ABSTRACT
Objective: Zuranolone is a positive allosteric modulator of both synaptic and extrasynaptic γ-aminobutyric acid (GABA) type A receptors and a neuroactive steroid approved in the United States as an oral, once-daily, 14-day treatment course for adults with postpartum depression and under investigation for adults with major depressive disorder (MDD). Interim results from the open-label, longitudinal, phase 3 SHORELINE Study (NCT03864614) that evaluated the long-term safety and efficacy of zuranolone in adults with MDD are reported.
Methods: This interim report includes patients who were enrolled and had the opportunity to be on study for up to 1 year between February 2019 and September 2021. Adults aged 18–75 years with MDD diagnosed per DSM-5 criteria and a 17-item Hamilton Rating Scale for Depression (HAMD-17) total score ≥ 20 received an initial 30-mg or 50-mg 14-day zuranolone course. HAMD-17 responders (≥ 50% reduction from baseline) at Day (D)15 of the initial treatment period were allowed to continue in the study beyond D28 and were followed up for ≤ 1 year, during which repeat treatment courses were permitted. The primary endpoint was safety and tolerability of the initial and repeat treatment courses through 1 year. Secondary endpoints included change from baseline (CFB) in HAMD-17 total score and need for repeat treatment course(s).
Results: As of September 2021, among patients in the 30-mg (n = 725) and 50-mg (n = 199) Cohorts who received a zuranolone dose, 493 (68.0%) and 137 (68.8%), respectively, reported a treatment-emergent adverse event (TEAE); most patients who experienced TEAEs reported mild/moderate events (30-mg Cohort, 90.9% [448/493]; 50-mg Cohort, 85.4% [117/137]). Mean (standard deviation) CFB HAMD-17 total score at D15 of the initial treatment period was −15.2 (7.1) and −16.0 (6.0) for the 30-mg and 50-mg Cohorts, respectively; similar improvements were observed after repeat treatment courses. The proportion of patients who received only 1 treatment course during their time on study was 42.9% (210/489) in the 30-mg Cohort and 54.8% (80/146) in the 50-mg Cohort; 57.1% (279/489) and 45.2% (66/146) patients, respectively, received 2–5 total treatment courses. The majority of patients who initially responded to zuranolone received ≤ 2 total treatment courses (30-mg Cohort, 68.5% [335/489]; 50-mg Cohort, 79.5% [116/146]).
Conclusions: Of patients who experienced TEAEs, most reported mild or moderately severe events, and responders to zuranolone experienced improvements in depressive symptoms with initial and repeat treatment courses.
Trial Registration: ClinicalTrials.gov identifier: NCT03864614
J Clin Psychiatry 2024;85(1):23m14845
Author affiliations are listed at the end of this article.
Major depressive disorder (MDD) is a serious mental health disorder associated with major depressive episodes that affected over 21 million US adults in 2021.1–8 Current standard-of-care (SOC) antidepressant therapies (ADTs) may take weeks to take effect and may be associated with treatment-limiting adverse events (AEs), such as sexual dysfunction, weight gain, fatigue, and insomnia.9–14 These factors commonly lead to nonadherence and relapse.15,16 Despite treatment, some patients experience suboptimal or no response, requiring use of adjunctive therapies or frequent medication changes.17 Even with treatment, many patients with MDD may experience impaired functioning and diminished quality of life (QoL).15,16,18,19 Therefore, newer therapies with novel mechanisms of action that may address concerns leading to nonadherence are needed for patients with MDD.
Zuranolone is a neuroactive steroid (NAS) and a positive allosteric modulator (PAM) of synaptic and extrasynaptic γ-aminobutyric acid (GABA) type A receptors (GABAAR) approved in the United States as an oral, once-daily, 14-day treatment course for adults with postpartum depression (PPD) and under investigation for patients with MDD.20–24 Zuranolone is hypothesized to rapidly restore network balance in brain networks dysregulated in depression by up-regulating GABAAR expression and enhancing GABAergic signaling.21 In a preclinical study, oral zuranolone led to rapid increases in beta-band electroencephalogram power, a biomarker of GABAAR PAM activity, in less than 1 hour.21 Three completed placebo-controlled clinical studies in MDD (the phase 2 MDD-201b [NCT03000530], phase 3 WATERFALL [NCT04442490], and phase 3 CORAL [NCT04476030] Studies) demonstrated significantly greater improvements with zuranolone vs placebo as assessed by the change from baseline (CFB) in 17-item Hamilton Rating Scale for Depression (HAMD-17) total score at Day 3 or Day 15.25–27 Self-reported improvements in QoL were observed in post hoc analyses of the WATERFALL Study, and across studies, the safety profile of zuranolone was consistent, with predominantly mild or moderate severity treatment-emergent AEs (TEAEs).25–30 One phase 3 study (MOUNTAIN [NCT03672175]) evaluating zuranolone 20 and 30 mg did not demonstrate significantly greater improvements in depressive symptoms vs placebo at either dose, as assessed by CFB in HAMD-17 total score at Day 15, but post hoc analyses of patients with measurable plasma levels of zuranolone and/or severe depression (HAMD-17 total score ≥ 24) showed nominally significant improvements with zuranolone 30 mg vs placebo at Day 15.25 Notably, zuranolone 20 mg did not separate from placebo at any time point, suggesting that zuranolone 30 mg may be the minimum dose required to elicit significant improvements in depressive symptoms.25 Thus, subsequent studies shifted to include a higher dose of zuranolone 50 mg.
All completed clinical studies of zuranolone evaluated efficacy and safety of a single 14-day treatment course at 30 mg or 50 mg; however, the long-term safety and efficacy of repeat treatment courses of zuranolone (if needed) have not been assessed. The open-label SHORELINE Study was designed to evaluate the long-term safety of and need for repeat treatment course(s) with zuranolone 30 or 50 mg in adults with MDD over ≤ 1 year. Interim results are reported here.
METHODS
Study Design and Participants
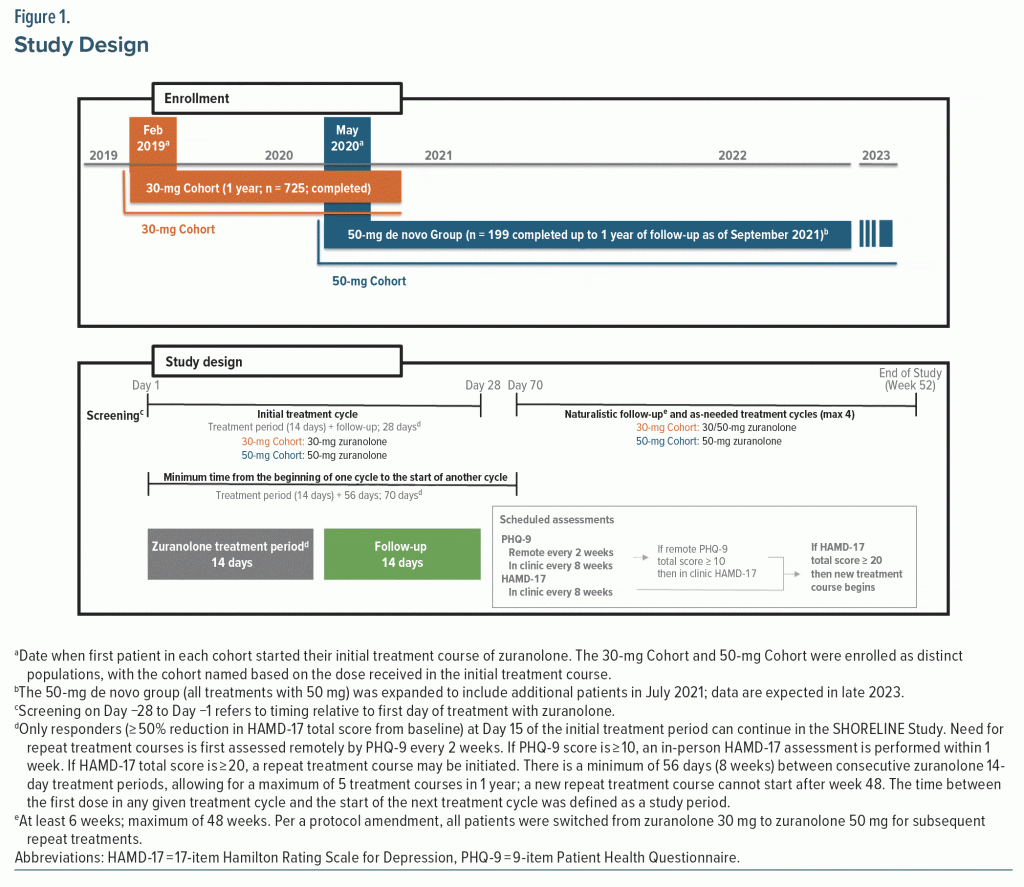
SHORELINE (NCT03864614) was an open-label, phase 3, observational study. The study had institutional review board approval and was conducted in accordance with the Declaration of Helsinki and consistent with the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use and Good Clinical Practice guidelines. The study started enrolling patients in February 2019, and a second cohort of patients began enrollment in May 2020 (Figure 1). Included in this analysis are data collected from de novo patients who completed or had the opportunity to complete 1 year of follow-up as of September 2021. Eligible patients were 18–75 years old with a diagnosis of MDD per Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, criteria for ≥ 4 weeks, HAMD-17 total score ≥ 20, and Montgomery-Asberg Depression Rating Scale total score ≥ 28 at screening and before dosing at Day 1.30,31 Determination of having MDD with elevated anxiety was based on HAMD-17 anxiety subscale standardized score ≥ 39 (raw score ≥ 7). Patients could take SOC ADTs if they were on a stable dose for ≥ 60 days before their first zuranolone dose and intended to continue the ADT through Day 28. Exclusion criteria included a history of bipolar disorder, schizophrenia, active psychosis, attempted suicide or significant risk of suicide in the current episode, or treatment-resistant depression, defined as persistent depressive symptoms despite treatment with adequate doses of antidepressants within the current major depressive episode (excluding antipsychotics) from 2 different classes for at least 4 weeks of treatment. Additionally, regular use of benzodiazepines, barbiturates, or other GABAAR modulators within 28 days prior to enrollment and dosing on Day 1 was exclusionary. Women with PPD were excluded due to specific parturition-related pathology and because use of NASs for PPD is considered complete after a single treatment course.32–34 Full eligibility criteria are available in Supplementary Appendix 1. All patients provided written informed consent.
Patients were screened for ≤ 4 weeks, followed by a 14-day treatment period and 14-day follow-up (Figure 1). A treatment cycle was defined as the 14-day treatment period and 14-day follow-up (Day 28). Only patients who achieved HAMD-17 response (≥ 50% reduction in HAMD-17 total score from baseline) at Day 15 of the initial treatment course (responders) were eligible to continue in the study. Responders who remained in the study through Day 28 could enter an observational period of ≤ 48 weeks.
Initially, SHORELINE included a single zuranolone 30-mg Cohort. After a protocol amendment, the starting dose was changed to 50 mg. For patients in the 30-mg Cohort still in the study, any future repeat courses were to be administered at 50 mg. Thus, a subset of patients in the 30-mg Cohort received the initial treatment course with zuranolone 30 mg and future treatment courses (if needed) with zuranolone 50 mg. A minimum of 56 days was required between consecutive treatment courses, allowing for a maximum of 5 treatment cycles in a 1-year period. This made Day 70 the earliest eligible date for a repeat treatment course (14-day treatment course + 56 days). The terminal date on study (end of study visit) was defined as the week 52 visit.
Need for repeat treatment courses after the initial treatment cycle was determined by remote assessment of 9-item Patient Health Questionnaire (PHQ-9) every 2 weeks and in-clinic HAMD-17 every 8 weeks.35 If the PHQ-9 score was ≥ 10, the patient returned to the clinic within 1 week for the clinician-administered HAMD-17. If the follow-up HAMD-17 total score was ≥ 20 and the minimum 56 days had elapsed since the last dose of zuranolone, the patient was considered qualified to receive a repeat treatment course. Patients who relapsed prior to Day 70 could receive a dose adjustment of an existing ADT or start a new ADT at the investigator’s discretion. Supplementary Appendices 1 and 2 include additional detail.
Procedures
Patients were instructed to take zuranolone once daily at night within 1 hour of consuming fat-containing food for 14 days. If a patient missed a dose, they were to skip that dose and take the next scheduled dose. Dose reduction from 30 mg to 20 mg or 50 mg to 40 mg was permitted based on tolerability. Safety, tolerability, and efficacy assessments occurred at screening; on Day 1, Day 8, Day 15, and Day 28 of each treatment cycle; and Day 70, Day 126, Day 182, Day 238, Day 294, Day 350, and Day 364 of each observational period (visit days reset to Day 1 if patients entered an additional treatment course). Clinically significant postbaseline physical examination abnormalities were reported as treatment-emergent AEs (TEAEs).
Endpoints
The primary endpoint was safety and tolerability of the initial and repeat zuranolone treatment courses through 1 year, assessed by incidence of AEs; CFB in clinical laboratory measures, vital signs, and electrocardiograms (ECGs); and changes in suicidal ideation/behavior using the Columbia-Suicide Severity Rating Scale.36 Secondary endpoints included need for repeat treatment courses and efficacy at the end of each treatment period. Need for repeat treatment courses was evaluated as time to first repeat treatment course, number of patients qualifying for repeat treatment, and number of total treatment courses received. Efficacy was assessed by CFB in HAMD-17 total score and Clinical Global Impressions-Severity (CGI-S) score, percentages of patients experiencing HAMD-17 response, HAMD-17 remission (HAMD-17 total score ≤ 7), and CGI-Improvement (CGI-I) response (“much improved” or “very much improved”).30,37 Patient-reported depressive symptoms measured by CFB in PHQ-9 score were an exploratory endpoint. Time to relapse among those who responded at Day 15 was defined as the time after Day 15 of any given treatment cycle when HAMD-17 total score was ≥ 20 within 10 days of a PHQ-9 score ≥ 10. Depressive symptom severity by study visit was categorized by PHQ-9 score (minimal, 0–4; mild, 5–9; moderate, 10–14; moderately severe, 15–19; severe, 20–27. A post hoc analysis of categorical CGI-S score (minimal/mild, ≤ 3; moderate, 4; severe, 5; very severe, 6; extreme, 7) and PHQ-9 score also assessed depressive symptom severity at study exit.
Statistical Analysis
Data for continuous endpoints were reported with summary statistics unless otherwise specified. Categorical data were summarized with counts and percentages. Times to events were evaluated by Kaplan-Meier (KM) estimates. No formal sample size calculation was performed; the target sample size of 1,200 de novo patients was chosen to have ≥ 675 patients complete 24 weeks and 235 patients complete 56 weeks. The safety set included all patients who received ≥ 1 dose of zuranolone. The full analysis set (FAS) included all dosed patients who achieved a HAMD-17 response at Day 15 in the initial treatment cycle and continued in the study beyond Day 28. The mean number of repeat treatment courses was calculated based on the FAS. Patients who remained in the study for the minimum 56 days between potential treatment courses but did not need any additional treatment courses (based on the HAMD-17 ≥ 20 threshold) were counted as 0 in the calculation of mean repeat treatment courses.
RESULTS
Patient Disposition, Demographics, and Baseline Characteristics
As of September 2021, 924 patients (30-mg Cohort [n = 725]; 50-mg Cohort [n = 199]) received ≥ 1 dose of zuranolone (Supplementary Figure 1). At Day 15 of the initial treatment cycle, 73.5% (505/687) and 80.5% (149/185) of patients who had nonmissing HAMD-17 scores in the 30-mg and 50-mg Cohorts, respectively, achieved a HAMD-17 response, with 23.9% (173/725) and 17.6% (35/199), respectively, withdrawing due to a lack of response. Most patients in the FAS completed 1 full year in the study (30-mg Cohort, 63.6% [311/489]; 50-mg Cohort, 65.1% [95/146]).
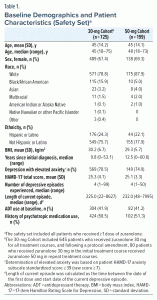
Most patients in both cohorts were White, were female, and had MDD with elevated anxiety at baseline (Table 1). Baseline mean (standard deviation [SD]) HAMD-17 total score was 25.3 (4.1) and 25.1 (3.3) in the 30-mg and 50-mg Cohorts, respectively. The median (range) time since initial MDD diagnosis was 9.8 (0–53.1) years (30-mg Cohort) and 12.5 (0–60.6) years (50-mg Cohort), with 11.0% (80/725) and 6.5% (13/199) of patients, respectively, reporting this as their first depressive episode. Approximately 10% of patients were > 65 years of age. The median (range) number of depressive episodes was 4 (1–99) and 4 (1–50) in the 30-mg and 50-mg Cohorts, respectively; 41.9% (304/725) and 41.2% (82/199) of patients were receiving an ADT at baseline, respectively.
Safety and Tolerability
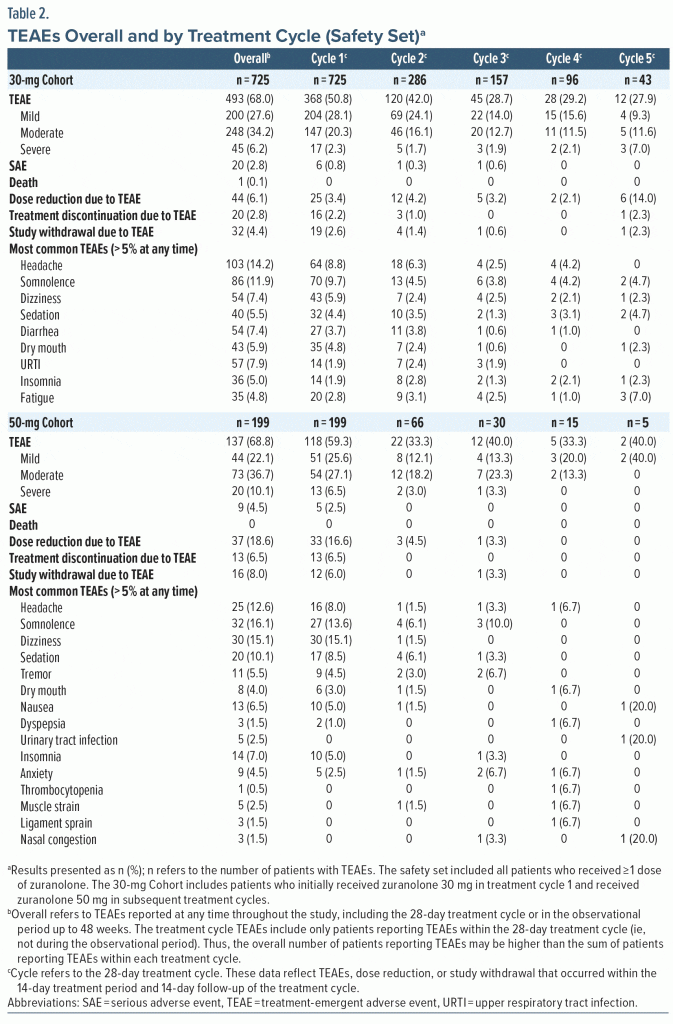
In the safety set, ≥ 1 TEAE was reported by 68.0% (493/725) and 68.8% (137/199) of patients in the 30-mg and 50-mg Cohorts, respectively (Table 2). Most patients who experienced TEAEs reported events that were mild or moderate in severity (30-mg Cohort, 90.9% [448/493]; 50-mg Cohort, 85.4% [117/137]). Sixty-five patients reported severe TEAEs (30-mg Cohort, 6.2% [45/725]; 50-mg Cohort, 10.1% [20/199]). Of these patients with severe TEAEs, 25 reported a total of 32 severe TEAEs that were assessed by the investigator as related to zuranolone, and all were reported while on treatment. Overall, the TEAEs reported by ≥ 10% of patients were headache (14.2% [103/725]) and somnolence (11.9% [86/725]) in the 30-mg Cohort and somnolence (16.1% [32/199]), dizziness (15.1% [30/199]), headache (12.6% [25/199]), and sedation (10.1% [20/199]) in the 50-mg Cohort. The proportion of patients with TEAEs leading to dose reduction was 6.1% (44/725, 30-mg Cohort) and 18.6% (37/199, 50-mg Cohort). The TEAEs leading to dose reduction (occurring in ≥ 2 patients) were somnolence, fatigue, dizziness, anxiety, lethargy, nausea, sedation, tremor, headache, and attention disturbance in the 30-mg Cohort and dizziness, somnolence, sedation, headache, nausea, tremor, fatigue, asthenia, gait disturbance, agitation, and blurred vision in the 50-mg Cohort. The proportion of patients with TEAEs leading to study withdrawal was 4.4% (32/725; 30-mg Cohort) and 8.0% (16/199; 50-mg Cohort). The TEAEs leading to study withdrawal (occurring in ≥ 2 patients) were anxiety, depression, suicidal ideation, dizziness, confusional state, insomnia, nausea, and pulmonary embolism.
Patients reported TEAEs primarily while on treatment in treatment cycles 1 and 2, and no new or unexpected safety findings were identified with repeat treatment courses (Table 2; Supplementary Table 1). Among patients in the 30-mg Cohort, the most common TEAEs (reported by ≥ 5% of patients) during the first 28-day treatment cycle (ie, treatment cycle TEAEs) were somnolence (9.7% [70/725]), headache (8.8% [64/725]), and dizziness (5.9% [43/725]); 25 patients (3.4%) had their dose reduced and 19 (2.6%) withdrew from the study due to treatment cycle TEAEs. Among patients who received a second treatment course, the most common treatment cycle TEAE was headache (6.3% [18/286]); the rate of dose reduction due to TEAEs was 4.2% (12/286), and 4 patients (1.4%) withdrew due to treatment cycle TEAEs. There were ≤ 6 patients with dose reduction or study withdrawal due to treatment cycle TEAEs in study periods 3, 4, or 5. Headache and somnolence were the most frequently reported treatment cycle TEAEs among patients who received a third treatment course (somnolence, 3.8% [6/157]; headache, 2.5% [4/157]) and a fourth treatment course (somnolence, 4.2% [4/96]; headache, 4.2% [4/96]). Of 43 patients in the 30-mg Cohort who received a fifth treatment course, the most common treatment cycle TEAE was fatigue (7.0% [n = 3]).
Among the 199 patients in the 50-mg Cohort, the most common TEAEs during the initial 28-day treatment cycle were dizziness (15.1% [30/199]), somnolence (13.6% [27/199]), sedation (8.5% [17/199]), headache (8.0% [16/199]), and nausea (5.0% [10/199]); 33 patients (16.6%) had their dose reduced and 12 (6.0%) withdrew due to treatment cycle TEAEs (Table 2). Of these 199 patients, 66 later qualified for and received a second treatment course. The most common treatment cycle TEAEs in treatment cycle 2 were somnolence (6.1% [n = 4]) and sedation (6.1% [n = 4]). The rate of dose reduction due to TEAEs in the second treatment cycle was 4.5% (3/66), and no patients withdrew due to treatment cycle TEAEs. There was ≤ 1 patient with dose reduction or study withdrawal due to TEAEs in treatment cycles 3, 4, or 5. In treatment cycle 3, the most common TEAEs were somnolence (10.0% [3/30]), anxiety (6.7% [2/30]), and tremor (6.7% [2/30]). Fifteen patients in the 50-mg Cohort received a fourth treatment course, and 5 reported treatment cycle TEAEs, which included headache, dry mouth, and dyspepsia (6.7% [n = 1 each]). Of 5 patients who received a fifth treatment course, 1 each reported nausea, nasal congestion, and urinary tract infection during the treatment cycle. Overall, the incidence and timing of TEAEs were not notably different between patients who received zuranolone alone vs those who received zuranolone and a stable dose of ADTs at baseline (Supplementary Table 2).
The overall incidence of serious AEs was 2.8% (20/725; 30-mg Cohort) and 4.5% (9/199; 50-mg Cohort), and most were observed in the initial study period (ie, during the initial 28-day treatment cycle or ≤ 48-week observational period). One patient in the 30-mg Cohort died due to herpes simplex encephalitis and intracranial hemorrhage; the death occurred > 150 days after the last dose and was determined by the investigator to be unrelated to zuranolone. Findings surrounding patient vital sign parameters, ECGs, withdrawal symptoms, and suicidal ideation/behavior are reported in the Supplementary Material (Supplementary Appendix 2, Supplementary Figure 2).
Efficacy
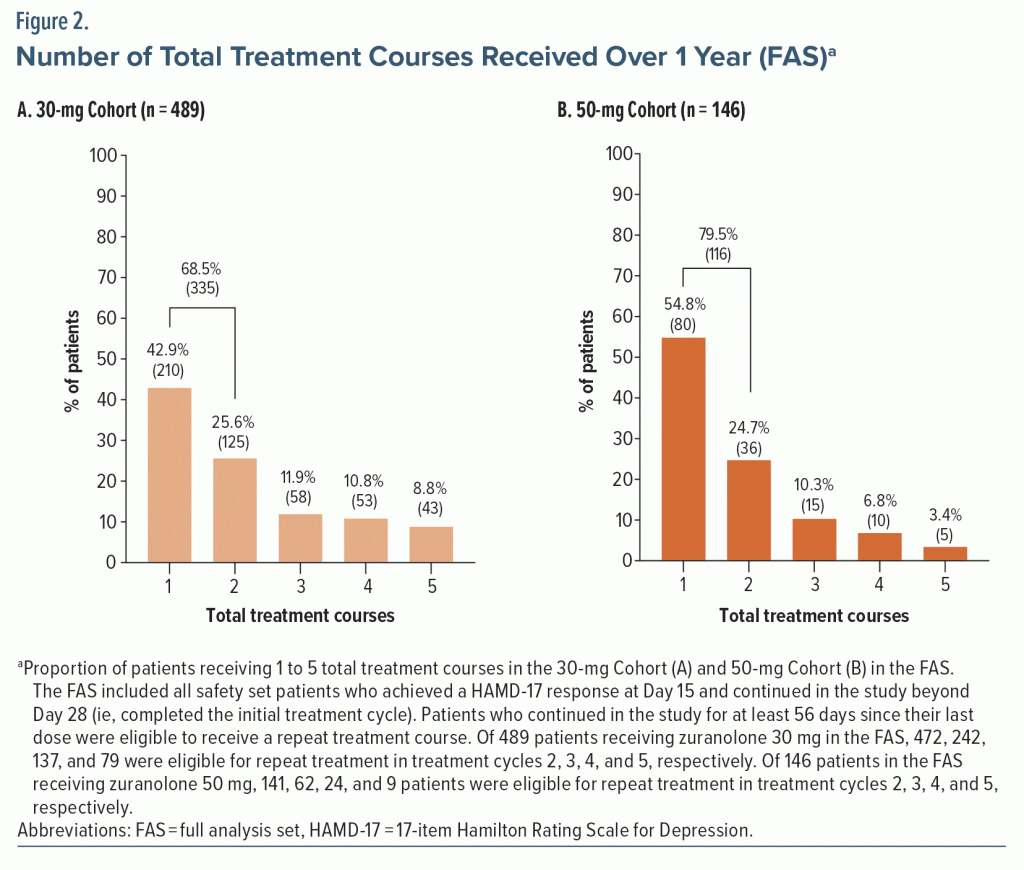
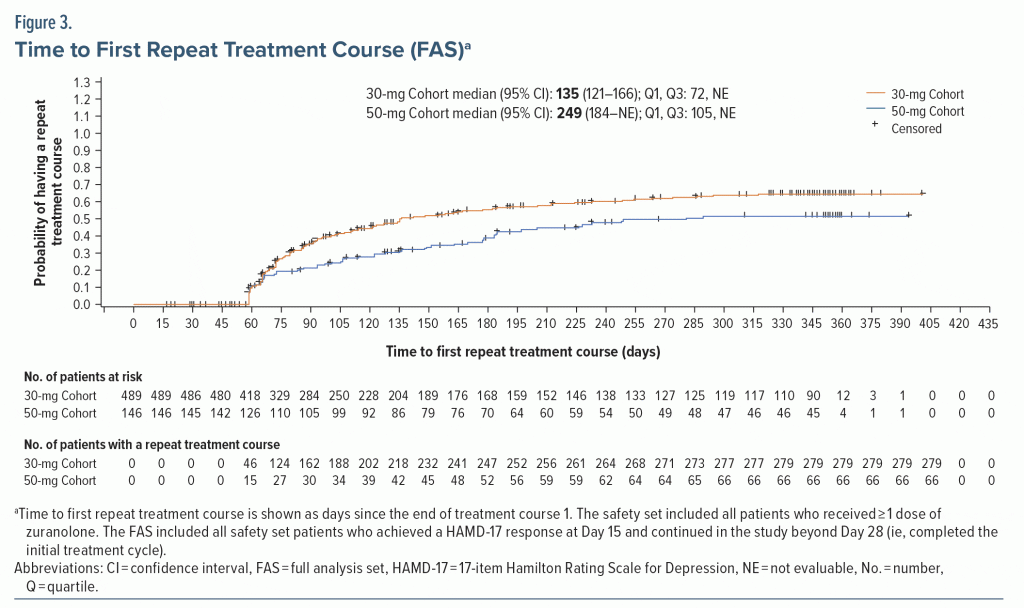
Most dosed patients responded at Day 15 of the initial treatment cycle and remained in the study beyond Day 28 (FAS; 30-mg Cohort, 67.4% [489/725]; 50-mg Cohort, 73.4% [146/199]). Among responders, 42.9% (210/489) of the 30-mg Cohort and 54.8% (80/146) of the 50-mg Cohort received only the initial 14-day treatment course with zuranolone during their time in the study up to 1 year (Figure 2). The proportion of responders to the initial treatment course who received exactly 2 total treatment courses during their time on study was 25.6% (125/489; 30-mg Cohort) and 24.7% (36/146; 50-mg Cohort). The proportion of responders to the initial treatment course who received 2 to 5 total treatment courses (ie, received at least 1 repeat treatment course during their time in the study) was 57.1% (279/489) in the 30-mg Cohort and 45.2% (66/146) in the 50-mg Cohort. Most patients received 1 or 2 total treatment courses (no repeat treatment course or 1 repeat treatment course) throughout up to 1 year in the study (30-mg Cohort, 68.5% [335/489]; 50-mg Cohort, 79.5% [116/146]). Overall, the mean (SD) number of repeat treatment courses among patients in the FAS was 1.2 (1.3) and 0.8 (1.1) for the 30-mg and 50-mg Cohorts, respectively. The number of total treatment courses was comparable between patients who received zuranolone with and without baseline ADT (Supplementary Figure 3). The median time to first repeat treatment course based on KM estimation was 135 and 249 days for the 30-mg and 50-mg Cohorts, respectively (Figure 3). In the initial study period, the estimated median time to relapse based on the KM method was 109 and 224 days for the 30-mg and 50-mg Cohorts, respectively (Supplementary Figure 4).
At Day 15 of the initial treatment period, patients experienced improvements from baseline in depressive symptoms across efficacy endpoints (Supplementary Table 3). In the overall population, improvements in depressive symptoms, as assessed by mean (SD) CFB in HAMD-17 total score, were observed at Day 15 of the initial treatment period (safety set; 30-mg Cohort, –15.2 [7.1]; 50-mg Cohort, –16.0 [6.0]). Improvements in HAMD-17 total score at Day 70 in those who remained on study were sustained beyond Day 28 (FAS, mean [SD] CFB; 30-mg Cohort, −12.2 [9.1]; 50-mg Cohort, −14.0 [7.2]). Most patients in both cohorts in the safety set achieved a CGI-I response at Day 8 that was sustained at all study visits through Day 70 in study period 1 in the FAS (Supplementary Figure 5). Patient-reported depressive symptoms as assessed by mean PHQ-9 score were consistent with results from the investigator-reported HAMD-17 (Supplementary Figure 6A). At Day 15 of the initial treatment period, the majority of dosed patients in both cohorts reported minimal or mild depressive symptoms as assessed by PHQ-9 (30-mg Cohort, 71.1% [456/641]; 50-mg Cohort, 76.6% [134/175]).
After the initial treatment course, improvements in depressive symptoms, as assessed by change from period-specific baseline in HAMD-17 total score, mean PHQ-9 score, and categorical depression severity as assessed by PHQ-9, were also observed with repeat treatment courses for patients who received them (Supplementary Table 4; Supplementary Figures 6–8). Of the patients who were eligible for a repeat treatment (ie, patients in the FAS who were on study for the minimum 56 days between treatment courses), the majority in both cohorts reached the threshold for a repeat treatment as defined by a PHQ-9 score ≥ 10 (30-mg Cohort, 69.7% [329/472]; 50-mg Cohort, 58.9% [83/141]). The proportion of patients who reached the threshold defined as a HAMD-17 total score ≥ 20 was similar: 61.4% (290/472) in the 30-mg Cohort and 48.2% (68/141) in the 50-mg Cohort. The proportion of patients in the 30-mg Cohort who received a second treatment course and achieved HAMD-17 response at Day 15 following the repeat treatment course was 64.3% (171/266; Supplementary Table 5). The proportion of patients in the 30-mg Cohort who achieved response at Day 15 following subsequent treatment courses was 63.6% (96/151; treatment cycle 3), 66.7% (62/93; treatment cycle 4), and 71.8% (28/39; treatment cycle 5). In the 50-mg Cohort, the proportion of patients who received a second treatment course and achieved response was 64.6% (42/65). The proportion of patients who achieved response in the 50-mg Cohort at Day 15 following repeat treatment courses was 50.0% (14/28), 40.0% (6/15), and 0/5 patients for treatment cycles 3, 4, and 5, respectively; of note, after the second treatment course, 30 or fewer patients needed and received any additional treatments (Supplementary Table 5).
The proportion of patients in the 30-mg Cohort who achieved remission at Day 15 following the second treatment course was 33.5% (89/266). The rates of remission in treatment cycles 3, 4, and 5, respectively, were 31.1% (47/151), 38.7% (36/93), and 48.7% (19/39; Supplementary Table 5). In the 50-mg Cohort, the proportion of patients who achieved remission at Day 15 following a second treatment course was 33.8% (22/65). Remission was lower at Day 15 for patients who received a third, fourth, or fifth treatment course in the 50-mg Cohort (treatment cycle 3, 21.4% [6/28]; treatment cycle 4, 26.7% [4/15]; treatment cycle 5, 0/5; Supplementary Table 5). At study exit, most patients in the FAS had minimal/mild depressive symptoms, as assessed by CGI-S score, regardless of whether they completed the study (30-mg Cohort, 84.9% [264/311]; 50-mg Cohort, 88.4% [84/95]) or withdrew prematurely (30-mg Cohort, 71.3% [127/178]; 50-mg Cohort, 74.5% [38/51]). Similar results were observed at study exit as assessed by PHQ-9 score, with most patients reporting minimal or mild depressive symptoms regardless of whether they completed the study (30-mg Cohort, 74.6% [232/311]; 50-mg Cohort, 86.3% [82/95]) or withdrew early (30-mg Cohort, 60.7% [108/178]; 50-mg Cohort, 72.5% [37/51]).
DISCUSSION
In the interim results from SHORELINE, most TEAEs reported by patients receiving a once-daily, 14-day treatment with oral zuranolone 30 mg or 50 mg were mild/moderate in severity, consistent with the safety profile of previous placebo-controlled trials of zuranolone.32,38–42 The extended follow-up period of this study allowed for the first assessment of long-term safety of zuranolone. The most common TEAEs (somnolence, dizziness, headache, and sedation) were consistent with those reported in previous studies of zuranolone and occurred primarily while on treatment, with no evidence of increased suicidal ideation throughout the study.32,42 The safety profile of zuranolone was generally consistent in repeat treatment courses, with no notable trends in the frequency of reporting or the types of TEAEs reported by patients who received multiple treatment courses compared with the initial treatment course. Overall, 5.2% (48/924) of patients withdrew from the study due to TEAEs (30-mg Cohort, 4.4% [32/725]; 50-mg Cohort, 8.0% [16/199]), with most withdrawals occurring in treatment cycle 1.
These data may also highlight the potential long-term efficacy of zuranolone in adults with MDD, although the open-label nature of the study must be noted as a limitation. About half of patients in both dose cohorts received only the initial treatment course, while the other half received at least 1 additional treatment course during their time in the study. Notably, while symptoms of elevated anxiety were present at baseline in nearly 80% of patients in SHORELINE, improvements in depressive symptoms were still observed in initial and repeat treatment courses. This is consistent with randomized, double-blinded studies, in which treatment with zuranolone was associated with improvements in depressive symptoms vs placebo in patients with MDD, including those with elevated anxiety (CORAL and integrated data from MDD-201b, MOUNTAIN, and WATERFALL).27,43 The majority of patients who initially responded to zuranolone reached the threshold for repeat treatment as assessed only by a self-reported PHQ-9 score ≥ 10 in both dose cohorts, and similar results were observed based on the investigator-reported HAMD-17 criterion. KM estimates suggest potentially sustained effects of zuranolone, with a median time to the first repeat treatment course of approximately 4 months in the 30-mg Cohort and 8 months in the 50-mg Cohort. However, while the 50-mg dose was associated with a longer time to first repeat treatment course compared with the 30-mg dose, patients in the 50-mg Cohort were approximately 3 times as likely to require dose reduction due to TEAEs and were twice as likely to withdraw from the study due to TEAEs.
Most responders to the initial treatment course in the 30-mg Cohort regained HAMD-17 response if they received a second treatment course—and a similar trend was observed for subsequent treatment courses—despite the fact that period-specific baseline HAMD-17 total scores were generally lower after treatment cycle 1. Patient-reported PHQ-9 results were consistent with these observations, showing that most patients in the 30-mg Cohort reported minimal or mild symptoms at Day 15 and at later study visits. Response was also regained if initial responders in the 50-mg Cohort received a second treatment course. Response and remission rates decreased with repeat treatment courses among patients in the 50-mg Cohort, although a relatively small number of patients in this cohort (n ≤ 30) received more than 2 total treatment courses with zuranolone 50 mg, which limits the interpretation of these data. In both dose cohorts, those who needed and received 3 or more treatment courses were generally more likely to report moderate/severe depressive symptoms (assessed by PHQ-9) at Day 15 and later study visits compared with those who only needed 1 or 2 total treatment courses, although results varied by study visit, which may be due to the smaller sample size for patients who received 3 or more treatment courses. Regardless of whether responders completed a full year in the study or withdrew early, most exited the study with at most mild depressive symptoms, as assessed both by patient-reported PHQ-9 and by investigator-reported CGI-S score, potentially suggesting that lack of efficacy was not a primary driver in patient dropout.
A limitation of the open-label SHORELINE Study is that it was not designed for comparative safety and efficacy assessments, in part due to the lack of randomization. Additionally, the sample size of the 50-mg Cohort reported here was smaller than that of the 30-mg Cohort, especially in study periods 3 to 5, which limits the interpretation of results. Additional data from the completed 50-mg Cohort will be reported on study completion.
Overall, SHORELINE adds evidence supporting the emergent strategy of targeting GABAergic signaling to treat MDD, which is based on the hypothesis that decreased GABA activity plays a fundamental role in the network dysregulation thought to contribute to the clinical presentation of depression.20,44–48 In preclinical models, NASs can lead to rapid and sustained regulation of brain networks implicated in depression, consistent with antidepressant activity.49 Mechanistically, this is thought to be related to the biochemistry of NASs, which bind to both synaptic and extrasynaptic GABAARs and increase surface receptor expression in vitro, consistent with both rapid and sustained signaling.50,51
In conclusion, the interim results from SHORELINE show that most TEAEs reported were mild/moderate, and patients who received zuranolone showed improvement in depressive symptoms with initial and repeat treatment courses, with a safety profile that was consistent with previous placebo-controlled studies of zuranolone. In both dose cohorts, improvements in depressive symptoms were potentially sustained for months, with the possibility to recapture response with repeat treatment courses, if needed.
Article Information
Published Online: December 27, 2023. https://doi.org/10.4088/JCP.23m14845
© 2023 Physicians Postgraduate Press, Inc.
Submitted: February 24, 2023; accepted October 13, 2023.
To Cite: Cutler AJ, Mattingly GW, Kornstein SG, et al. Long-term safety and efficacy of initial and repeat treatment courses with zuranolone in adult patients with major depressive disorder: interim results from the open-label, phase 3 SHORELINE study. J Clin Psychiatry. 2024;85(1):23m14845.
Author Affiliations: Department of Psychiatry, SUNY Upstate Medical University, Syracuse, New York (Cutler); Department of Psychiatry, Washington University School of Medicine, St. Louis, Missouri (Mattingly); Department of Psychiatry, Virginia Commonwealth University School of Medicine, Richmond, Virginia (Kornstein); Institute for Advanced Diagnostics and Therapeutics, Sheppard Pratt, Baltimore, Maryland (Aaronson); Sage Therapeutics, Cambridge, Massachusetts (Lasser, Zhang, Rana, Brown, Doherty); Biogen Inc., Cambridge, Massachusetts (Levin, Miller, Kotecha, Forrestal).
Corresponding Author: Andrew J. Cutler, MD, 8429 Lorraine Rd #350, Lakewood Ranch, FL 34202 ([email protected]).
Author Contributions: All authors contributed to the acquisition, analysis, or interpretation of the data and critically revised the manuscript for important intellectual content. Drs Lasser and Doherty contributed to concept and design. Drs Cutler, Aaronson, Lasser, Zhang, Miller, Kotecha, and Forrestal contributed to drafting the manuscript. Dr Forrestal provided input into the study methodology. Dr Zhang performed statistical analysis and has directly accessed and verified the underlying data reported in the manuscript.
Relevant Financial Relationships: Dr Cutler serves as a consultant to AbbVie, Acadia, AiCure, Alfasigma, Alkermes, Allergan, Cognitive Research, Intra-Cellular Therapies, Janssen, Jazz Pharmaceuticals, Lundbeck, MedAvante-Prophase, Neurocrine, Noven, Otsuka, Sage Therapeutics, Inc., Sunovion, Supernus, Takeda, Terran Biosciences, and Teva; serves as a speaker for/receives promotional honoraria from AbbVie, Acadia, Alfasigma, Alkermes, Allergan, Intra-Cellular Therapies, Janssen, Lundbeck, Neurocrine, Noven, Otsuka, Sunovion, Supernus, Takeda, and Teva; has received research grants from Acadia, Alkermes, Allergan, Axsome, Biohaven, Intra-Cellular Therapies, Janssen, Lilly, Lundbeck, Novartis, Otsuka, Sage Therapeutics, Inc., Sunovion, and Takeda; and is an employee and board member of the Neuroscience Education Institute. Dr Mattingly serves as a researcher for AbbVie, Akili, Alkermes, Axsome, Boehringer, Genentech, Janssen, Lundbeck, Medgenics, NLS Pharma, Otsuka, Reckitt Benckiser, Roche, Sage Therapeutics, Inc., Sunovion, Supernus, Takeda, Taisho, and Teva; serves as a consultant for AbbVie, Alkermes, Alfasigma, Ironshore, Janssen, Lundbeck, Major League Baseball, Otsuka, National Football League, Neos, NLS Pharma, Purdue, Rhodes, Sage Therapeutics, Inc., Sunovion, Supernus, Takeda, Teva, and Vanda; and serves as a speaker for AbbVie, Alkermes, Ironshore, Janssen, Lundbeck, Otsuka, Neos, Shire, Sunovion, Takeda, and Teva. Dr Kornstein has received research support from Pfizer, Marinus, the National Science Foundation, and the National Institutes of Health. She has served on advisory boards for Lilly, Sage Therapeutics, Inc., Janssen, Reunion Neuroscience, Relmada, Sunovion, AbbVie, and Astellas. Dr Aaronson serves as a consultant to Sage Therapeutics, Inc., Janssen, Neuronetics, LivaNova, and Genomind. He has received research support from Compass and Neuronetics. Dr Brown is an employee of Sage Therapeutics, Inc., and may hold stock and/or stock options. Drs Lasser, Zhang, Rana, and Doherty were employees of Sage Therapeutics, Inc. at the time of study conduct, and may hold stock and/or stock options. Drs Levin, Miller, Kotecha, and Forrestal are employees of Biogen Inc. and may hold stock.
Funding/Support: This study was sponsored by Sage Therapeutics, Inc., and Biogen Inc.
Role of the Funders/Sponsors: Sage Therapeutics, Inc., and Biogen Inc. contributed to the concept, study design, data collection, and analysis.
Previous Presentation: Cutler AJ, et al. Efficacy of Zuranolone 50 mg and Need for Repeat Treatment Courses in the Open-Label, Phase 3 SHORELINE Study of Adult Patients With Major Depressive Disorder (MDD). Poster presented at the Society of Biological Psychiatry Annual Meeting, April 28–30, 2022, and the Psych Annual Congress, September 17–20, 2022.
Acknowledgments: The authors thank the patients and their families for helping them reimagine brain health. They also thank the clinical trial investigators and their staff at sites throughout the country. Medical writing and editorial support were provided by Ryan Coleman, PhD, of AlphaBioCom, a Red Nucleus company, and funded by Sage Therapeutics, Inc., and Biogen Inc.
Supplementary Material: Available at Psychiatrist.com.
Clinical Points
- This study provides the first evidence of the long-term safety of 14-day treatment course(s) with zuranolone. The side effects of zuranolone in this study were consistent with those observed in previous clinical studies and were not notably different among patients who later relapsed and received repeat treatment courses, suggesting that the 14-day treatment course of zuranolone can potentially be repeated, if needed.
- Most patients achieved treatment response following a 14-day treatment course with zuranolone. Importantly, for patients who responded to their first treatment, the time to first repeat treatment was over 4 months in both dose groups, and they were likely to respond again if they later relapsed and needed additional treatment course(s).
References (51)

- Kessler RC, Wang PS. The descriptive epidemiology of commonly occurring mental disorders in the United States. Annu Rev Public Health. 2008;29(1):115–129. PubMed CrossRef
- Mokdad AH, Ballestros K, Echko M, et al; US Burden of Disease Collaborators. The state of US Health, 1990–2016: burden of diseases, injuries, and risk factors among US states. JAMA. 2018;319(14):1444–1472. PubMed CrossRef
- James S, Abate D, Hassen K, et al; GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. PubMed CrossRef
- Malhi GS, Mann JJ. Depression. Lancet. 2018;392(10161):2299–2312. PubMed CrossRef
- Kessler RC, Berglund P, Demler O, et al; National Comorbidity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095–3105. PubMed CrossRef
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. American Psychiatric Publishing, Inc; 2013.
- Major Depression. National Institute of Mental Health. Updated January 2022. Accessed December 3, 2023. https://www.nimh.nih.gov/health/statistics/major-depression
- National Survey on Drug Use and Health. Substance Abuse and Mental Health Services Administration. Accessed February 10, 2023. https://www.samhsa.gov/data/report/2021-nsduh-detailed-tables
- Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. PubMed CrossRef
- Trivedi MH, Rush AJ, Wisniewski SR, et al; STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. PubMed CrossRef
- Ferguson JM. SSRI antidepressant medications: adverse effects and tolerability. Prim Care Companion J Clin Psychiatry. 2001;3(1):22–27. PubMed CrossRef
- Armitage R. The effects of antidepressants on sleep in patients with depression. Can J Psychiatry. 2000;45(9):803–809. PubMed CrossRef
- Higgins A, Nash M, Lynch AM. Antidepressant-associated sexual dysfunction: impact, effects, and treatment. Drug Healthc Patient Saf. 2010;2:141–150. PubMed CrossRef
- Keks N, Hope J, Keogh S. Switching and stopping antidepressants. Aust Prescr. 2016;39(3):76–83. PubMed CrossRef
- Romera I, Pérez V, Ciudad A, et al. Residual symptoms and functioning in depression, does the type of residual symptom matter? a post-hoc analysis. BMC Psychiatry. 2013;13(1):51. PubMed CrossRef
- Nierenberg AA, Husain MM, Trivedi MH, et al. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol Med. 2010;40(1):41–50. PubMed CrossRef
- Arnaud ASE, DeRienzo L, Zhang X, et al. Frequent treatment changes in the course of antidepressant therapy: real-world evidence of unmet needs in the pharmacotherapy of major depressive disorder. JMCP. 2019;25(10):F11.
- Luo Y, Kataoka Y, Ostinelli EG, et al. National prescription patterns of antidepressants in the treatment of adults with major depression in the US between 1996 and 2015: a population representative survey based analysis. Front Psychiatry. 2020;11:35. PubMed CrossRef
- Semahegn A, Torpey K, Manu A, et al. Psychotropic medication non-adherence and its associated factors among patients with major psychiatric disorders: a systematic review and meta-analysis. Syst Rev. 2020;9(1):17. PubMed CrossRef
- Sarawagi A, Soni ND, Patel AB. Glutamate and GABA homeostasis and neurometabolism in major depressive disorder. (Review) Front Psychiatry. 2021;12:637863. PubMed CrossRef
- Althaus AL, Ackley MA, Belfort GM, et al. Preclinical characterization of zuranolone (SAGE-217), a selective neuroactive steroid GABAA receptor positive allosteric modulator. Neuropharmacology. 2020;181:108333. PubMed CrossRef
- Martinez Botella G, Salituro FG, Harrison BL, et al. Neuroactive steroids. 2. 3α-hydroxy-3β-methyl-21-(4-cyano-1H-pyrazol-1′-yl)-19-nor-5β-pregnan-20-one (SAGE-217): a clinical next generation neuroactive steroid positive allosteric modulator of the (γ-aminobutyric acid) a receptor. J Med Chem. 2017;60(18):7810–7819. PubMed CrossRef
- Hoffmann E, Nomikos GG, Kaul I, et al. SAGE-217, a novel GABA(A) receptor positive allosteric modulator: clinical pharmacology and tolerability in randomized phase I dose-finding studies. Clin Pharmacokinet. 2020;59(1):111–120. PubMed CrossRef
- Zurzuvae. Package insert. Cambridge, MA: Sage Therapeutics, Inc and Biogen Inc; 2023.
- Clayton AH, Lasser R, Nandy I, et al. Zuranolone in major depressive disorder: results from MOUNTAIN-a phase 3, multicenter, double-blind, randomized, placebo-controlled trial. J Clin Psychiatry. 2023;84(2):22m14445. PubMed CrossRef
- Clayton AH, Lasser R, Parikh SV, et al. Zuranolone for the treatment of adults with major depressive disorder: a randomized, placebo-controlled phase 3 trial. Am J Psychiatry. 2023;180(9):676–684. PubMed CrossRef
- Parikh SV. Efficacy and safety of zuranolone co-initiated with an antidepressant in adults with major depressive disorder: results from the phase 3, randomized, double-blind, placebo-controlled CORAL study. Poster presented at: American Society of Clinical Psychopharmacology May 31–June 3 2022; Scottsdale, AZ.
- Huang M-Y. Zuranolone in major depressive disorder (MDD): minimal important difference (MID) and meaningful change threshold (MCT) on the 17-item Hamilton Rating Scale for Depression (HAMD-17). Poster presented at: Psych Congress; September 17–20 2022; New Orleans, LA.
- Clayton AH. Short form-36 quality of life data from the zuranolone clinical development program in major depressive disorder and postpartum depression. Poster presented at: Psych Congress; September 17–20 2021; New Orleans, LA.
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. PubMed CrossRef
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382–389. PubMed CrossRef
- Deligiannidis KM, Meltzer-Brody S, Gunduz-Bruce H, et al. Effect of zuranolone vs placebo in postpartum depression: a randomized clinical trial. JAMA Psychiatry. 2021;78(9):951–959. PubMed CrossRef
- Kanes S, Colquhoun H, Gunduz-Bruce H, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017;390(10093):480–489. PubMed CrossRef
- Kanes SJ, Colquhoun H, Doherty J, et al. Open-label, proof-of-concept study of brexanolone in the treatment of severe postpartum depression. Hum Psychopharmacol. 2017;32(2):e2576. PubMed CrossRef
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. PubMed CrossRef
- Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266–1277. PubMed CrossRef
- Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4(7):28–37. PubMed
- Sage Therapeutics reports topline results from pivotal phase 3 MOUNTAIN study of SAGE-217 in major depressive disorder. Press Release. 2019.
- Sage Therapeutics and Biogen announce positive pivotal phase 3 results for zuranolone, an investigational two-week, once-daily therapeutic being evaluated for major depressive disorder. Press Release. 2021.
- Sage Therapeutics and Biogen announce the phase 3 CORAL study met its primary and key secondary endpoints - comparing zuranolone 50 mg co-initiated with standard of care antidepressant vs standard of care co-initiated with placebo in people with MDD. Press Release. 2022.
- Sage Therapeutics and Biogen announce that the phase 3 SKYLARK study of zuranolone in postpartum depression met its primary and all key secondary endpoints. Press Release. 2022.
- Gunduz-Bruce H, Silber C, Kaul I, et al. Trial of SAGE-217 in patients with major depressive disorder. N Engl J Med. 2019;381(10):903–911. PubMed CrossRef
- Czysz AH, Schatzberg CNA, Lasser R, et al. Improvement in depressive symptoms with zuranolone treatment in patients with major depressive disorder with or without elevated anxiety: integrated analysis from the MDD-201B, MOUNTAIN, and WATERFALL studies. Poster presented at: American College of Neuropsychopharmacology (ACNP) 2022 Annual Meeting; December 4–7 2022; Phoenix, AZ.
- Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16(4):383–406. PubMed CrossRef
- Yan C-G, Chen X, Li L, et al. Reduced default mode network functional connectivity in patients with recurrent major depressive disorder. Proc Natl Acad Sci U S A. 2019;116(18):9078–9083. PubMed CrossRef
- Spellman T, Liston C. Toward circuit mechanisms of pathophysiology in depression. Am J Psychiatry. 2020;177(5):381–390. PubMed CrossRef
- Fischer AS, Keller CJ, Etkin A. The clinical applicability of functional connectivity in depression: pathways toward more targeted intervention. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1(3):262–270. PubMed CrossRef
- Fogaça MV, Duman RS. Cortical GABAergic dysfunction in stress and depression: new insights for therapeutic interventions. Front Cell Neurosci. 2019;13:87. PubMed CrossRef
- Zorumski CF, Paul SM, Covey DF, et al. Neurosteroids as novel antidepressants and anxiolytics: GABA-A receptors and beyond. Neurobiol Stress. 2019;11:100196. PubMed CrossRef
- Abramian AM, Comenencia-Ortiz E, Modgil A, et al. Neurosteroids promote phosphorylation and membrane insertion of extrasynaptic GABAA receptors. Proc Natl Acad Sci U S A. 2014;111(19):7132–7137. PubMed CrossRef
- Jacob TC, Michels G, Silayeva L, et al. Benzodiazepine treatment induces subtype-specific changes in GABA(A) receptor trafficking and decreases synaptic inhibition. Proc Natl Acad Sci U S A. 2012;109(45):18595–18600. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite