J Clin Psychiatry 2023;84(2):22cr14598
To cite: Wallner MA, Eloge JC, Mackey IV, et al. Safety and tolerability of concomitant intranasal esketamine treatment with irreversible, nonselective MAOIs: a case series. J Clin Psychiatry. 2023;84(2):22cr14598.
To share: https://doi.org/10.4088/JCP.22cr14598
© 2023 Physicians Postgraduate Press, Inc.
aRush Medical College, Chicago, Illinois
bRush University Medical Center, Chicago, Illinois
*Corresponding author: Margarete A. Wallner, BS, Rush Medical College, 600 S Paulina St, Ste 403, Chicago, IL 60612 ([email protected]).
Intranasal esketamine has been approved to treat adults with treatment-resistant depression (TRD) and depressive symptoms in adults with major depressive disorder with acute suicidal ideation or behavior in conjunction with an oral antidepressant. However, per esketamine prescribing information, concomitant use with monoamine oxidase inhibitors (MAOIs) may increase blood pressure.1 This case series reviews the safety of 3 female patients with TRD who received intranasal esketamine in conjunction with MAOI pharmacotherapy.
Case 1
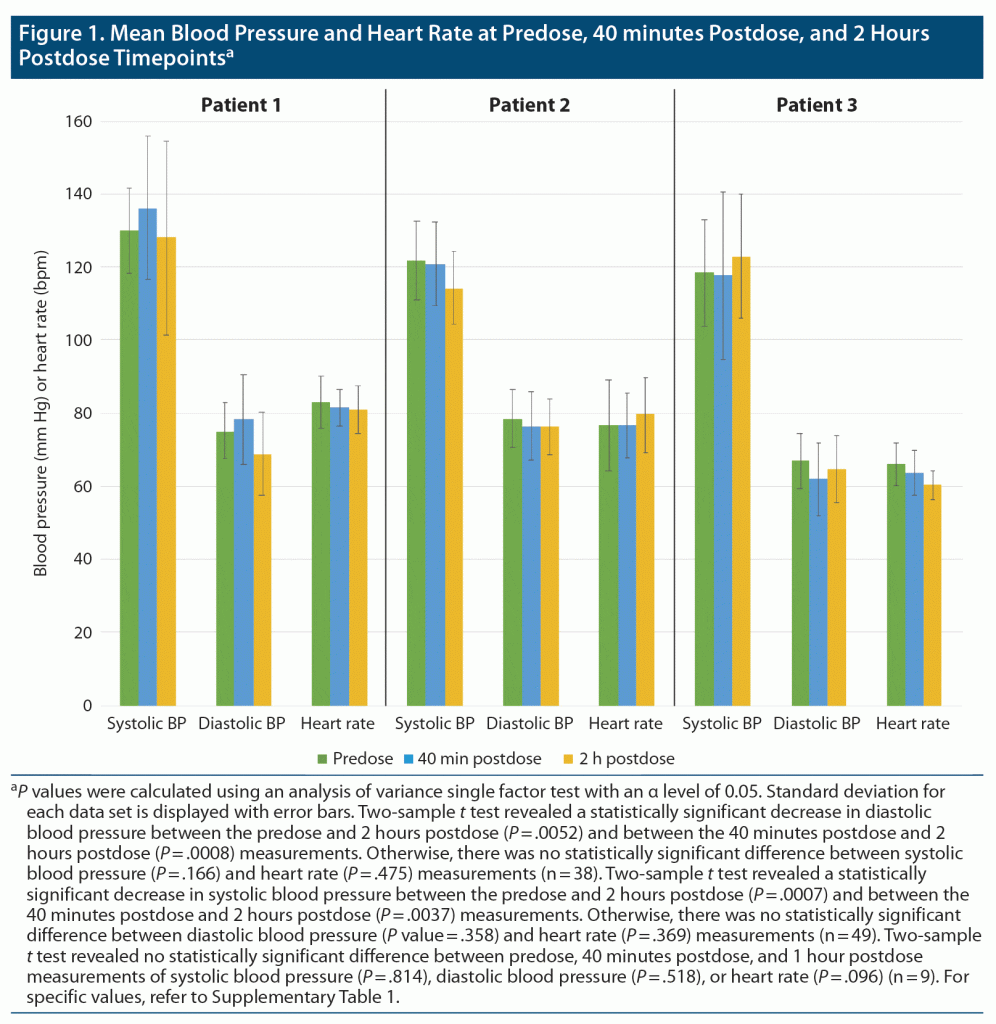
The first patient, a 74-year-old woman, was initiated on phenelzine in November 2020. The patient did not achieve remission, and thus she started augmentation with esketamine in April 2021. During the first treatment with esketamine 56 mg, the patient’s blood pressures at baseline, 40 minutes postdose, and 2 hours postdose were 116/79 mm Hg, 137/85 mm Hg, and 200/99 mm Hg, respectively. Although the patient was asymptomatic, she was given amlodipine 10 mg. A repeat blood pressure measurement approximately 3 hours postdose was 110/66 mm Hg. During the patient’s next esketamine treatment 4 days later, her afternoon dose (15 mg) of phenelzine was held and she received 84 mg of intranasal esketamine. The patient’s baseline, 40 minutes postdose, and 2 hours postdose blood pressures were 101/59 mm Hg, 128/80 mm Hg, and 142/78 mm Hg, respectively. The patient switched from phenelzine to tranylcypromine after a taper in February 2022 due to cognitive issues, such as confusion, and unsteadiness, as well as due to a lack of response to phenelzine therapy at a tolerable level. Throughout her remaining 37 treatments with esketamine and either phenelzine or tranylcypromine over approximately 11 months, she showed no significantly elevated blood pressure readings (Figure 1).
Case 2
The second patient, a 32-year-old woman, was initiated on esketamine in June 2019. After 73 esketamine treatments, the patient started augmentation with tranylcypromine for persistent suicidal ideation in November 2020. Throughout the patient’s concomitant 49 treatments with esketamine and tranylcypromine over approximately 14 months, she had no significantly elevated blood pressure readings (Figure 1).
Case 3
The third patient, a 61-year-old woman, was initiated on esketamine in March 2017, as a participant in the SUSTAIN-3 study, an esketamine open-label long-term extension study (NCT02782104). After 152 treatments, the patient did not achieve remission and thus started augmentation with tranylcypromine in March 2020. Throughout the patient’s subsequent 9 treatments with intranasal esketamine and MAOI therapies over approximately 2 months, she showed no significantly elevated blood pressure readings (Figure 1).
Discussion
Treatment of TRD with esketamine is approved to be initiated with an oral antidepressant. However, the clinical trials that led to the approval of esketamine for TRD excluded patients currently taking MAOIs, and there are limited data regarding esketamine and MAOI interactions.2 It is not uncommon for individuals with TRD to be taking MAOIs after failure of many other therapies3; however, given the lack of safety data and risk of elevated blood pressures, prescribers are often hesitant to use MAOIs in combination with other antidepressant therapies.4
One previously published case report5 showed no evidence of significantly elevated blood pressures with concomitant esketamine and tranylcypromine. Additionally, a recent retrospective cohort study6 that investigated cardiovascular changes when administering subcutaneous or intravenous esketamine with tranylcypromine for TRD found no clinically significant changes in blood pressure or heart rate. To our knowledge, this is the first case series that reviews the safety and tolerability of concomitant intranasal esketamine therapy with various MAOI therapies, specifically phenelzine and tranylcypromine.
These preliminary data suggest that when clinically indicated, concomitant use of intranasal esketamine with an irreversible, nonselective MAOI may be safe in some subjects acutely and long-term. However, careful blood pressure monitoring is necessary as evidenced by blood pressure elevation seen with phenelzine, but not tranylcypromine, in our first patient. It is important to consider that this phenomenon of blood pressure elevation specifically seen during this patient’s first esketamine administration could be due to intranasal esketamine alone.7 Given that this case series includes only 3 patients, there is still a need for more data to show that esketamine and MAOIs can be safely used in combination. However, given the role of MAOIs and esketamine in TRD, clinicians may need to consider the risks and benefits of treatments including this combination. Further studies should be pursued to examine the safety of combining intranasal esketamine and MAOI pharmacotherapies in TRD.
Published online: February 1, 2022.
Relevant financial relationships: Dr Zajecka serves on the advisory board of Alkermes, Alpha Sigma, Elminda, Ltd, Janssen/Johnson & Johnson, LivaNova, Lundbeck, Seelos Therapies, and Takeda; has received grant/research support from Alkermes, Allergan, Boehringer-Ingelheim, the Cheryl T. Herman Foundation, Elminda Ltd, Hoffman-LaRoche, Janssen/Johnson & Johnson, LivaNova, Lundbeck, Novartis, Neurocrine, Otsuka, Praxis, Sage Therapeutics, and Takeda; and has received other financial support from the Cheryl T. Herman Foundation. Mr Mackey is a compensated speaker for Janssen Pharmaceuticals and Teva Pharmaceuticals. Ms Wallner and Dr Eloge report no financial affiliation or other relationship relevant to the subject of this case series.
Funding/support: None.
Patient consent: Consent was received from the patients to publish the case reports, and information has been de-identified to protect anonymity.
Supplementary material: Available at Psychiatrist.com.
References (7)

- SPRAVATO [prescribing information]. Janssen Pharmaceuticals, Inc; July 2020.
- Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893–903. PubMed CrossRef
- Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. PubMed CrossRef
- Flockhart DA. Dietary restrictions and drug interactions with monoamine oxidase inhibitors: an update. J Clin Psychiatry. 2012;73(suppl 1):17–24. PubMed CrossRef
- Dunner DL, Fugate RM, Demopulos CM. Safety and efficacy of esketamine nasal spray in a depressed patient who was being treated with tranylcypromine: a case report. Neurol Psychiatry Brain Res. 2020;36:30–31. CrossRef
- Ludwig VM, Sauer C, Young AH, et al. Cardiovascular effects of combining subcutaneous or intravenous esketamine and the MAO inhibitor tranylcypromine for the treatment of depression: a retrospective cohort study. CNS Drugs. 2021;35(8):881–892. PubMed CrossRef
- Williamson DJ, Gogate JP, Kern Sliwa JK, et al. Longitudinal course of adverse events with esketamine nasal spray: a post hoc analysis of pooled data from phase 3 trials in patients with treatment-resistant depression. J Clin Psychiatry. 2022;83(6):21m14318. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top