ABSTRACT
Objective: Aripiprazole 2-month ready-to-use 960 mg (Ari 2MRTU 960) is a new long-acting injectable antipsychotic formulation for administration every 2 months. A randomized, open-label, 32-week trial evaluated the safety, tolerability, and pharmacokinetics of Ari 2MRTU 960 in clinically stable adults with schizophrenia or bipolar I disorder (per DSM-5 criteria). This secondary analysis evaluated the safety and efficacy of Ari 2MRTU 960 in the subpopulation of patients with schizophrenia.
Methods: Patients were randomized to receive Ari 2MRTU 960 every 56 ± 2 days (4 injections scheduled) or aripiprazole once-monthly 400 mg (AOM 400) every 28 ± 2 days (8 injections scheduled). Data were collected during August 2019–July 2020 across 16 US sites. Primary endpoints included safety and tolerability, evaluated throughout. Secondary endpoints for efficacy in patients with schizophrenia included change from baseline at week 32 in Positive and Negative Syndrome Scale, Clinical Global Impression – Severity, and Subjective Well-being under Neuroleptic Treatment – Short Form scores, along with Clinical Global Impression – Improvement at week 32.
Results: Patients with schizophrenia were randomized to Ari 2MRTU 960 (n = 92) or AOM 400 (n = 93). The incidence of treatment-emergent adverse events (TEAEs) was similar between Ari 2MRTU 960 (66.3%) and AOM 400 (63.4%). The most frequently reported TEAE was increased weight (Ari 2MRTU 960: 21.7%; AOM 400: 18.3%). Patients in both treatment groups remained clinically stable throughout, with minimal change from baseline observed in efficacy parameters at week 32.
Conclusions: Ari 2MRTU 960 was well tolerated in clinically stable patients with schizophrenia, with efficacy similar to AOM 400.
Trial Registration: ClinicalTrials.gov identifier: NCT04030143
J Clin Psychiatry 2023;84(5):23m14873
Author affiliations are listed at the end of this article.
Schizophrenia is a complex chronic disorder characterized by continuous or relapsing episodes of psychosis.1 The domains of dysfunction in schizophrenia include positive symptoms, cognition, and negative symptoms.2
Consistent pharmacologic treatment is important for achieving and maintaining symptom control in schizophrenia,3,4 but adherence to oral medication is often poor in this patient population, with ~30%–60% of patients not taking their medication as prescribed.5–7
Real-world evidence suggests that, compared with oral antipsychotics, long-acting injectable (LAI) formulations of antipsychotics are associated with improvements in treatment adherence in patients with schizophrenia.8–10 In addition, compared with oral antipsychotics, LAI use in schizophrenia is associated with lower odds of hospitalization, fewer hospitalizations, and fewer visits to emergency departments,11,12 and may also be associated with improved functioning and improved quality of life,11,12 particularly in clinically stable patients.13
Aripiprazole once-monthly 400 mg (AOM 400) is an extended-release aripiprazole monohydrate suspension for administration every 28 days via intramuscular injection.14,15 In the US, AOM 400 has been approved by the Food and Drug Administration (FDA) for the treatment of schizophrenia in adults and the maintenance monotherapy treatment of bipolar I disorder (BP-I) in adults.14 In Europe, AOM 400 has been approved for the maintenance treatment of schizophrenia in adult patients stabilized with oral aripiprazole.15
Aripiprazole 2-month ready-to-use 960 mg (Ari 2MRTU 960) is a new LAI formulation containing 960 mg of aripiprazole monohydrate, supplied in a pre-filled syringe for gluteal administration once every 2 months. In April 2023, Ari 2MRTU 960 received FDA approval for the treatment of schizophrenia and for maintenance monotherapy treatment of BP-I in adults in the US.16 The development of LAIs with different dosing intervals, and ready-to-use delivery systems, may increase the breadth of treatment options available to patients with schizophrenia.17,18
A randomized, multiple-dose, parallel-arm, pivotal trial was conducted to evaluate the safety, tolerability, and pharmacokinetics of multiple doses of Ari 2MRTU 960 compared with AOM 400 in clinically stable adult patients with schizophrenia or BP-I. The outcomes of this trial in the full patient population have been published elsewhere.19 Data are reported here for the safety, tolerability, and efficacy of multiple doses of Ari 2MRTU 960 compared with AOM 400 for the subpopulation of patients with schizophrenia, to help guide clinical decisions concerning the treatment of adult patients with schizophrenia. Data for the subpopulation of patients with BP-I are reported elsewhere.20
METHODS
Study Design and the Subpopulation of Patients With Schizophrenia
This was an open-label, multiple-dose, randomized, parallel-arm, multicenter trial, enrolling patients across 16 sites in the United States.19 The trial started on August 1, 2019, and was completed on July 8, 2020.19 Study design is shown in Supplementary Figure 1; full details of the study design have been published elsewhere.19 The study was conducted in accordance with the International Council for Harmonization Good Clinical Practice guidelines and local regulatory requirements. The study protocol was approved by the governing institutional review board or independent ethics committee for each investigational site, and the study was registered at ClinicalTrials.gov (identifier: NCT04030143). All patients provided written informed consent prior to the start of the study.
Key inclusion criteria for the subpopulation of patients with schizophrenia were age 18–64 years; current diagnosis of schizophrenia (as defined by Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [DSM-5] criteria)21; body mass index of 18–35 kg/m2; good physical health; clinical stability on an atypical antipsychotic medication (except for clozapine, which was not permitted) for ≥ 2 months prior to screening; and prior history of tolerating oral aripiprazole and/or AOM 400 (according to the investigator’s judgment). Patients without a history of tolerating aripiprazole received 3 single oral aripiprazole doses of 10 mg on 3 consecutive days (30 mg in total, in addition to their current oral antipsychotic) during the screening period to establish tolerability.
Key exclusion criteria for the subpopulation of patients with schizophrenia were substance use disorder (as defined by DSM-5 criteria)21 within the past 180 days, or a positive test for drugs of abuse (excluding nicotine, alcohol, and marijuana with clinical rationale, including rationale/indication for use, review of patterns and frequency of use, and justification to support compliance with the protocol for the intended duration of treatment); use of any cytochrome P450 (CYP)2D6 and CYP3A4 inhibitors or CYP3A4 inducers within 14 days (fluoxetine or fluoxetine/olanzapine within 28 days) prior to dosing, for the duration of the trial, and 30 days after the last study drug dose; current acute relapse of schizophrenia; current DSM-5 diagnosis other than schizophrenia21; a significant risk of committing suicide (based on history, routine psychiatric status examination, investigator’s judgment, or a “yes” answer to questions 4 or 5 on the Columbia-Suicide Severity Rating Scale [C-SSRS] questionnaire [active suicidal ideation with some intent to act, without specific plan, or with specific plan and intent, currently or over the last 6 months])22; treatment resistance to an atypical antipsychotic medication; and history of neuroleptic malignant syndrome or clinically significant tardive dyskinesia (as assessed by the investigator).
Study Interventions
Study interventions were delivered as a single injection in the gluteal muscle, administered by an investigator at a clinical trial site every 56 ± 2 days for Ari 2MRTU 960 (injection volume 3.2 mL; 4 injections scheduled in total) or every 28 ± 2 days for AOM 400 (injection volume 2.0 mL; 8 injections scheduled in total) over the course of 32 weeks. In case of safety and tolerability issues, a 1-time dose decrease to 660 mg for Ari 2MRTU 960 and to 300 mg for AOM 400 was allowed, along with a 1-time subsequent increase back to 960 mg for Ari 2MRTU 960 and to 400 mg for AOM 400.
For patients stabilized on oral antipsychotic treatment, overlapping oral antipsychotic treatment was administered for 7 days after the first administration of Ari 2MRTU 960 or for 14 days after the first administration of AOM 400. For patients stabilized on a non-aripiprazole oral antipsychotic, they either continued to receive their current oral antipsychotic for the period of overlapping oral antipsychotic treatment or switched to 10–20 mg oral aripiprazole per day, depending on which pharmacokinetic sampling schedule they were assigned to (sparse or robust). There was no oral overlap for participants stabilized on AOM 400. For additional detail on the study design and interventions, see Supplementary Figure 1.
Endpoints
Safety endpoints were evaluated as a primary objective of the study and were based on reported adverse events (AEs; all AEs were coded by system organ class and the Medical Dictionary for Regulatory Activities [MedDRA] preferred term); investigator’s assessment of most recent injection site for symptoms of pain, swelling, redness, and induration; Visual Analog Scale (VAS)23 scores for patient-reported rating of pain at the most recent injection site (range 0 [no pain] to 100 [extreme pain]); motoric (extrapyramidal) symptoms, assessed by the Simpson–Angus Scale (SAS) for parkinsonian adverse effects,24 Abnormal Involuntary Movement Scale (AIMS) for dyskinetic movements,25 and Barnes Akathisia Rating Scale (BARS) for akathisia26; vital signs, electrocardiograms (ECGs), clinical laboratory monitoring (serum chemistry, hematology, and urinalysis), physical examinations; and suicidality (assessed by the C-SSRS).22
Efficacy was evaluated as a secondary objective of the study, to determine whether patients remained clinically stable throughout the study. Scales used included the Positive and Negative Syndrome Scale (PANSS)27 and Clinical Global Impression – Severity (CGI-S).25 Patients were also evaluated using the Clinical Global Impression – Improvement (CGI-I)25 and Subjective Well-being under Neuroleptic Treatment – Short Form (SWN-S).28 Change from baseline at week 32 was evaluated for all scales, except for the CGI-I; CGI-I score was evaluated at week 32. For more detail on the scales used to evaluate study treatment efficacy, see Supplementary Table 1. A schedule of all assessments is shown in Supplementary Table 2.
Statistical Analysis
This was a post hoc analysis to evaluate the safety, tolerability, and efficacy of multiple administrations of Ari 2MRTU 960 versus AOM 400 in the subpopulation of patients with schizophrenia from an open-label, multiple-dose, randomized trial.19
All randomized patients with schizophrenia who received at least 1 study drug dose, regardless of any protocol violation, were included in the safety analysis.
All randomized patients with schizophrenia who received at least 1 study drug dose and had at least 1 efficacy assessment were included in the efficacy analysis. As the study enrolled clinically stable patients, minimal change from baseline in efficacy outcomes was expected, and the study was not powered to show statistical significance of any change from baseline in efficacy outcomes.
Differences between the two treatment groups in safety outcomes, including study completion rate, were evaluated using a t test (for continuous variables) or a Fisher exact test (for categorical variables).
The last observation carried forward (LOCF) method was used to impute missing data for the motoric assessment scales at post-baseline visits. Change from baseline in the PANSS, CGI-S, and SWN-S scores was summarized using descriptive statistics and evaluated using a mixed model for repeated measures (MMRM) with a restricted maximum likelihood approach, including categorically fixed effects of treatment, trial week, and treatment-by–trial week interaction, pharmacokinetic sampling schedule (sparse or robust) for determining the concentration of aripiprazole in patients’ plasma, as well as the covariates of baseline-score-by-week interaction in addition to patient as random effect. An unstructured covariance matrix was used to model the within-patient errors, and the Kenward-Roger degree of freedom was used to test the fixed effects. The P values from the MMRM analysis were used to assess the difference in the estimated treatment effects between the Ari 2MRTU 960 and the AOM 400 groups.
RESULTS
Patients
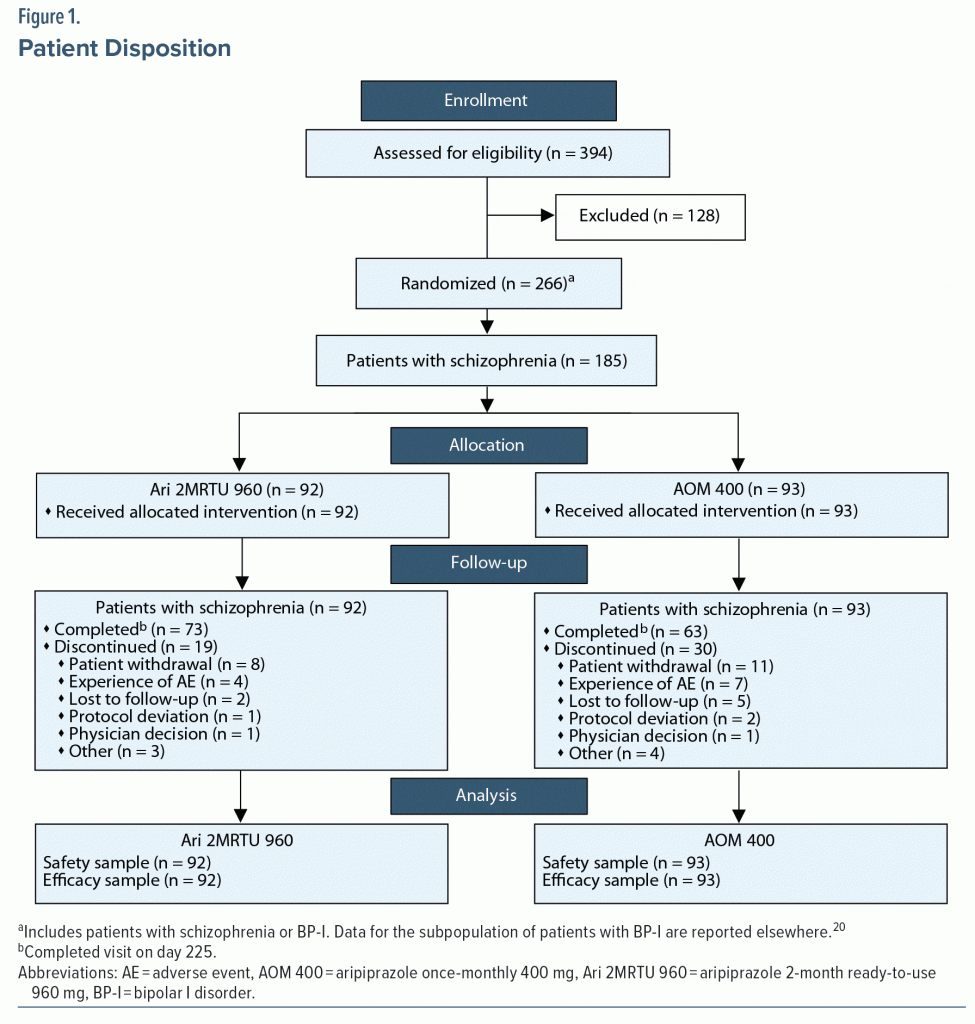
Of the 394 patients who were screened, 266 were enrolled into the study, including 185 patients with schizophrenia. Patient disposition is shown in Figure 1. Data for the subpopulation of patients with BP-I (n = 81) are reported elsewhere.20 Patients with schizophrenia were randomized to receive Ari 2MRTU 960 (n = 92) or AOM 400 (n = 93).
Study completion rate was 79.3% (73/92 patients) in the Ari 2MRTU 960 group and 67.7% (63/93 patients) in the AOM 400 group (P = .0954). In both groups, the most common reason for study discontinuation was patient withdrawal, reported in 8.7% (8/92) of patients in the Ari 2MRTU 960 group and in 11.8% (11/93) of patients in the AOM 400 group (P = .6293; Figure 1).
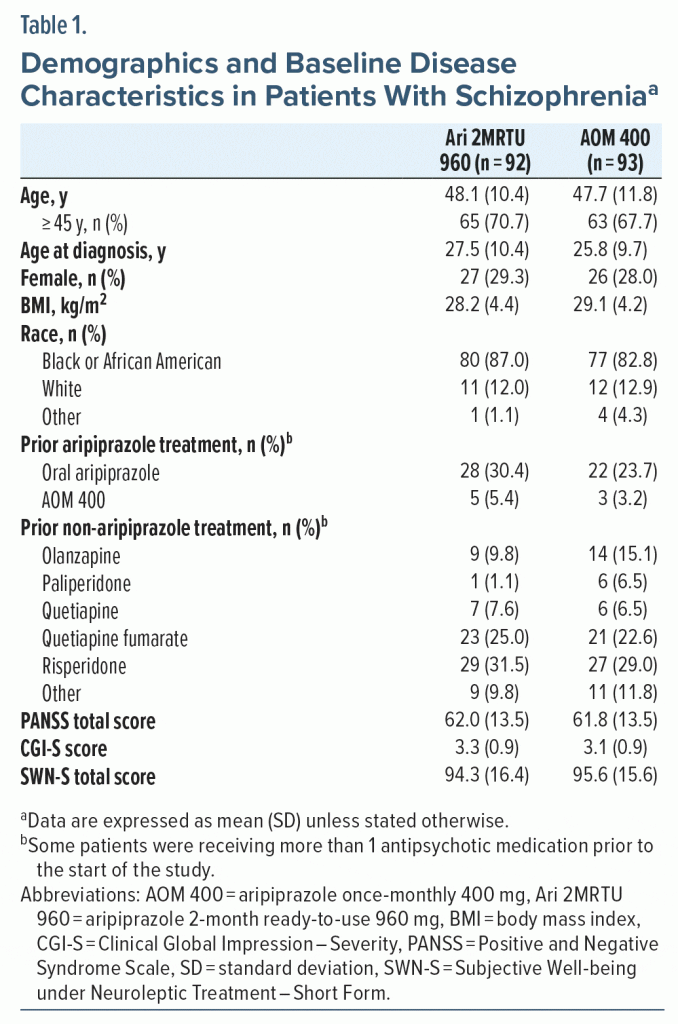
Demographic characteristics and baseline disease characteristics were well balanced between groups (Table 1). No statistically significant differences were observed between the two treatment groups in the number of patients receiving prior aripiprazole or non-aripiprazole treatment (data not shown).
Safety
All 185 randomized patients with schizophrenia received at least 1 study drug dose and were included in the safety analyses. Multiple doses of Ari 2MRTU 960 into the gluteal muscle were generally well tolerated. No patient in either treatment group required a dose adjustment during the study.
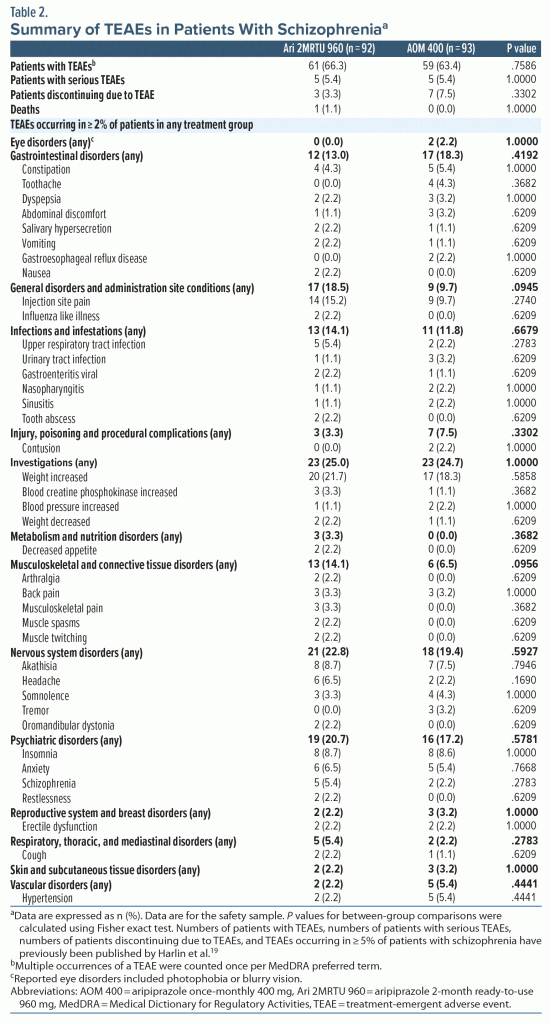
Overall, 64.9% of patients with schizophrenia (120/185) experienced treatment-emergent AEs (TEAEs; 66.3% [61/92] in the Ari 2MRTU 960 group and 63.4% [59/93] in the AOM 400 group). The overall incidence of TEAEs and serious TEAEs was similar between the two treatment groups. Most TEAEs in either treatment group occurred following the first injection, with a lower incidence of TEAEs with subsequent injections of Ari 2MRTU 960 or AOM 400. Most TEAEs were mild or moderate in severity. A summary of TEAEs is presented in Table 2.
Serious TEAEs (ie, TEAEs resulting in hospitalization, prolonged hospitalization, life endangerment, persistent or significant disability, or death)29 occurred in 5 patients in each treatment group. No one type of serious TEAE occurred in more than 1 patient. In the Ari 2MRTU 960 group, serious TEAEs were cardiac arrest, cellulitis, akathisia, auditory hallucination, and psychotic disorder (ie, worsening of psychosis). In the AOM 400 group, serious TEAEs were acute cholecystitis, septic shock, adenocarcinoma of colon, psychotic disorder (ie, worsening of psychosis), schizophrenia (ie, worsening of schizophrenia), and a suicide attempt. No trends were observed with respect to timing of serious TEAE onset and number of study drug injections received. There was 1 death (cardiac arrest) in a 52-year-old patient with schizophrenia in the Ari 2MRTU 960 group (on day 211, 41 days after the patient’s final dose of study drug), which was assessed as unrelated to the study drug by both the investigator and the sponsor. The participant’s relevant medical history (including hypertension, diabetes mellitus, tobacco consumption, postmenopausal status, and vitamin D deficiency) was considered a potential risk factor for the reported fatal event. During the trial, there were no changes to the medications for the ongoing treatment of hypertension, and there were no significant changes to the participant’s ECG findings during the study visits compared with their ECG findings during the screening period (Supplementary Table 3).
The most frequently reported TEAEs were increased weight, injection site pain, akathisia, and insomnia (Table 2). The incidence of increased weight, akathisia, and insomnia was comparable between treatment groups. None of the cases of increased weight, injection site pain, or insomnia were considered severe or serious by the investigator. At the end of the study, potentially clinically significant weight gain of ≥ 7% was reported in 39.7% of patients in the Ari 2MRTU 960 group (29 of the 73 patients for whom post-baseline weight assessment data were available) and in 38.1% of patients in the AOM 400 group (24 of the 63 patients for whom post-baseline weight assessment data were available; P = .8619).
Injection site pain was experienced by 15.2% of patients (14/92) in the Ari 2MRTU 960 group and 9.7% of patients (9/93) in the AOM 400 group (P = .2740). Any injection site pain experienced following an Ari 2MRTU 960 or an AOM 400 injection was rated as “mild” or “moderate” in severity, and all events occurred within 2 days of the injection. Most events occurred following the first injection of either study drug. No symptoms of swelling or induration were observed by the investigators after any injection. In > 90% of patients, the investigator’s assessment of the most recent injection site (reported using a 4-point categorical scale [absent, mild, moderate, severe]) was rated as “absent” for symptoms of pain and redness after the first and last injections in either treatment group.
Overall, mean (SD) and median VAS scores for pain were low and similar between the groups following the first and the last injection (VAS range: 0 to 100, where 0 represents no pain and 100 the worst possible pain). Mean (SD) VAS scores were 3.9 (9.96) following the first injection and 1.5 (4.58) following the last injection in the Ari 2MRTU 960 group, and 2.9 (5.40) following the first injection and 1.3 (2.79) following the last injection in the AOM 400 group. Median VAS scores following the last injection in both groups were 1. There was no significant difference between the two treatment groups in mean VAS scores after the first injection (P = .3859) or after the last injection (P = .7427).
Motoric TEAEs were reported in 15.2% of patients (14/92) in the Ari 2MRTU 960 group and in 11.8% of patients (11/93) in the AOM 400 group (P = .5265). The most frequently observed motoric adverse event was akathisia, reported in 8.7% of patients (8/92) in the Ari 2MRTU 960 group and 7.5% of patients (7/93) in the AOM 400 group (Table 2). In total, 13.0% of patients (12/92) in the Ari 2MRTU 960 group and 8.6% of patients (8/93) in the AOM 400 group received anticholinergic treatments for motoric TEAEs (P = .3544). No notable improvement or decline from baseline was observed in motoric rating scale scores in either treatment group (Supplementary Table 4).
No notable differences between groups were observed in laboratory test results, vital signs, or ECG parameters.
Suicidality, suicidal ideation, and emergence of suicidal ideation were reported in 3 patients in the Ari 2MRTU 960 group; suicidality, suicidal ideation, and the emergence of suicidal ideation were reported in 2 patients in the AOM 400 group, and suicidal behavior and emergence of suicidal behavior were reported in 1 patient in the AOM 400 group.
Efficacy
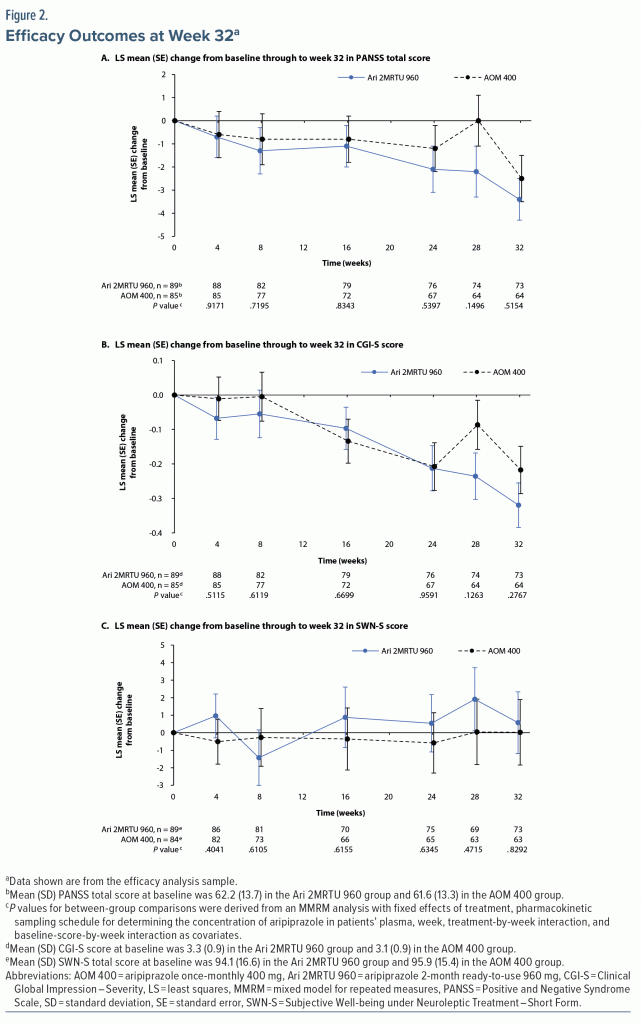
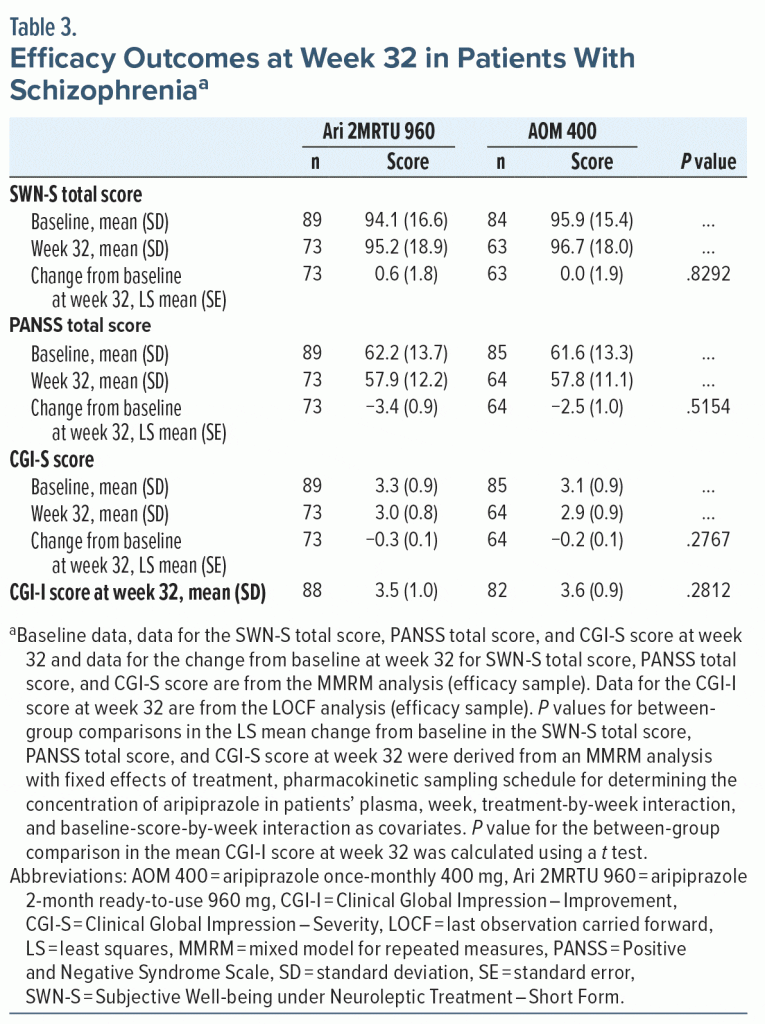
The study population evaluated in this secondary analysis consisted of patients with schizophrenia who were clinically stable at baseline. Assessments of efficacy at week 32 using the MMRM analysis showed that, on average, patients in both treatment arms remained clinically stable throughout the treatment period of this open-label study. The MMRM analysis showed that there was minimal change from baseline, and minimal difference between treatment groups, in the PANSS total, CGI-S, and SWN-S (Figure 2 and Table 3). Mean (SD) CGI-I score at week 32 was similar in the two groups, with a score of 3.5 (1.0) reported in the Ari 2MRTU 960 group and 3.6 (0.9) reported in the AOM 400 group (Table 3).
DISCUSSION
In this study, Ari 2MRTU 960 was well tolerated by patients with schizophrenia, with a discontinuation rate similar to that observed for AOM 400 in previous trials.30–32 While the study completion rate was nominally higher in the Ari 2MRTU 960 group (79.3% of patients [73/92]) than in the AOM 400 group (67.7% of patients [63/93]), the difference between the groups was not statistically significant (P = .0954). The overall incidence rates of TEAEs or serious TEAEs with Ari 2MRTU 960 were comparable with those observed in the AOM 400 treatment group and in previous trials evaluating AOM 400.30–32
Minimal change from baseline was observed at the end of the study in either treatment group in the efficacy endpoints assessed, which was the expected outcome given the trial population of clinically stable patients with schizophrenia. SWN-S was included among the efficacy endpoints to provide an outcome measure evaluating patients’ health-related quality of life and treatment satisfaction, as patients’ subjective feeling of well-being has been shown to have a major impact on medication adherence.28 An analysis of SWN-S scores of 212 patients with schizophrenia showed a low correlation (r = –0.19) with their PANSS scores,28 suggesting that the subjective well-being of patients with schizophrenia cannot be predicted from the outcomes of objective psychopathology assessments. SWN-S scores of ~95 were observed in either treatment group at baseline and at week 32, which indicate a generally positive attitude of patients toward their prior treatment that did not change with the initiation of Ari 2MRTU 960. SWN-S scores observed in this study are comparable to those previously reported for AOM 400 in the QUALIFY study that compared AOM 400 with paliperidone palmitate.33
Overall, the results of the present secondary analysis suggest that Ari 2MRTU 960 and AOM 400 are comparable in their safety, tolerability, and efficacy in the treatment of clinically stable patients with schizophrenia. Maintenance of clinical stability in schizophrenia is important, as outcomes worsen with repeated relapses34; LAIs are associated with a reduced risk of relapse compared with oral antipsychotics.12 No trend was observed with respect to schizophrenia symptoms emerging toward the end of the 2-month dosing interval. Patients may prefer an LAI with a 2-month dosing interval, such as Ari 2MRTU 960, as longer intervals between administrations may provide greater convenience and reduce any discomfort associated with injection site pain or fear of needles,35 with more time between injections to focus on self-care and relationships.36–38 These could all be possible reasons behind the numerically lower discontinuation rate for Ari 2MRTU 960 compared with AOM 400 in this study.
The symptomatic stability observed throughout the study in both treatment groups is paralleled by the similar pharmacokinetic profiles reported for Ari 2MRTU 960 and AOM 400 in the full study population of patients with schizophrenia or BP-I.19 Mean aripiprazole plasma exposure over the study period was comparable between treatment groups, and mean aripiprazole plasma concentrations remained above the previously determined aripiprazole efficacy threshold of ≥ 95 ng/mL,19 which has been associated with a reduced risk of relapse in patients with schizophrenia,39 and within the therapeutic reference range for aripiprazole (100–350 ng/mL).40
There are several limitations of this study. It was open-label in design, and it was not powered to show statistical significance of change from baseline in efficacy outcomes, as efficacy was a secondary objective of the study. In addition, the study was conducted in the US only. Study strengths include the detailed evaluation of safety and tolerability parameters, and the large patient sample included.
CONCLUSION
Multiple doses of Ari 2MRTU 960 were well tolerated in patients with schizophrenia, with a safety profile consistent with that of AOM 400. Ari 2MRTU 960 and AOM 400 showed comparable efficacy in maintaining symptomatic stability in clinically stable patients with schizophrenia. Study completion rate was higher with Ari 2MRTU 960 than with AOM 400.
Due to its extended dosing interval compared with that of AOM 400 and the associated reduction in burden on patients and clinicians, Ari 2MRTU 960 has the potential to improve adherence to antipsychotic treatment and enhance the care available to patients with schizophrenia. Ari 2MRTU 960 will be provided in a ready-to-use pre-filled syringe, which is expected to be an easy-to-use method of administration.
Article Information
Published Online: September 4, 2023. https://doi.org/10.4088/JCP.23m14873
© 2023 Physicians Postgraduate Press, Inc.
Submitted: March 20, 2023; accepted July 11, 2023.
To Cite: Citrome L, Such P, Yildirim M, et al. Safety and efficacy of aripiprazole 2-month ready-to-use 960 mg: secondary analysis of outcomes in adult patients with schizophrenia in a randomized, open-label, parallel-arm, pivotal study. J Clin Psychiatry. 2023;84(5):23m14873
Author Affiliations: Department of Psychiatry and Behavioral Sciences, New York Medical College, Valhalla (Citrome); H. Lundbeck A/S, Valby, Denmark (Such, Yildirim, Larsen); Otsuka Pharmaceutical Development & Commercialization Inc., Princeton, New Jersey (Madera-McDonough, Zhang, Harlin); Otsuka Pharmaceutical Europe Ltd., Wexham, United Kingdom (Beckham).
Corresponding Author: Matthew Harlin, MS, Otsuka Pharmaceutical Development & Commercialization Inc., 508 Carnegie Center Dr, Princeton, NJ 08540 ([email protected]).
Author Contributions: Trial conception and design: (Harlin, Larsen). Data collection and/or analysis: (all authors). Drafting or critical review of the article: (all authors). All authors gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Relevant Financial Relationships: Dr Citrome has served as consultant to AbbVie/Allergan, Acadia, Adamas, Alkermes, Angelini, Astellas, Avanir, Axsome, BioXcel, Boehringer Ingelheim, Cadent Therapeutics, Cerevel, Clinilabs, COMPASS, Eisai, Enteris BioPharma, HLS Therapeutics, Idorsia, Impel, INmune Bio, Intra-Cellular Therapies, Janssen, Karuna, Lundbeck, Lyndra, Marvin, Medavante-ProPhase, Merck, Mitsubishi-Tanabe Pharma, Neurelis, Neurocrine, Novartis, Noven, Otsuka, Ovid, Praxis, Recordati, Relmada, Reviva, Sage, Sunovion, Supernus, Teva, University of Arizona, and Vanda and has done one-off ad hoc consulting for individuals/entities conducting marketing, commercial, or scientific scoping research. He has served as speaker for AbbVie/Allergan, Acadia, Alkermes, Angelini, Axsome, BioXcel, Eisai, Idorsia, Intra-Cellular Therapies, Janssen, Lundbeck, Neurocrine, Noven, Otsuka, Recordati, Sage, Sunovion, Takeda, and Teva and contributed to CME activities organized by medical education companies such as Medscape, NACCME, NEI, Vindico, and universities and professional organizations/societies. He holds a small number of shares of common stock with Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Merck, and Pfizer (purchased >10 years ago). He currently has options for acquiring stocks with Reviva. He has received royalties or income from publishing from Taylor & Francis (Editor-in-Chief, Current Medical Research and Opinion, 2022–date), Wiley (Editor-in-Chief, International Journal of Clinical Practice, through end 2019), UpToDate (reviewer), Springer Healthcare (book), and Elsevier (Topic Editor, Psychiatry, Clinical Therapeutics). Drs Madera-McDonough and Zhang and Mr Harlin are full-time employees of Otsuka Pharmaceutical Development & Commercialization Inc. Ms Beckham is a full-time employee of Otsuka Pharmaceutical Europe Ltd. Drs Yildirim, Such, and Larsen are full-time employees of H. Lundbeck A/S.
Funding/Support: This work was sponsored by Otsuka Pharmaceutical Development & Commercialization Inc. (Princeton, New Jersey, USA) and H. Lundbeck A/S (Valby, Denmark).
Role of the Funders/Sponsors: The sponsors were involved in the design of the study, the collection, analysis and interpretation of data, the writing and reviewing of this article, and the decision to submit the article for publication.
Acknowledgments: Writing support was provided by Babette Jamieson, MSc, assisted by her colleagues at Cambridge – a Prime Global Agency (Knutsford, UK), and funded by Otsuka Pharmaceutical Development & Commercialization Inc. and H. Lundbeck A/S. The authors thank Calvin Liu, MS, Otsuka Pharmaceutical Development & Commercialization Inc., for programming support in data analysis.
Previous Presentation: This is a secondary analysis of study drug safety and efficacy outcomes from study NCT04030143 (https://clinicaltrials.gov/ct2/show/NCT04030143), in a subpopulation of patients with schizophrenia; results for the full study population, comprising patients with schizophrenia or bipolar I disorder, have been published in Harlin et al. CNS Drugs. 2023;37(4):337–350. Data for the subpopulation of patients with bipolar I disorder are reported elsewhere (McIntyre et al. Curr Med Res Opin. 2023;39[7]:1021–1030). Some of the data in this article have previously been reported in a poster presented at the NEI Congress 2022, November 3–6, 2022; Colorado Springs, Colorado, USA; Poster 60.
Additional Information: To submit inquiries related to Otsuka clinical research, or to request access to individual participant data (IPD) associated with any Otsuka clinical trial, please visit https://clinical-trials.otsuka.com/. For all approved IPD access requests, Otsuka will share anonymized IPD on a remotely accessible data sharing platform.
ORCID: Leslie Citrome: https://orcid.org/0000-0002-6098-9266; Pedro Such: https://orcid.org/0000-0003-1905-1142; Murat Yildirim: https://orcid.org/0000-0001-6192-1047; Jessica Madera-McDonough: https://orcid.org/0000-0003-2887-7480; Clodagh Beckham: https://orcid.org/0009-0003-2351-2519; Zhen Zhang: https://orcid.org/0000-0001-8448-5663; Frank Larsen: https://orcid.org/0000-0002-8318-1681; Matthew Harlin: https://orcid.org/0000-0001-7087-2734
Supplementary Material: Available at Psychiatrist.com.
Clinical Points
- Aripiprazole 2-month ready-to-use 960 mg (Ari 2MRTU 960) is a new long-acting injectable (LAI) antipsychotic, administered every 2 months. Its safety, tolerability, and efficacy were compared with the LAI aripiprazole once-monthly 400 mg (AOM 400).
- Ari 2MRTU 960 was well tolerated in clinically stable patients with schizophrenia, with efficacy similar to AOM 400, and may enhance treatment of schizophrenia.
References (40)

- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition, Text Revision. American Psychiatric Association; 2022.
- Tamminga CA. Domains of dysfunction in schizophrenia: implications for diagnosis. World Psychiatry. 2008;7(1):34–35. PubMed CrossRef
- Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39–59. PubMed CrossRef
- American Psychiatric Association. Practice Guideline for the Treatment of Patients With Schizophrenia. 3rd ed. American Psychiatric Association; 2021.
- Yaegashi H, Kirino S, Remington G, et al. Adherence to oral antipsychotics measured by electronic adherence monitoring in schizophrenia: a systematic review and meta-analysis. CNS Drugs. 2020;34(6):579–598. PubMed CrossRef
- Tiihonen J, Haukka J, Taylor M, et al. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011;168(6):603–609. PubMed CrossRef
- Thieda P, Beard S, Richter A, et al. An economic review of compliance with medication therapy in the treatment of schizophrenia. Psychiatr Serv. 2003;54(4):508–516. PubMed CrossRef
- Yan T, Greene M, Chang E, et al. Medication adherence and discontinuation of aripiprazole once-monthly 400 mg (AOM 400) versus oral antipsychotics in patients with schizophrenia or bipolar I disorder: a real-world study using US claims data. Adv Ther. 2018;35(10):1612–1625. PubMed CrossRef
- Rubio JM, Taipale H, Tanskanen A, et al. Long-term continuity of antipsychotic treatment for schizophrenia: a nationwide study. Schizophr Bull. 2021;47(6):1611–1620. PubMed CrossRef
- Weiser M, Davis JM, Brown CH, et al. Differences in antipsychotic treatment discontinuation among veterans with schizophrenia in the U.S. Department of Veterans Affairs. Am J Psychiatry. 2021;178(10):932–940. PubMed CrossRef
- Lin D, Thompson-Leduc P, Ghelerter I, et al. Real-world evidence of the clinical and economic impact of long-acting injectable versus oral antipsychotics among patients with schizophrenia in the United States: a systematic review and meta-analysis. CNS Drugs. 2021;35(5):469–481. PubMed CrossRef
- Kishimoto T, Hagi K, Kurokawa S, et al. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry. 2021;8(5):387–404. PubMed CrossRef
- Montemagni C, Frieri T, Rocca P. Second-generation long-acting injectable antipsychotics in schizophrenia: patient functioning and quality of life. Neuropsychiatr Dis Treat. 2016;12:917–929. PubMed
- Abilify Maintena (aripiprazole). US package insert. Otsuka America Pharmaceutical, Inc.; June 2020.
- Abilify Maintena (aripiprazole). EU package insert. Otsuka Pharmaceutical Netherlands B.V.; October 2021.
- Abilify Asimtufii (aripiprazole). US package insert. Otsuka America Pharmaceutical, Inc.; April 2023.
- Citrome L. Long-acting injectable antipsychotics update: lengthening the dosing interval and expanding the diagnostic indications. Expert Rev Neurother. 2017;17(10):1029–1043. PubMed CrossRef
- Citrome L. Long-acting injectable antipsychotics: what, when, and how. CNS Spectr. 2021;26(2):118–129. PubMed CrossRef
- Harlin M, Yildirim M, Such P, et al. A randomized, open-label, multiple-dose, parallel-arm, pivotal study to evaluate the safety, tolerability, and pharmacokinetics of aripiprazole 2-month long-acting injectable in adults with schizophrenia or bipolar I disorder. CNS Drugs. 2023;37(4):337–350. PubMed CrossRef
- McIntyre RS, Such P, Yildirim M, et al. Safety and efficacy of aripiprazole 2-month ready-to-use 960 mg: secondary analysis of outcomes in adult patients with bipolar I disorder in a randomized, open-label, parallel-arm, pivotal study. Curr Med Res Opin. 2023;39(7):1021–1030. PubMed CrossRef
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. American Psychiatric Association; 2013.
- Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266–1277. PubMed CrossRef
- Delgado DA, Lambert BS, Boutris N, et al. Validation of digital Visual Analog Scale pain scoring with a traditional paper-based Visual Analog Scale in adults. J Am Acad Orthop Surg Glob Res Rev. 2018;2(3):e088. PubMed CrossRef
- Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand suppl. 1970;45(S212):11–19. PubMed CrossRef
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Revised. National Institute of Mental Health; 1976.
- Barnes TRE. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154(5):672–676. PubMed CrossRef
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. PubMed CrossRef
- Naber D, Moritz S, Lambert M, et al. Improvement of schizophrenic patients’ subjective well-being under atypical antipsychotic drugs. Schizophr Res. 2001;50(1–2):79–88. PubMed CrossRef
- US Food & Drug Administration. What Is a Serious Adverse Event? Updated February 2, 2016. Accessed November 18, 2022. https://www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event
- Kane JM, Sanchez R, Perry PP, et al. Aripiprazole intramuscular depot as maintenance treatment in patients with schizophrenia: a 52-week, multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2012;73(5):617–624. PubMed CrossRef
- Fleischhacker WW, Sanchez R, Perry PP, et al. Aripiprazole once-monthly for treatment of schizophrenia: double-blind, randomised, non-inferiority study. Br J Psychiatry. 2014;205(2):135–144. PubMed CrossRef
- Naber D, Hansen K, Forray C, et al. QUALIFY: a randomized head-to-head study of aripiprazole once-monthly and paliperidone palmitate in the treatment of schizophrenia. Schizophr Res. 2015;168(1-2):498–504. PubMed CrossRef
- Potkin SG, Loze JY, Forray C, et al. Multidimensional assessment of functional outcomes in schizophrenia: results from QUALIFY, a head-to-head trial of aripiprazole once-monthly and paliperidone palmitate. Int J Neuropsychopharmacol. 2017;20(1):40–49. PubMed CrossRef
- Correll CU, Lauriello J. Using long-acting injectable antipsychotics to enhance the potential for recovery in schizophrenia. J Clin Psychiatry. 2020;81(4):MS19053AH5C. PubMed CrossRef
- Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1–24. PubMed CrossRef
- Pietrini F, Albert U, Ballerini A, et al. The modern perspective for long-acting injectables antipsychotics in the patient-centered care of schizophrenia. Neuropsychiatr Dis Treat. 2019;15:1045–1060. PubMed CrossRef
- Pungor K, Sanchez P, Pappa S, et al. The Patient, Investigator, Nurse, Carer Questionnaire (PINC-Q): a cross-sectional, retrospective, non-interventional study exploring the impact of less frequent medication administration with paliperidone palmitate 3-monthly as maintenance treatment for schizophrenia. BMC Psychiatry. 2021;21(1):300. PubMed CrossRef
- Rise MB, Stølan LO, Erdner A, et al. Patients’ perspectives on three-monthly administration of antipsychotic treatment with paliperidone palmitate - a qualitative interview study. Nord J Psychiatry. 2021;75(4):257–265. PubMed CrossRef
- Wang X, Raoufinia A, Bihorel S, et al. Population pharmacokinetic modeling and exposure-response analysis for aripiprazole once monthly in subjects with schizophrenia. Clin Pharmacol Drug Dev. 2022;11(2):150–164. PubMed CrossRef
- Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(01-02):9–62. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top