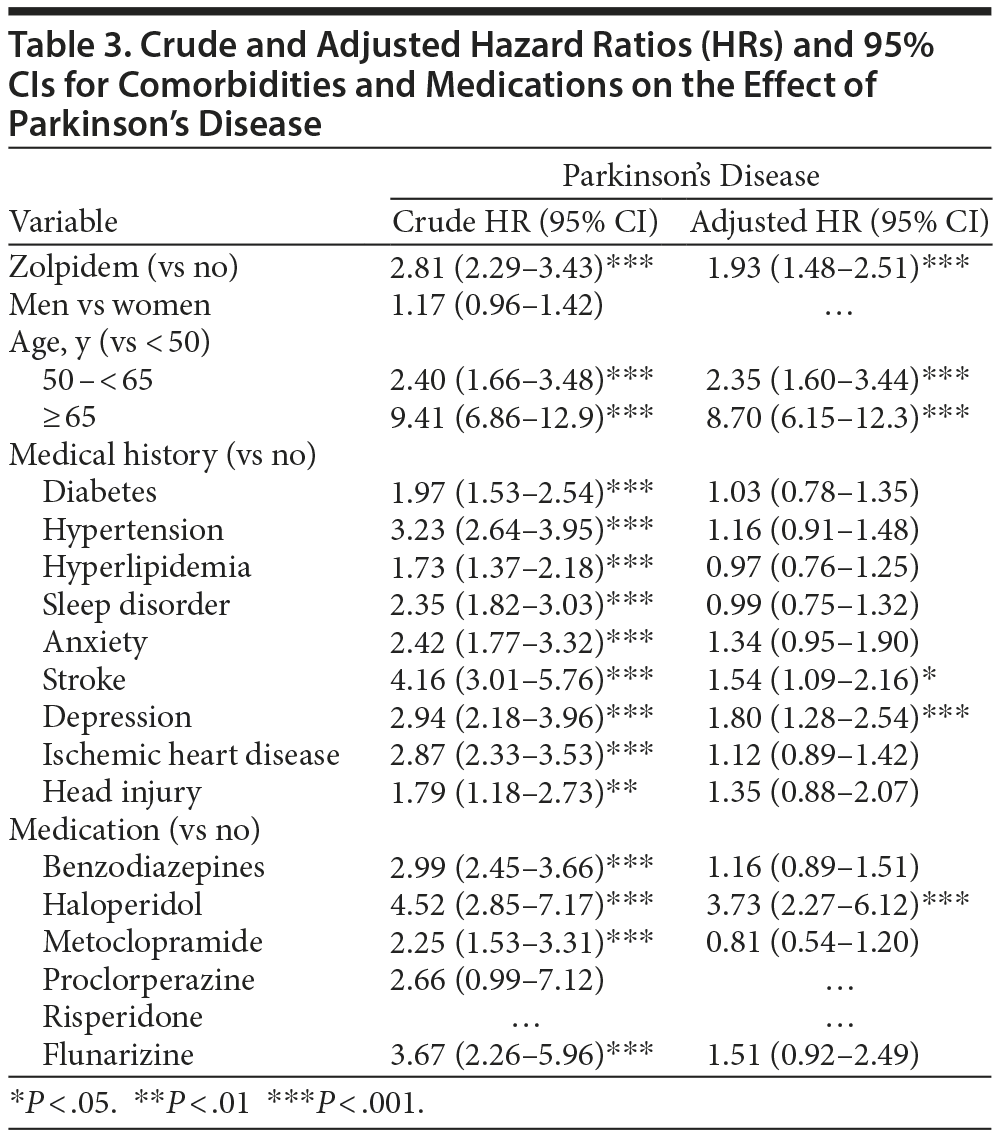
Table 3 represents the effect of comorbidities and medication use on the risk of developing Parkinson’s disease. The HR of Parkinson’s disease increased in subjects with depression (HR = 1.80; 95% CI, 1.28-2.54; P < .001) compared to that in subjects without depression. The HR of Parkinson’s disease also increased with haloperidol use (HR = 3.73; 95% CI, 2.27-6.12; P < .001) compared to haloperidol nonuse.
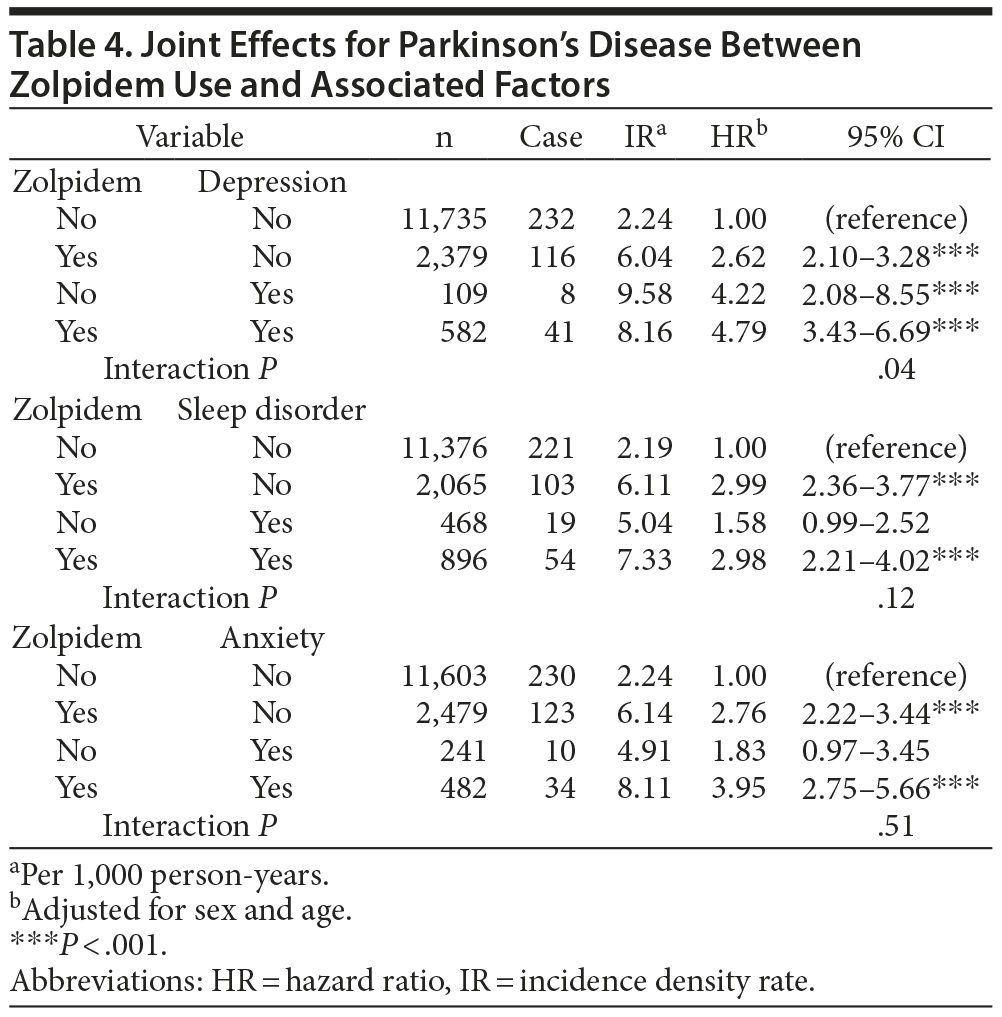
The joint effects of zolpidem use and a psychiatric comorbidity like depression, sleep disorder, or anxiety on the risk of Parkinson’s disease are represented in Table 4. The HR of Parkinson’s disease was 2.62 (95% CI, 2.10-3.28) among subjects who used zolpidem only and 4.22 (95% CI, 2.08-8.55) among those with depression only. The HR increased to 4.79 (95% CI, 3.43-6.69) in patients with depression who used zolpidem (interaction P = .04). The risk of Parkinson’s disease was also significantly higher among subjects with anxiety who used zolpidem (HR = 3.95; 95% CI, 2.75-5.66; P < .04) than that of subjects without concurrent anxiety who did not use zolpidem. The incidence of Parkinson’s disease was lower among subjects with sleep disorder (HR = 1.58; 95% CI, 0.99-2.52) than that in zolpidem users (HR = 2.99; 95% CI, 2.36-3.77). The incidence of Parkinson’s disease among subjects with sleep disorder who used zolpidem (HR = 2.98; 95% CI, 2.21-4.02) was not significantly different than that of subjects without sleep disorder who used zolpidem (P = .12).
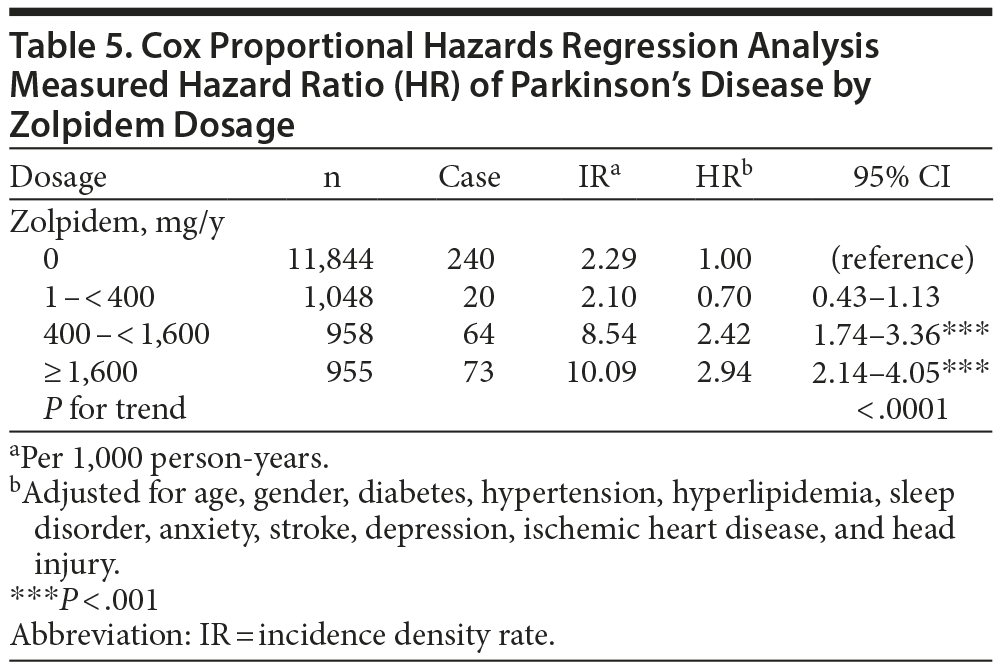
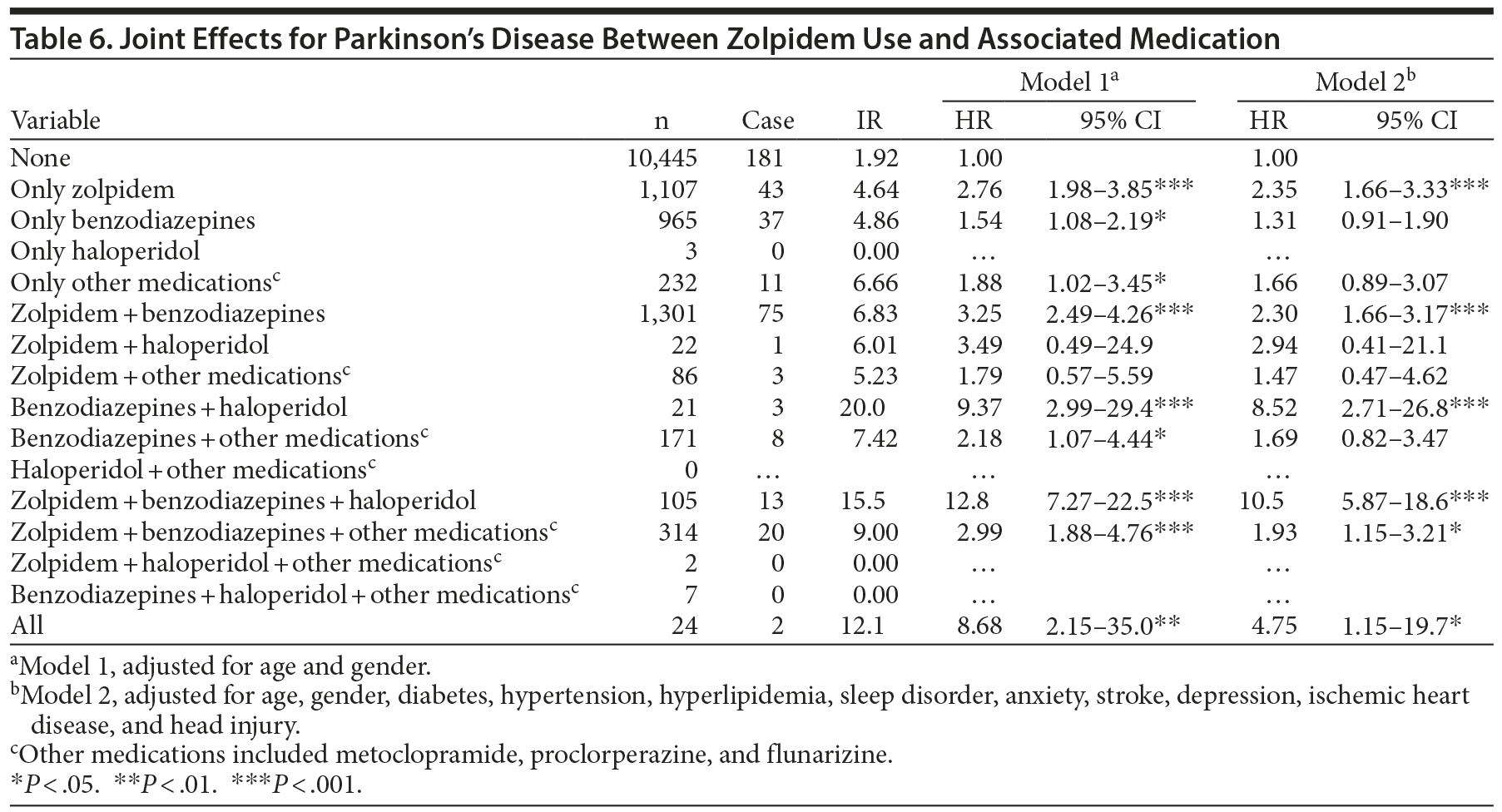
There was a dose-dependent relationship between zolpidem use and Parkinson’s disease (Table 5). The HR of Parkinson’s disease increased with higher zolpidem dosage (HR = 0.70; 95% CI, 0.43-1.13 for 1 to < 400 mg/y; HR = 2.42; 95% CI, 1.74-3.36 for 400 to < 1,600 mg/y; HR = 2.94; 95% CI, 2.14-4.05 for ≥ 1,600 mg/y). The joint effects of the use of zolpidem, benzodiazepines, haloperidol, or drugs that induce extrapyramidal symptoms (EPSs), including risperidone, metoclopramide, prochlorpramide, and flunarizine, on the risk of Parkinson’s disease are presented in Table 6. Subjects who used zolpidem only had a higher incidence of Parkinson’s disease than those who took benzodiazepines only or EPS-inducing drugs only (HRs = 2.35, 1.31, and 1.66, respectively).
DISCUSSION
This is the first population-based study of Parkinson’s disease among zolpidem users in Taiwan (n = 2,961). The results reveal a significant association between zolpidem use of longer duration than 3 months and increase in the incidence of Parkinson’s disease (Table 2). The Kaplan-Meier estimate of the cumulative incidence for Parkinson’s disease shows that zolpidem users have a significantly higher incidence for Parkinson’s disease than the comparison cohort, with a 4% increase in the cumulative incidence of Parkinson’s disease (Figure 1). Zolpidem users aged 50 years or younger have the largest IRR, which decreases gradually with increasing age. In contrast, the HR of Parkinson’s disease increases with increasing age. The incidence of Parkinson’s disease increases by 41.7-fold with increasing age (from 0.16 to 6.67 per 1,000 person-years) among zolpidem nonusers and by 3.69-fold among zolpidem users (3.52 to 12.98 per 1,000 person-years).
These results suggest that zolpidem use may have a greater impact on the increasing incidence of Parkinson’s disease among the younger population. On the other hand, the effect of zolpidem use on Parkinson’s disease incidence among older patients may have been masked by the effects of other factors10 because age is also a risk factor in developing Parkinson’s disease. The increase in HR of Parkinson’s disease with increasing age indicates the combined effect of zolpidem use and age-related factors. Furthermore, it is intriguing to find that the zolpidem impact is most obvious if the study period is less than 5 years but quenched if the period is longer than 5 years. Although the reasons for this phenomenon are uncertain, one possible explanation may be that the impact of the aging process on the development of Parkinson’s disease is greater in longer study periods and this effect may culminate in “catch-up” of the control cohort in Parkinson’s disease incidence compared to the ceiling or saturated Parkinson’s disease incidence of the zolpidem group. On the other hand, another reason might be that zolpidem use exacerbates parkinsonian symptoms to allow earlier diagnosis. In other words, zolpidem use might unmask the preclinical Parkinson’s disease.
Since zolpidem users have a higher prevalence of diabetes, hypertension, hyperlipidemia, sleep disorder, anxiety, stroke, depression, ischemic heart disease, and head injury than the comparison cohort (Table 1), the effects of comorbidities on Parkinson’s disease were also analyzed (Table 3). Depression and zolpidem use have greater effects on Parkinson’s disease than those of other comorbidities (Table 3), with no difference in incidence of Parkinson’s disease between them (Table 4). The combination effect of depression and zolpidem use on Parkinson’s disease is increased when compared to depression alone (Table 4). Depression has been suggested to precede the development of Parkinson’s disease by years.10,11 Furthermore, around 20%-40% of patients with Parkinson’s disease are diagnosed with depression during their disease course, and depression among such patients is most likely the result of endogenous factors.10 Thus, it is conceivable that depression increases the incidence of Parkinson’s disease by itself and in conjunction with zolpidem use. In other words, it is possible that zolpidem use in patients with depression unmasks Parkinson’s disease.
Sleep disturbances are one of the most frequent nonmotor manifestations of Parkinson’s disease,12 and they can be broadly categorized as excessive daytime sleepiness or nocturnal sleep disturbance, which includes insomnia, sleep apnea, restless-leg syndrome, and rapid-eye-movement sleep behavior disorder. Recent studies11,12 have shown that sleep disorders may develop years before the onset of motor symptoms and may be the first clinical manifestation of Parkinson’s disease. Therefore, the association observed between zolpidem use and Parkinson’s disease risk may reflect a higher prevalence of zolpidem use in a subgroup of sleep disorder patients who are in the premotor stage of early, subclinical Parkinson’s disease. However, if this is the case, the HR of sleep disorder alone will likely be equal to or greater than that of zolpidem use alone, but our analysis here shows that the HR of zolpidem alone is higher than that of sleep disorder alone (Table 4). Consequently, sleep disorder is more likely to be a confounding factor instead.
The prevalence of Parkinson’s disease increases with increasing dosage of zolpidem, indicating that zolpidem use increases the incidence of Parkinson’s disease and is dose dependent (Table 5). Mierzejewski et al13 reported that zolpidem can induce dose-dependent catalepsy in Wistar rats. Catalepsy refers to the extrapyramidal symptom of delaying correction of an abnormal posture in an animal model of Parkinson’s disease or parkinsonism.13 Moreover, this response can also be observed in haloperidol administration, with dose-dependent and long-lasting catalepsy, but not in the case of benzodiazepines like diazepam or midazolam.13 Those results are similar to our findings (Table 3 and Table 6).
The HR for Parkinson’s disease among zolpidem users is higher than that of benzodiazepine users, and the combined effects of the use of zolpidem, benzodiazepines, and haloperidol on the subsequent risk of Parkinson’s disease are greater than that of zolpidem use alone (Table 6). The difference between the effects of zolpidem and benzodiazepines may be related to their different mechanisms of action. The benzodiazepines are mediated by GABAA receptors, containing the α1, α2, α3, α5, and γ2 subunits, and exert hypnotic, anxiolytic, anticonvulsant, and myorelaxant effects.14 Unlike benzodiazepines, zolpidem exhibits high selectivity at α1 subunit-containing GABAA receptors. Milic et al14 showed that benzodiazepines produce significant ataxia and muscle relaxation in rats, but zolpidem mediates ataxia mainly. Licata et al15 reported that nonselective benzodiazepine drugs result in ataxia-like and myorelaxant-like effects in squirrel monkeys. However, the α1 subunit-preferring agonist zolpidem has significant ataxia-like effects, but not myorelaxant-like effects.15 The results show that α1 subunit GABAA receptors are mainly involved in deteriorating motor control, which is the main symptom of Parkinson’s disease, while other subunits were involved more in muscle relaxing.
Although short-term use of zolpidem may improve the motor symptoms of Parkinson’s disease3-6 through the selective enhancement of GABAergic inhibition of the internal globus pallidus and substantia nigra, restoring the influence of the thalamus and motor cortex, larger doses of zolpidem may result in worse motor control, thereby allowing earlier diagnosis of Parkinson’s disease. Whether this influence contributes to an increased risk of Parkinson’s disease among long-term zolpidem users remains unclear and warrants future investigations.
Limitations
This study has some limitations. First, the registries in NHI claims are primarily intended for administrative billing purposes and do not undergo scientific verification. The diagnosis of Parkinson’s disease in our database is based on the criteria of ICD-9-CM, which is composed of paralysis agitans and parkinsonism. Although the majority of parkinsonism patients have Parkinson’s disease,16 there are some parkinsonism subjects in the study data who cannot be ruled out according to their history or neurologic examination because of the anonymity ensured by the system. However, if the diagnosis of Parkinson’s disease could fulfill the UK Parkinson’s Disease Brain Bank Criteria for idiopathic Parkinson’s disease, the specificity of this study would be higher. Moreover, subjects cannot be contacted directly to obtain more information on their use of zolpidem, and the reason why zolpidem was prescribed independent of sleep disorder might be related to some undocumented factors in this database. Second, the NHIRD does not provide detailed information regarding patients’ smoking habits, alcohol consumption, body mass index, level of physical activity, socioeconomic status, mortality, or family history of Parkinson’s disease, all of which are possible confounding factors in the analysis of Parkinson’s disease risk. Third, this study investigated the development of Parkinson’s disease after zolpidem use only, rather than changes in motor symptoms among zolpidem users with Parkinson’s disease. Fourth, the evidence derived from a retrospective cohort study is generally of a lower methodological quality than that obtained from randomized trials because its design is subject to biases related to adjustments for confounders. Despite the meticulous study design and adequate control of confounding factors, bias stemming from possible unmeasured or unknown confounders may influence the results. Lastly, although Parkinson’s disease is a neurodegenerative disorder with a long preclinical period, the subjects have only been followed up for 10 years, and the prescription data before 1998 have been excluded from the analysis. Both of these factors may result in the underestimation of the prevalence of Parkinson’s disease and the cumulative dosage of zolpidem, thus weakening the observed association between zolpidem use and Parkinson’s disease. Although the data for zolpidem prescriptions and the diagnosis of Parkinson’s disease are highly reliable, larger, population-based, unbiased, and randomized clinical trials are required to confirm the findings.
CONCLUSION
This population-based cohort study reveals a significant association between zolpidem use and increased incidence of Parkinson’s disease in 5 years. The findings here should raise awareness among clinicians and the general public as regards the importance of monitoring any sign of Parkinson’s disease when zolpidem is prescribed, especially in younger patients. Since zolpidem use may unmask preclinical Parkinson’s disease, especially in patients with depression, the diagnosis of Parkinson’s disease can be made as early as possible, and early treatment can be initiated.
Drug names: diazepam (Diastat, Valium, and others), haloperidol (Haldol and others), metoclopramide (Reglan, Metozolv, and others), prochlorperazine (Compro and others), risperidone (Risperdal and others), zolpidem (Ambien, Edluar, and others).
Author affiliations: Graduate Institute of Clinical Medical Science (Drs Huang, Tsai, Sung, and Kao), China Medical University; Neuroscience Laboratory and Department of Neurology (Drs Huang, Tsai, Lin, and Lu), Management Office for Health Data (Drs Muo and Sung), and Department of Nuclear Medicine and PET Center (Dr Kao), China Medical University Hospital; and Graduate Institute of Neural and Cognitive Sciences (Dr Tsai), School of Medicine, College of Medicine (Drs Huang, Tsai, Lin, Lu, Sung, and Kao), China Medical University, Taichung, Taiwan.
Author contributions: Drs Huang and Tsai contributed equally. Conception/design: Drs Huang, Tsai, and Kao; provision of study material or patients: Drs Lin, Lu, and Sung; collection and/or assembly of data: Dr Muo; data analysis and interpretation: Drs Huang, Tsai, and Kao; manuscript writing: Drs Huang, Tsai, Muo, and Kao; final approval of manuscript: all authors.
Potential conflicts of interest: None reported.
Funding/support: The study was supported in part by grants from the Ministry of Science and Technology (MOST 103-2314-B-039 -017 -MY2), Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW103-TDU-B-212-113002), Health and Welfare Surcharge of Tobacco Products, China Medical University Hospital Cancer Research Center of Excellence (MOHW103-TD-B-111-03, Taiwan), “Aim for the Top University Plan” of the National Chiao Tung University and Ministry of Education, and China Medical University Hospital (DMR-104-049), Taiwan.
Role of the sponsors: The funders had no role in study design, data collection and analysis; decision to publish; or preparation of the manuscript. There was no additional external funding received for this study.
Disclaimer: The views expressed in this article do not necessarily represent the views of China Medical University and Hospital.
Acknowledgments: The authors thank Chia-Ing Li, PhD, of China Medical University Hospital, Taichung, Taiwan, for statistical consulting. Dr Li has no conflicts of interest to report.
Additional information: The National Health Insurance Research Database (http://nhird.nhri.org.tw/date_01.html) is derived from a single-payer National Health Insurance Program by the Bureau of National Health Insurance, Taiwan, and is maintained by the National Health Research Institutes, Taiwan.
REFERENCES
1. Tanner CM, Goldman SM. Epidemiology of Parkinson’s disease. Neurol Clin. 1996;14(2):317-335. PubMed doi:10.1016/S0733-8619(05)70259-0
2. Luchetti S, Huitinga I, Swaab DF. Neurosteroid and GABA-A receptor alterations in Alzheimer’s disease, Parkinson’s disease and multiple sclerosis. Neuroscience. 2011;191:6-21. PubMed doi:10.1016/j.neuroscience.2011.04.010
3. Daniele A, Albanese A, Gainotti G, et al. Zolpidem in Parkinson’s disease. Lancet. 1997;349(9060):1222-1223. PubMed doi:10.1016/S0140-6736(05)62416-6
4. Evidente VG. Zolpidem improves dystonia in “Lubag” or X-linked dystonia-parkinsonism syndrome. Neurology. 2002;58(4):662-663. PubMed doi:10.1212/WNL.58.4.662
5. Chen YY, Sy HN, Wu SL. Zolpidem improves akinesia, dystonia and dyskinesia in advanced Parkinson’s disease. J Clin Neurosci. 2008;15(8):955-956. PubMed doi:10.1016/j.jocn.2007.07.082
6. Huang HY, Hsu YT, Wu YC, et al. Zolpidem improves neuropsychiatric symptoms and motor dysfunction in a patient with Parkinson’s disease after deep brain stimulation. Acta Neurol Taiwan. 2012;21(2):84-86. PubMed
7. Kao CH, Sun LM, Liang JA, et al. Relationship of zolpidem and cancer risk: a Taiwanese population-based cohort study. Mayo Clin Proc. 2012;87(5):430-436. PubMed doi:10.1016/j.mayocp.2012.02.012
8. Cheng CL, Kao YH, Lin SJ, et al. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20(3):236-242. PubMed doi:10.1002/pds.2087
9. Kang JH, Chen YH, Lin HC. Comorbidity profiles among patients with ankylosing spondylitis: a nationwide population-based study. Ann Rheum Dis. 2010;69(6):1165-1168. PubMed
10. Aarsland D, Pahlhagen S, Ballard CG, et al. Depression in Parkinson disease-epidemiology, mechanisms and management. Nat Neurol. 2012;8(1):35-47. PubMed
11. Bhidayasiri R, Truong DD. Therapeutic strategies for nonmotor symptoms in early Parkinson’s disease: the case for a higher priority and stronger evidence. Parkinsonism Relat Disord. 2012;18(suppl 1):S110-S113. PubMed doi:10.1016/S1353-8020(11)70035-9
12. Zoccolella S, Savarese M, Lamberti P, et al. Sleep disorders and the natural history of Parkinson’s disease: the contribution of epidemiological studies. Sleep Med Rev. 2011;15(1):41-50. PubMed doi:10.1016/j.smrv.2010.02.004
13. Mierzejewski P, Kolaczkowski M, Nowak N, et al. Pharmacological characteristics of zolpidem-induced catalepsy in the rat. Neurosci Lett. 2013;556:99-103. doi:10.1016/j.neulet.2013.10.011 PubMed
14. Milić M, Divljaković J, Rallapalli S, et al. The role of α1 and α5 subunit-containing GABAA receptors in motor impairment induced by benzodiazepines in rats. Behav Pharmacol. 2012;23(2):191-197. PubMed doi:10.1097/FBP.0b013e3283512c85
15. Licata SC, Platt DM, Cook JM, et al. Contribution of alpha1 subunit-containing gamma-aminobutyric acidA (GABAA) receptors to motor-impairing effects of benzodiazepines in squirrel monkeys. Psychopharmacology (Berl). 2009;203(3):539-546. PubMed doi:10.1007/s00213-008-1401-7
16. Muangpaisan W, Mathews A, Hori H, et al. A systematic review of the worldwide prevalence and incidence of Parkinson’s disease. J Med Assoc Thai. 2011;94(6):749-755. PubMed
Please sign in or purchase this PDF for $40.00.
Save
Cite