Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
To the Editor: Posttraumatic stress disorder (PTSD) is a heterogeneous condition comprising threat/fear (eg, intrusions, avoidance, hypervigilance) and loss/dysphoria (eg, numbing, dysphoric arousal) symptoms. Contemporary scientific efforts in psychiatry, such as the National Institute of Mental Health Research Domain Criteria project, are encouraging studies of the neurobiological and behavioral underpinnings of transdiagnostic aspects of mental disorders, such as threat and loss symptoms, with the goal of developing novel, mechanism-based classifications of psychopathology, which can be used to develop more targeted treatments.
In line with such efforts, we recently found that PTSD is associated with greater cannabinoid type 1 (CB1) receptor availability4 and that greater CB1 receptor availability in the amygdala was associated with increased threat, but not loss, symptoms in trauma survivors.
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
The rs1049353 Polymorphism in the CNR1 Gene Interacts With Childhood Abuse to Predict Posttraumatic Threat Symptoms
To the Editor: Posttraumatic stress disorder (PTSD) is a heterogeneous condition comprising threat/fear (eg, intrusions, avoidance, hypervigilance) and loss/dysphoria (eg, numbing, dysphoric arousal) symptoms.1,2 Contemporary scientific efforts in psychiatry, such as the National Institute of Mental Health Research Domain Criteria project, are encouraging studies of the neurobiological and behavioral underpinnings of transdiagnostic aspects of mental disorders, such as threat and loss symptoms, with the goal of developing novel, mechanism-based classifications of psychopathology, which can be used to develop more targeted treatments.3
In line with such efforts, we recently found that PTSD is associated with greater cannabinoid type 1 (CB1) receptor availability4 and that greater CB1 receptor availability in the amygdala was associated with increased threat, but not loss, symptoms in trauma survivors.5 Variation in the cannabinoid receptor type 1 (CNR1) gene may also contribute to risk for PTSD, with the A allele of rs1049353 associated with increased likelihood of PTSD.6 Additionally, rs1049353 has been found to interact with childhood physical abuse (CPA), one type of trauma that might impact the endocannabinoid system, to predict anhedonia.7 However, no study has examined associations between rs1049353 genotype—alone or interactively with CPA—and the phenotypic expression of PTSD symptoms.
Using data from the Detroit Neighborhood Health Study8,9 (DNHS), an epidemiologic study of predominantly African-American adults from urban Detroit for which data were collected from June 2008 to December 2013, we examined how rs1049353 genotype—alone and interactively with CPA—relates to severity of posttraumatic threat and loss symptoms. We hypothesized that rs1049353 genotype would specifically underlie threat, but not loss, symptoms.5 The University of Michigan Institutional Review Board approved the DNHS, and participants provided written informed consent.
Method. A total of 487 adults (mean age = 53.3 [SD = 15.6] years; 57.7% female; 82.7% African-American) provided valid data for rs1049353 and completed the PTSD Checklist10 and the Conflict Tactics Scale11 measure of CPA. We conducted a multivariate analysis of covariance (MANCOVA) to examine the relation between rs1049353 genotype (AA/AG vs GG) and CPA (alone and in interaction) as predictors of threat (sum of PTSD Checklist intrusion, avoidance, and anxious arousal) and loss (sum of numbing and dysphoric arousal) symptoms. Age, sex, lifetime trauma burden, and the first 2 principal components from a multidimensional scaling analysis of genome-wide data were included as covariates.
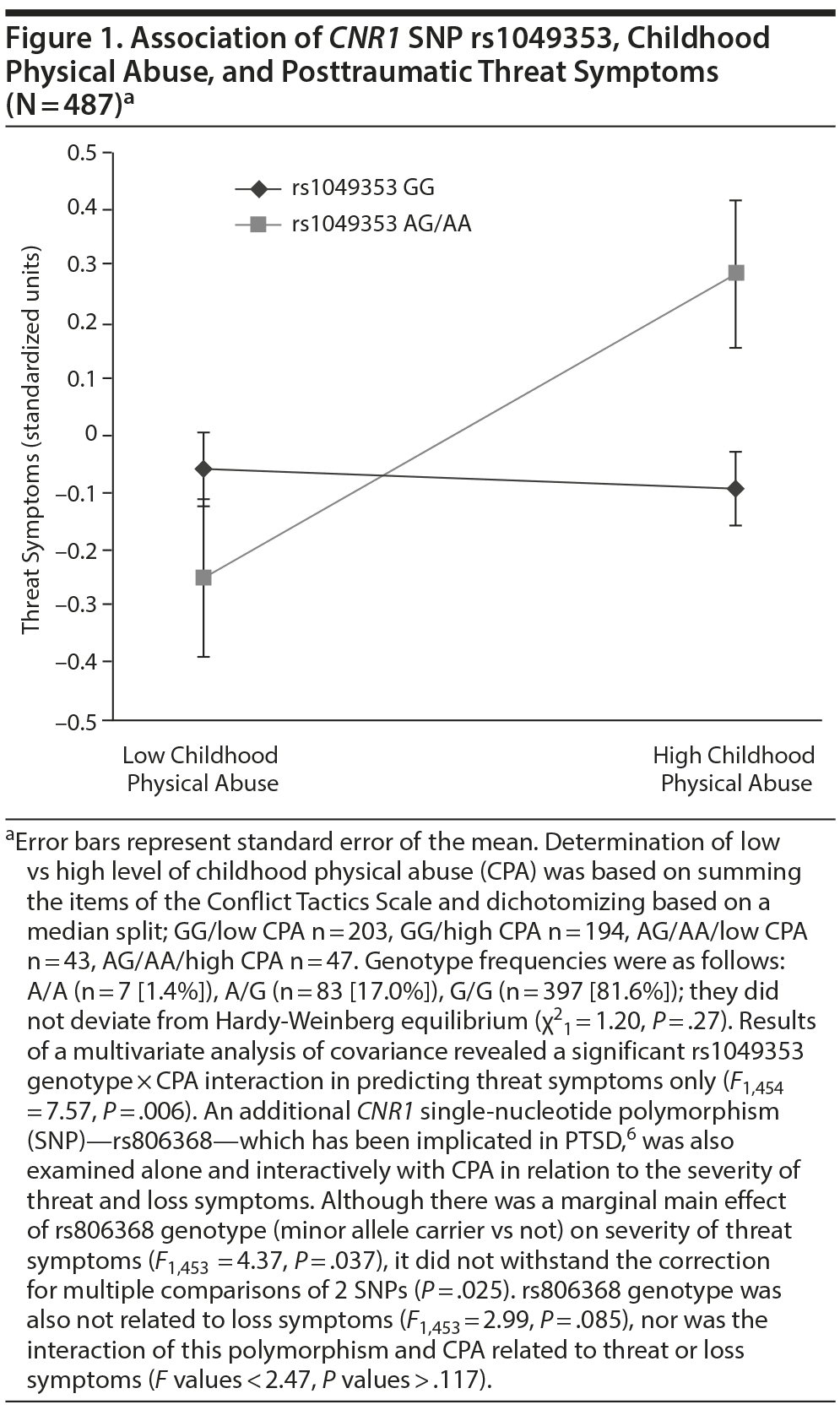
Results. CPA predicted threat symptoms (F1,454 = 5.59, P = .018), but main effects of rs1049353 genotype were nonsignificant (threat: F1,454 = 0.69, P = .41; loss: F1,454 = 0.51, P = .47). There was also a significant rs1049353 genotype ×— CPA interaction for threat symptoms only (F1,454 = 7.57, P = .006); minor A allele carriers with high levels of CPA reported greater threat symptoms (Figure 1). In a separate MANCOVA of the 5 PTSD symptom dimensions (reexperiencing, avoidance, anxious arousal, numbing, dysphoric arousal), this interaction also emerged for 2 of 3 dimensions of threat symptoms: avoidance (F1,454 = 6.13, P = .014) and reexperiencing (F1,454 = 6.13, P = .014).
The current findings extend our prior work implicating the endocannabinoid system in PTSD.4,5 The rs1049353 single-nucleotide polymorphism (SNP) has been found to interact with CPA to contribute to decreased anhedonia,7 and here we demonstrate that rs1049353 genotype interacts with CPA to increase severity of threat/fear, but not loss/dysphoria, symptoms of PTSD. The rs1049353 SNP is exonic, but synonymous, and may cause alterations in CNR1 protein formation during translation.7 Notably, a previous study7 observed a protective effect of the minor A allele in rs1049353 and CPA interaction in relation to anhedonia symptoms. One explanation for these results is differing demographic (eg, racial) compositions or trauma exposure characteristics of the samples; another is that the rs1049353 genotype interacts with level of CPA to predict posttraumatic threat symptoms in some CPA survivors and anhedonic symptoms in other samples of CPA survivors. Further research is needed to replicate these results and investigate underlying mechanisms.
References
1. Armour C, Carragher N, Elhai JD. Assessing the fit of the Dysphoric Arousal model across two nationally representative epidemiological surveys: the Australian NSMHWB and the United States NESARC. J Anxiety Disord. 2013;27(1):109-115. PubMed doi:10.1016/j.janxdis.2012.10.006
2. Pietrzak RH, Rosenheck RA, Cramer JA, et al. Elucidating the transdiagnostic dimensional structure of trauma-related psychopathology: findings from VA cooperative study 504 – Risperidone Treatment for Military Service Related Chronic Post Traumatic Stress Disorder. J Affect Disord. 2014;172C:331-336. PubMed
3. Cuthbert BN. The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13(1):28-35. PubMed doi:10.1002/wps.20087
4. Neumeister A, Normandin MD, Pietrzak RH, et al. Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: a positron emission tomography study. Mol Psychiatry. 2013;18(9):1034-1040. PubMed doi:10.1038/mp.2013.61
5. Pietrzak RH, Huang Y, Corsi-Travali S, et al. Cannabinoid type 1 receptor availability in the amygdala mediates threat processing in trauma survivors. Neuropsychopharmacology. 2014;39(11):2519-2528. PubMed doi:10.1038/npp.2014.110
6. Lu AT, Ogdie MN, Jפrvelin M-R, et al. Association of the cannabinoid receptor gene (CNR1) with ADHD and post-traumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(8):1488-1494. PubMed doi:10.1002/ajmg.b.30693
7. Agrawal A, Nelson EC, Littlefield AK, et al. Cannabinoid receptor genotype moderation of the effects of childhood physical abuse on anhedonia and depression. Arch Gen Psychiatry. 2012;69(7):732-740. PubMed doi:10.1001/archgenpsychiatry.2011.2273
8. Uddin M, Aiello AE, Wildman DE, et al. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proc Natl Acad Sci U S A. 2010;107(20):9470-9475. PubMed doi:10.1073/pnas.0910794107
9. Lowe SR, Meyers JL, Galea S, et al. RORA and posttraumatic stress trajectories: main effects and interactions with childhood physical abuse history. Brain Behav. 2015;5(4):e00323. PubMed doi:10.1002/brb3.323
10. Weathers F, Litz B, Herman D, et al. The PTSD Checklist [PCL]: reliability, validity, and diagnostic utility. Paper presented at the Annual Convention of the International Society for Traumatic Stress Studies; 1993; San Antonio, TX.
11. Straus MA. Measuring intrafamily conflict and violence: the Conflict Tactics (CT) Scales. J Marriage Fam. 1979;41(1):75-88. doi:10.2307/351733
aUS Department of Veterans Affairs National Center for Posttraumatic Stress Disorder, Clinical Neurosciences Division, VA Connecticut Healthcare System, West Haven
bDepartment of Psychiatry, Yale University School of Medicine, New Haven, Connecticut
cDepartment of Epidemiology, Mailman School of Public Health, Columbia University, New York, New York
dMitsubishi Tanabe Pharma Development America, Jersey City, New Jersey
eDepartment of Psychology, Carol R. Woese Institute for Genomic Biology, University of Illinois-Urbana Champaign, Champaign
fDepartment of Epidemiology, Gillings School of Global Public Health, University of North Carolina, Chapel Hill
gCarl R. Woese Institute for Genomic Biology and Department of Molecular and Integrative Physiology, University of Illinois, Urbana
hBoston University School of Public Health, Boston, Massachusetts
iDepartment of Epidemiology, Harvard T. H. Chan School of Public Health, Boston, Massachusetts
jCurrent affiliation: Department of Clinical Health Psychology, University of Manitoba, Winnipeg, Manitoba, Canada
Potential conflicts of interest: Dr Neumeister has received consulting fees from Pfizer. Dr Pietrzak is a scientific consultant to Cogstate. The other authors report no potential conflict of interest.
Funding/support: This research was funded by National Institutes of Health grants (R01DA022720, R01DA022720-S1 [PhenX], R01DA022720-S1 [Supplement], and RC1MH088283). Preparation of this report was supported in part by the US Department of Veterans Affairs National Center for Posttraumatic Stress Disorder and a private donation.
Additional information: Investigators interested in collaborating on a project using data from the Detroit Neighborhood Health Study (DNHS) can obtain information from the DNHS website: http://dnhs.unc.edu/data-requests/.
J Clin Psychiatry 2015;76(12):e1622-e1623
dx.doi.org/10.4088/JCP.15l10084
© Copyright 2015 Physicians Postgraduate Press, Inc.
This PDF is free for all visitors!
Save
Cite