Objective: Collaborative care models for treatment of adolescent depression are rapidly evolving. However, a dearth of information exists regarding patient characteristics associated with positive outcomes. We explored the association between baseline scores on routine screening tools for substance abuse, mood disorders, and anxiety with depression remission and graduation from a collaborative care program in an outpatient pediatric practice.
Methods: Adolescents (aged 12-17 years) with Patient Health Questionnaire-9 Modified for Adolescents (PHQ-9A) score ≥ 10 and a diagnosis of depressive disorder based on DSM-IV criteria between July 2011 and August 2015 were eligible for enrollment in a collaborative care model and inclusion in this study. Remission was defined as a single PHQ-9A score < 5; the criterion for graduation was 3 consecutive months with PHQ-9A score < 5. Analyses compared baseline assessment scores with those at remission and graduation.
Results: Of the 182 patients included in the analysis, the overall remission rate was 55%; program graduation rate was 27%. There was no association between scores on baseline screening tools and remission. Graduation was associated with lower scores on a screening tool for substance abuse (unit odds ratio [OR] = 1.62; P = .01) and anxiety (unit OR = 1.03; P = .02). When the scores were examined as categorical variables, graduation was associated with negative assessments on screening tools for substance abuse (OR = 3.21; P = .003) and anxiety (OR = 2.35; P = .02).
Conclusions: Baseline substance abuse and anxiety assessments may have utility in identifying depressed adolescents who are less likely to maintain remission and graduate from a collaborative care program, suggesting that these patients may need additional intervention to achieve sustained remission.
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.

CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation. A $5 processing fee will apply.
CME Objective
After studying this article, you should be able to:
‘ ¢ Use screening tools in conjunction with clinical interviews in the collaborative care setting to assess adolescent patients for risk factors for difficult-to-treat depression
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Creditâ„¢. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Academy of Physician Assistants (AAPA) accepts certificates of participation for educational activities certified for AMA PRA Category 1 Creditâ„¢ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 1 hour of Category I credit for completing this program.
Release, Expiration, and Review Dates
This educational activity was published in July 2018 and is eligible for AMA PRA Category 1 Creditâ„¢ through August 31, 2020. The latest review of this material was July 2018.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Marlene P. Freeman, MD, Editor in Chief, has received research funding from JayMac and Sage, has been a member of the Independent Data Safety and Monitoring Committee for Janssen, has been medical editor for the Global Organization for EPA and DHA Omega-3s newsletter, and, as a Massachusetts General Hospital (MGH) employee, works with the MGH National Pregnancy Registry, which is sponsored by Alkermes, Otsuka, Actavis, and Sunovion, and works with the MGH Clinical Trials Network and Institute, which receives research funding from multiple pharmaceutical companies and the National Institute of Mental Health. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
An Examination of Screening Tools for Collaborative Care of Adolescent Depression
ABSTRACT
Objective: Collaborative care models for treatment of adolescent depression are rapidly evolving. However, a dearth of information exists regarding patient characteristics associated with positive outcomes. We explored the association between baseline scores on routine screening tools for substance abuse, mood disorders, and anxiety with depression remission and graduation from a collaborative care program in an outpatient pediatric practice.
Methods: Adolescents (aged 12-17 years) with Patient Health Questionnaire-9 Modified for Adolescents (PHQ-9A) score ≥ 10 and a diagnosis of depressive disorder based on DSM-IV criteria between July 2011 and August 2015 were eligible for enrollment in a collaborative care model and inclusion in this study. Remission was defined as a single PHQ-9A score < 5; the criterion for graduation was 3 consecutive months with PHQ-9A score < 5. Analyses compared baseline assessment scores with those at remission and graduation.
Results: Of the 182 patients included in the analysis, the overall remission rate was 55%; program graduation rate was 27%. There was no association between scores on baseline screening tools and remission. Graduation was associated with lower scores on a screening tool for substance abuse (unit odds ratio [OR] = 1.62; P = .01) and anxiety (unit OR = 1.03; P = .02). When the scores were examined as categorical variables, graduation was associated with negative assessments on screening tools for substance abuse (OR = 3.21; P = .003) and anxiety (OR = 2.35; P = .02).
Conclusions: Baseline substance abuse and anxiety assessments may have utility in identifying depressed adolescents who are less likely to maintain remission and graduate from a collaborative care program, suggesting that these patients may need additional intervention to achieve sustained remission.
J Clin Psychiatry 2018;79(4):17m11543
To cite: Ginsburg AD, Stadem PS, Takala CR, et al. An examination of screening tools for collaborative care of adolescent depression. J Clin Psychiatry. 2018;79(4):17m11543.
To share: https://doi.org/10.4088/JCP.17m11543
© Copyright 2018 Physicians Postgraduate Press, Inc.
aMayo Medical School, Mayo Clinic College of Medicine and Science, Rochester, Minnesota
bDivision of Child and Adolescent Psychiatry and Psychology, Mayo Clinic, Rochester, Minnesota
cDivision of Preventive, Occupational, and Aerospace Medicine, Mayo Clinic, Rochester, Minnesota
dDivision of Community Pediatrics and Adolescent Medicine, Mayo Clinic, Rochester, Minnesota
‘ ¡Messrs Ginsburg and Stadem contributed equally to this article.
*Corresponding author: John E. Huxsahl, MD, Division of Child and Adolescent Psychiatry and Psychology, Mayo Clinic, 200 First St SW, Rochester, MN 55905 ([email protected]).
Recent legislation and shifting health care strategies are transforming the provision of behavioral health care. These processes are adding incentives to integrated medical-behavioral health practices to improve access to high-quality treatment of behavioral health conditions, enhance patient outcomes, and contain costs.1 Health systems are increasingly using collaborative care to provide high-quality care to patients with complex diseases and presentations.
While collaborative care models are demonstrating success in treating depression in adults,2,3 few studies explore the use of collaborative care in pediatric populations with depression.4 Adolescent depression may be particularly well suited for collaborative care approaches because it has a high prevalence, confers profound morbidity, and presents a substantial societal economic burden. Further, the national shortage of child and adolescent psychiatrists (CAPs) will necessitate more efficient models of care in upcoming years.5,6 These factors coalesce to require an interdisciplinary team-based approach. Because most adolescents have preexisting relationships with pediatricians or family practice physicians, building the collaborative care model around primary care maximizes its access.
Little research has examined patient characteristics that lead to successful collaborative care interventions. The purpose of the present study was to assess the utility of screening tools for predicting clinical remission of depressive symptoms and graduation from a collaborative care program among adolescents. We hypothesized that elevated scores on screening assessments of depression, anxiety, bipolar disorder, and substance abuse would be associated with lower remission and graduation rates. By evaluating these assessments, we hoped to determine the screening tools that can best identify patients who are least likely to respond to collaborative care models so that additional resources may be provided early in their course of treatment.

- Little research is available regarding which patient characteristics lead to unsuccessful collaborative care interventions in adolescents with depression.
- Adolescents who score higher on anxiety and substance abuse screening tools may be less likely to reach long-term remission from depression in a collaborative care treatment model.
METHODS
EMERALD Program
In July 2011, the Early Management and Evidence-Based Recognition of Adolescents Living with Depression (EMERALD) program began as a pilot in a community pediatric and adolescent medicine primary care site at a large medical center in the Upper Midwest affiliated with Mayo Clinic in Rochester, Minnesota. This initiative has expanded the lessons learned from the Depression Improvement Across Minnesota Offering a New Direction program to design and implement a collaborative care model for the management of depression in adolescents aged 13 to 17 years (or both 18 years of age and in high school).7 To meet the goals of the Minnesota Community Measures, the decision was made to include 12-year-olds in December 2012.8 This program has the following goals: (1) to increase the number of adolescents in the primary care setting who received safe, effective, outcomes-based treatment of their depression; (2) to provide a framework to transition the primary care providers (PCPs) and behavioral health providers from a specialty referral-based model to a collaborative care model; and (3) to establish evidence to support a collaborative care model for adolescent depression in a primary care setting.
Eligibility Criteria for EMERALD Program
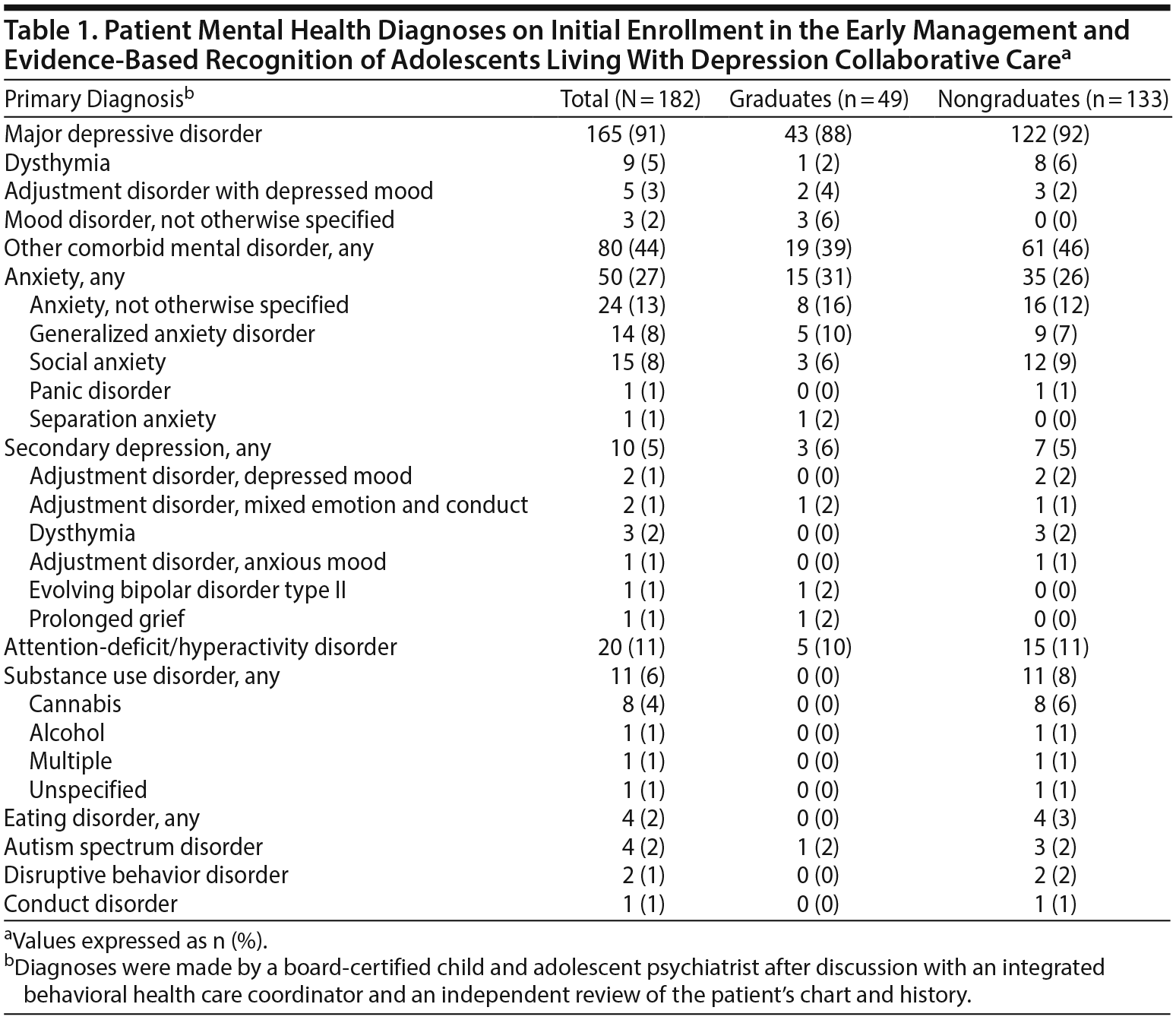
Adolescents aged 12 to 17 years with a Patient Health Questionnaire-9 Modified for Adolescents (PHQ-9A) score of 10 or greater were further assessed for EMERALD eligibility. Adolescents with a previous or new clinical diagnosis of a depressive disorder were eligible for EMERALD (see Table 1 for specific diagnoses of the included patients). Eligible adolescents and their parents were given the option of enrolling in the EMERALD program or receiving usual care, which consists of independent treatment by primary care or referral for behavioral health treatment or both. Adolescents with a previous diagnosis of bipolar disorder, severe cognitive disability, severe psychotic disorders, or substance use disorder requiring primary chemical dependency treatment in accordance with the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)9 were not eligible for EMERALD enrollment. Anxiety disorders, attention-deficit/hyperactivity disorder (ADHD), learning disorders, substance use disorders not requiring primary chemical dependency treatment, and disruptive behavior disorders did not affect eligibility.
Overview of EMERALD Care
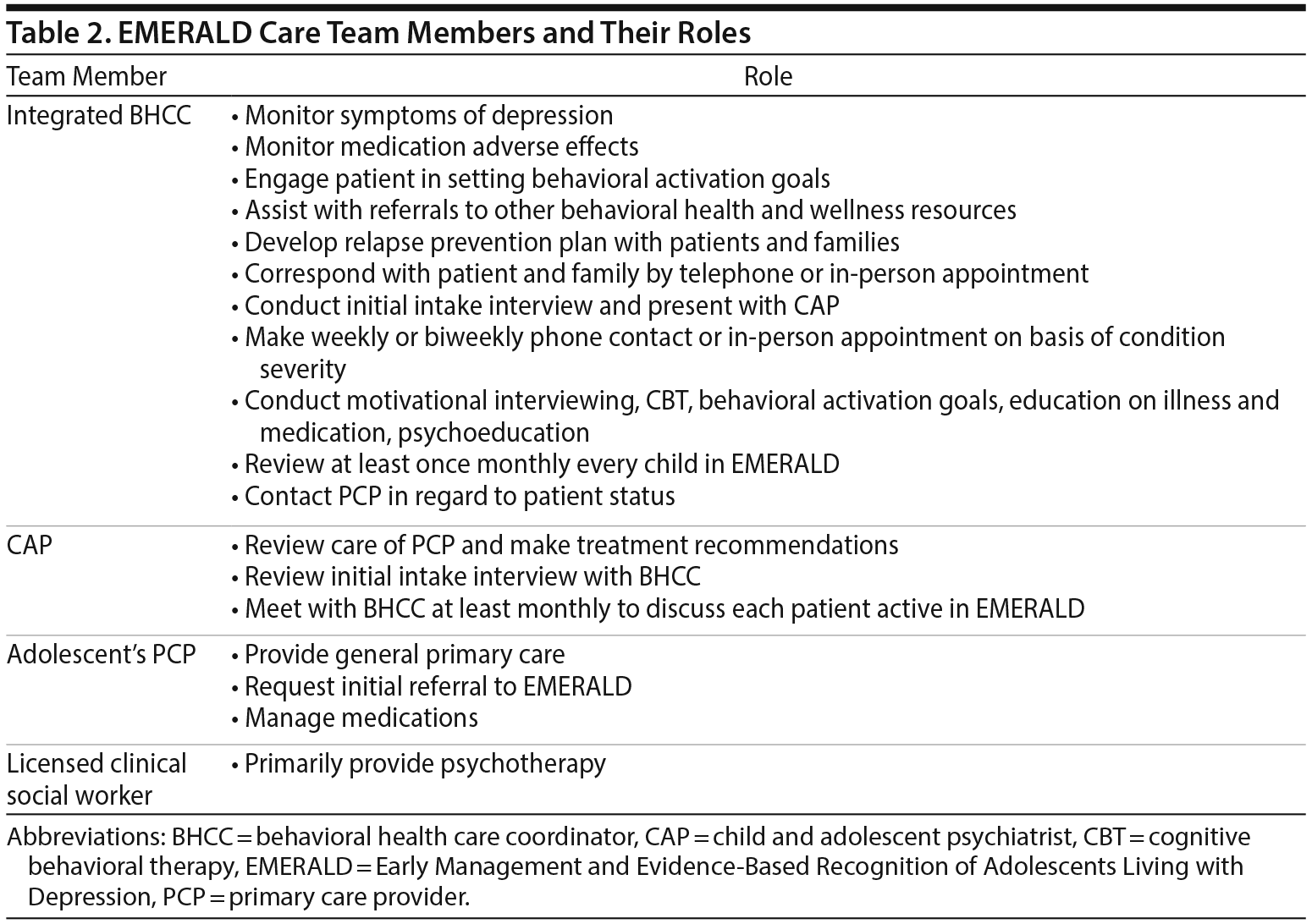
While collaborative care models vary, they typically consist of an integrated team of PCPs, psychiatrists, and nurses or allied health professionals serving as depression care managers. Through the depression care managers, these teams maintain frequent contact with patients to implement and adjust treatment strategies.10 In the EMERALD model, the role of depression care manager is undertaken by a registered nurse called a behavioral health care coordinator (BHCC). On enrollment, patients established their treatment goals with the BHCC. The BHCC communicated these treatment goals to both the patient’s PCP and CAP. Patients were initially contacted by the BHCC weekly or every 2 weeks during the first months of EMERALD care, with periods between contacts lengthening as treatment was established. Communication between the BHCC and the patient included telephone contacts, in-person appointments, and communication through the electronic health record portal system. Patient progress as documented by the BHCC was reviewed by a CAP at enrollment and at least once per month thereafter. In addition, the CAP completed a full review of the patient’s health record at EMERALD enrollment and reviewed ongoing care by the PCP. The CAP made treatment recommendations on an ongoing basis to the PCP for medication choice, dosage, and titration. Furthermore, the CAP made recommendations regarding psychotherapy.
Patients graduated from EMERALD when they had PHQ-9A scores of less than 5 for 3 consecutive months. Graduation criteria were prospectively set as 3 months with a PHQ-9A score less than 5, with the goals of operationalizing a more rigorous benchmark of remission in line with both contemporary conceptualizations (ie, to ensure the patient had at least a 2-month, symptom-free period) for research rigor and, more importantly, maximizing the long-term outcome of EMERALD patients.11 This 3-month period with PHQ-9A scores less than 5 was also deemed necessary in the context of clinical concerns that adolescent self-report measures of depression often have temporal instability.12 If graduation was reached, a remission plan was established, and EMERALD care was discontinued. Patients were removed from EMERALD if the BHCC was unable to contact the patient or the patient’s family for 2 consecutive months or the patient was enrolled in EMERALD without program graduation for 12 months. Patients who were enrolled in EMERALD for longer than 12 months were assumed to be nonresponders to the EMERALD collaborative care model and were referred for specialty care or provided resources as appropriate.
EMERALD Collaborative Care Team
The EMERALD care team consists of a BHCC, an adolescent PCP, a CAP, and a licensed clinical social worker. The roles of these team members are outlined in Table 2.
Study Practices
Eligible participants were located at a single community clinic affiliated with Mayo Clinic in Rochester, Minnesota, a large medical center in the Upper Midwest. This study was approved by the Mayo Clinic Institutional Review Board. All participants completed a Minnesota Research Authorization denoting permission to use deidentified data for the study in accordance with institutional review board policies.
Study Population
A total of 218 patients enrolled in EMERALD from July 2011 through August 2015. Patients were excluded from the analysis if they were currently receiving treatment in the EMERALD program (n = 29) or were pending enrollment in the EMERALD program (n = 3). Three patients were excluded because they were removed from the EMERALD program because of a change in their diagnosis to a nondepressive disorder. One patient was excluded because the patient did not meet the inclusion criteria for maximum age in EMERALD and was referred to adult providers. Most of the excluded patients (89%) were enrolled in EMERALD or pending enrollment at the time the data were obtained. Therefore, their outcomes could not be assessed, and their baseline characteristics were not compared with those of the study group. Cases of patients who were inactivated or dropped out of EMERALD before 3 months were included in the analysis as treatment failures.
Baseline Assessment
Before initiation of EMERALD care, patients were assessed for comorbid behavioral health conditions using the following screening tools.
CRAFFT is the most widely used and thoroughly studied standardized instrument for screening substance use in youth younger than 21 years. In addition, the American Academy of Pediatrics recommends it as the screening tool for adolescents 14 years and older.13 The acronym CRAFFT stands for the major keywords of the series of 6 questions in the tool, which are Car, Relax, Alone, Forget, Friends, and Trouble. The CRAFFT has a minimum score of 0 and a maximum score of 6.14
The Mood Disorder Questionnaire-modified for adolescents (MDQ-A) is a 13-item (yes/no) parent-reported screening tool for bipolar spectrum disorders that follows the DSM-IV criteria for bipolar disorder. A score of 5 or greater with co-occurrence of items, as well as impairment, is considered positive.13
The PHQ-9A is a 9-item self-report questionnaire that assesses depression symptoms and severity and has been validated for use with adolescents.15 Items based on DSM-IV criteria for depression are rated on a 4-point scale ranging from 0 (not at all) to 3 (nearly every day), with a maximum score of 27. The PHQ-9A includes minimal adjustments to the original Patient Health Questionnaire-9 (PHQ-9) to incorporate characteristics of depression among adolescents and age-appropriate language. Specifically, it includes irritability in the item assessing depressed mood, weight loss in the item assessing appetite, and additional self-harm and suicide items.15-18
The Spence Children’s Anxiety Scale (SCAS) is a behavior rating scale developed to measure the intensity of specific types of anxiety in children. SCAS has both child and parent versions, and each contains 38 questions, with 4 options (0-3) per question and a total maximum score of 114.19
All EMERALD patients were intended to be screened with each of the assessment tools described above at the initial intake. However, a small number of patients did not have results for all assessments. As a result, the sample analyzed for each screening tool was slightly less than the total study sample.
Primary Outcomes
Patients reached remission of depression when they had a single PHQ-9A score of less than 5, an outcome that was defined by the developers of the PHQ-9,20 has been frequently used in other studies,4,21-23 and is endorsed by Minnesota Community Measurement8 and the National Committee for Quality Assurance.24 Patients achieved graduation from EMERALD when their PHQ-9A score was less than 5 for 3 consecutive months.
Data Analysis
Screening tools were analyzed in comparison with remission and graduation through bivariate analysis using JMP software (SAS Institute Inc; Cary, NC). Screening tools were analyzed primarily as continuous variables. The P value is reported for the Wald χ2 test on the parameter of each screening tool. Since categorical thresholds have more clinical utility than continuous scores, the CRAFFT score and Spence Children’s Anxiety Scale-child version (SCAS-C) score were also examined as categorical variables in post hoc analysis. Due to the distribution of CRAFFT scores, scores of 0 were compared with scores greater than 0. The SCAS-C score positive values were 33 or greater for boys and 39 or greater for girls, in accordance with the published psychometric properties of the test.25
RESULTS
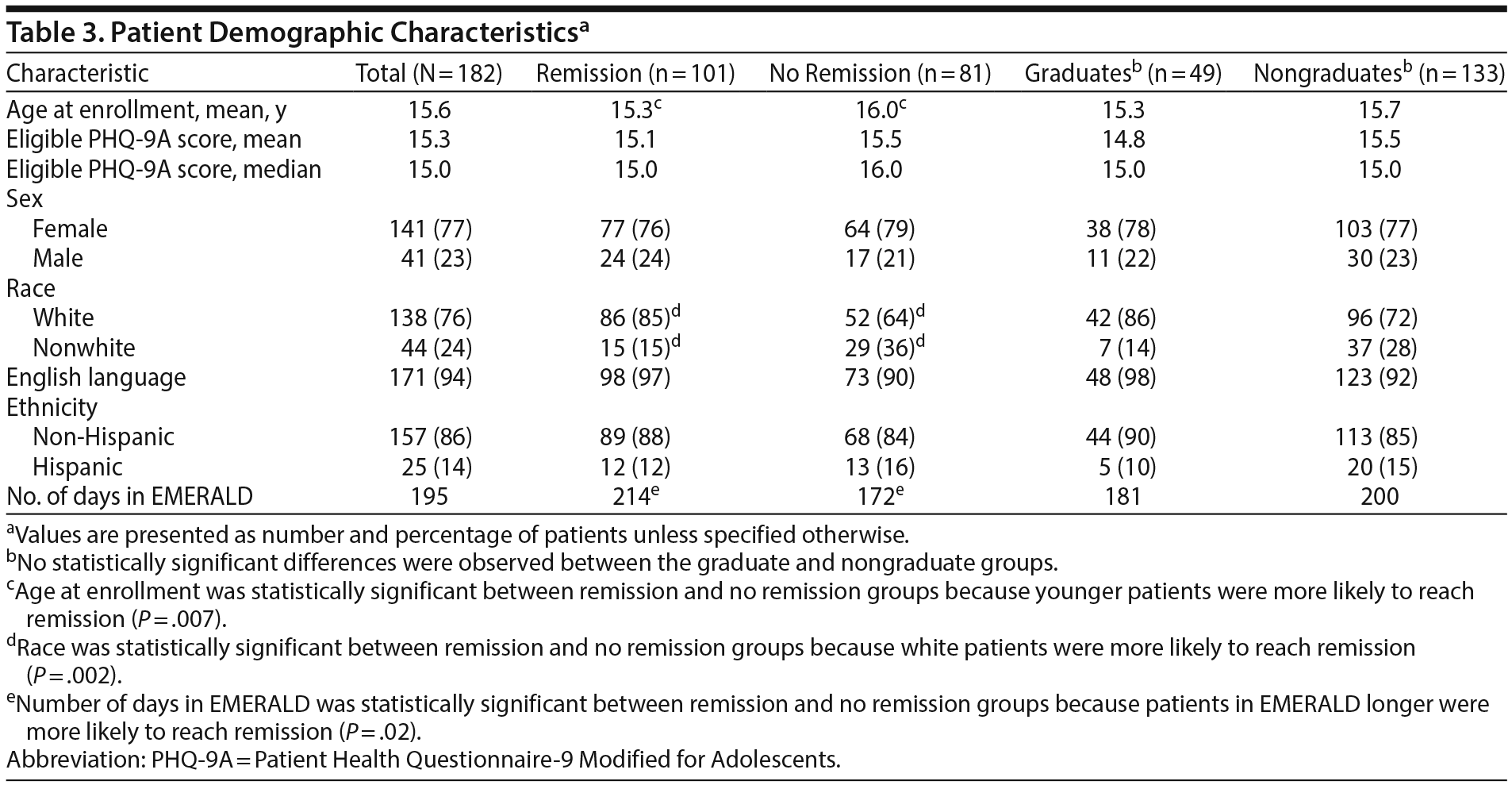
A total of 182 patients met inclusion criteria for the present study and were included in the analysis. One hundred one patients (55%) reached remission, and 49 patients (27%) achieved graduation from the EMERALD program. Of the other 133 patients who did not graduate, the reasons for inactivation included loss to follow-up (n = 85), treatment failure (active for 12 months without graduation) (n = 25), and patient’s choice (n = 23). Table 1 shows the initial patient mental health diagnoses and comorbidities. Table 3 shows the demographic information for remitters, nonremitters, graduates, and nongraduates. Of note, a statistically significant difference was found between remitters and nonremitters in age at enrollment (15.3 years vs 16.0 years, P = .007), race (85% white vs 64% white, P = .002), and number of days in EMERALD (214 days vs 172 days, P = .02). Although the difference in age at enrollment reached statistical significance, the magnitude of the difference was small and unlikely to be clinically meaningful. The difference in outcomes based on race most likely has clinical implications; however, because of a lack of economic and insurance data, it was not possible to further assess this relationship. Although the number of days in EMERALD differed between remitters and nonremitters, the relevance of time in the program was unclear. Importantly, there were no statistically significant differences between graduates and nongraduates.
Baseline Screening Measures and Graduation From EMERALD
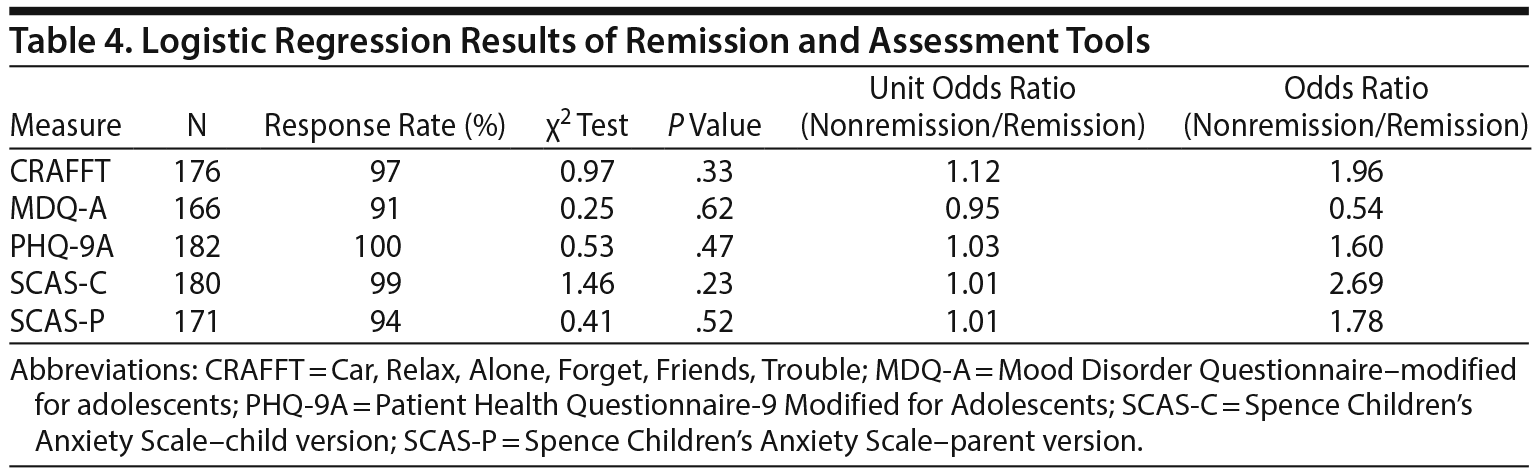
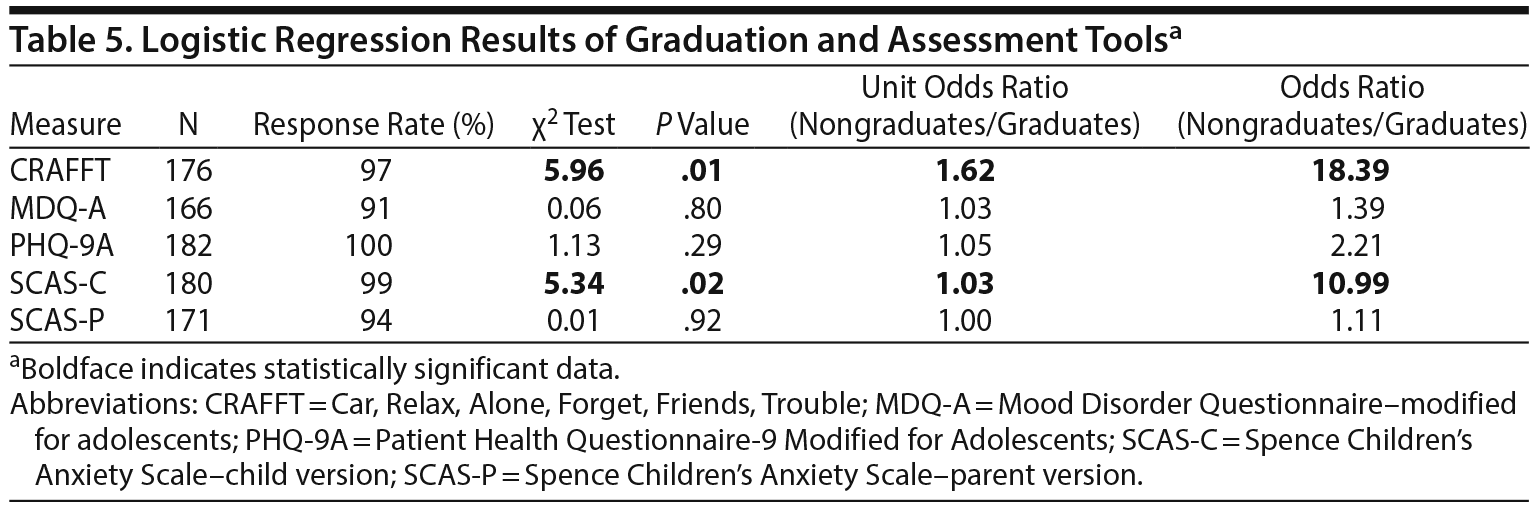
None of the screening tools used at baseline assessment had significant associations with remission (Table 4). Among patients who graduated, only CRAFFT and SCAS-C had significant associations with graduation (Table 5). Although CRAFFT scores for both groups were below the clinical threshold and the difference was of small magnitude, logistic regression analysis showed that higher scores decreased the likelihood of graduation (unit odds ratio [OR] = 1.62; P = .01). Indeed, as the CRAFFT score increased by 1 point, the odds of graduation were reduced by 62%. When the CRAFFT score was analyzed as a categorical variable (positive, ≥ 1), CRAFFT scores of 1 or greater significantly decreased the likelihood of graduation (OR = 3.21; P = .003). Similarly, higher scores on the SCAS-C had a small but significant inverse association with graduation (unit OR = 1.03; P = .02). When the SCAS-C was analyzed as a categorical variable (with positive considered as ≥ 33 in boys and ≥ 39 in girls), it was significantly associated with no graduation (OR = 2.35; P = .02). No significant difference was found between graduates and nongraduates in initial screening of MDQ-A, initial PHQ-9A, or SCAS-parent version.
DISCUSSION
A growing body of literature supports the use of collaborative care. Most of these studies have been conducted in adult populations and generally show a benefit in collaborative care on measures of depression.26-31 A much smaller group of studies has been conducted in pediatric populations. A recent meta-analysis32 compared collaborative care for children and adolescents with the usual care in treatment of behavioral health conditions. The authors found that collaborative care is superior to usual care in treatment, but not prevention, of mental health disorders (with the exception of substance abuse). However, multiple behavioral health conditions were grouped in that analysis, including ADHD, anxiety, depression, and substance abuse. In addition, the meta-analysis was limited because of the small number of studies and sample sizes that referenced adolescent populations, as well as study heterogeneity and primary outcome measurements.
In contrast to the 31 studies included in that meta-analysis,32 only 3 studies4,33,34 have focused specifically on collaborative care in the management of depression among children and adolescents. These studies have demonstrated varied results of collaborative care: 1 found no improvement in depression for adolescents receiving psychotherapy and pharmacotherapy administered in a collaborative manner compared with pharmacotherapy alone34; 1 found a small, significant improvement among patients receiving collaborative care compared with usual care33; and 1, a more recent study, demonstrated significant improvement at 12 months for adolescents in a collaborative care model.4
Whereas other studies of collaborative care models have defined remission as a single PHQ-9 score less than 5, our study adds a more stringent outcome—graduation—which we believe is more clinically applicable because it represents sustained remission. This is particularly important because adolescent self-report measures of depression such as the PHQ-9 quite likely have suboptimal temporal stability.12 The present study is an attempt to identify factors that are associated with graduation from a collaborative care model to help better identify patients who are likely to succeed in a collaborative care model and those who are not.
The results of the present study suggest that SCAS-C and CRAFFT are useful instruments for identifying patients who are less likely to achieve and maintain remission through collaborative care treatment for depression. These results suggest that EMERALD and perhaps other collaborative care models do not currently integrate resources necessary for patients with elevated scores on anxiety and substance abuse screening tools. Patients with elevated SCAS-C and CRAFFT scores quite likely need additional interventions specific to anxiety and substance abuse beyond the scope of the current EMERALD collaborative care model. By identifying these patients at the outset, the opportunity is greater for tailoring future treatment regimens for these patients.
These findings help delineate characteristics related to depression remission and graduation from the EMERALD collaborative care model. If the relationship between these characteristics and sustained remission of depression can be reproduced in future research, these data can be used to design collaborative care models for patient populations most likely to benefit and assist with the early identification of patients in need of more specialized care.
Limitations
This study has several limitations. Most importantly, patients were not systematically included in the EMERALD program. Although clear inclusion criteria have been identified for participation, many patients did not participate by choice or because of provider discretion. This factor could have created bias that affected our assessment of the screening tools.
The generalization of these findings is limited by variation in collaborative care models. Models at other institutions may differ markedly from the EMERALD program. It is therefore important that similar analyses of screening techniques are conducted on other collaborative care programs to learn whether the results are replicable.
Finally, collaborative care models have inherent internal variability due to the central role of the providers involved, particularly care coordinators. It is possible that graduation could be influenced by variation in the provision of care. Research under more controlled conditions would be helpful to determine characteristics that influence graduation regardless of provider variation.
CONCLUSION
This study suggests that use of CRAFFT and SCAS-C screening assessments at enrollment of collaborative care treatment of depression can aid in the early identification of patients at risk for treatment failure. Importantly, CRAFFT appears to have value even when patients are at apparently low risk for substance abuse disorders. Although elevated CRAFFT and SCAS-C scores were associated with a poor outcome in this sample, screening questionnaires should not replace a thorough clinical interview. However, these screening tools can be used in conjunction with such interviews to redirect resources to those patients who may be most at risk for treatment failure.
Submitted: February 22, 2017; accepted December 18, 2017.
Published online: July 17, 2018.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, no investigational information about pharmaceutical agents that is outside US Food and Drug Administration-approved labeling has been presented in this article.
Financial disclosure: Dr Croarkin has received grant support from the National Institute of Mental Health (K23 MH100266), the Brain and Behavior Research Foundation, Mayo Clinic, and Pfizer; has received in-kind support for equipment and supplies from Neuronetics for an investigator-initiated trial and is a site primary investigator for a Neuronetics-sponsored multicenter trial; and has received in-kind support for supplies and genotyping from Assurex Health for an investigator-initiated trial. Messrs Ginsburg and Stadem; Drs Takala, Billings, and Huxsahl; and Mss Mattson and Brennan have no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
Funding/support: This publication was supported by the grant UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). Dr Croarkin is supported by the National Institute of Mental Health (NIMH) of the National Institutes of Health (NIH) under award number K23MH100266.
Role of the sponsor: The funders (NCATS, NIH, and NIMH) had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Disclaimer: The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Previous presentation: Presented at the 63rd annual meeting of the American Academy of Child and Adolescent Psychiatry; October 27, 2016; New York, New York.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCES
1. Katon WJ, Unützer J. Health reform and the Affordable Care Act: the importance of mental health treatment to achieving the Triple Aim. J Psychosom Res. 2013;74(6):533-537. PubMed CrossRef
2. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10:CD006525. PubMed
3. Huffman JC, Niazi SK, Rundell JR, et al. Essential articles on collaborative care models for the treatment of psychiatric disorders in medical settings: a publication by the Academy of Psychosomatic Medicine Research and Evidence-Based Practice Committee. Psychosomatics. 2014;55(2):109-122. PubMed CrossRef
4. Richardson LP, Ludman E, McCauley E, et al. Collaborative care for adolescents with depression in primary care: a randomized clinical trial. JAMA. 2014;312(8):809-816. PubMed CrossRef
5. Fontanella CA, Hiance-Steelesmith DL, Phillips GS, et al. Widening rural-urban disparities in youth suicides, United States, 1996-2010. JAMA Pediatr. 2015;169(5):466-473. PubMed CrossRef
6. Thomas CR, Holzer CE 3rd. The continuing shortage of child and adolescent psychiatrists. J Am Acad Child Adolesc Psychiatry. 2006;45(9):1023-1031. PubMed CrossRef
7. Crain AL, Solberg LI, Unützer J, et al. Designing and implementing research on a statewide quality improvement initiative: the DIAMOND study and initiative. Med Care. 2013;51(9):e58-e66. PubMed CrossRef
8. MN Community Measurement. Data Collection Guide: 2016 Depression Care Measures. MNCommunity Measurement website. http://mncm.org/wp-content/uploads/2015/12/Depression-Care-Measures-2016-Data-Collection-Guide-FINAL-v1.pdf. 2015. Accessed July 14, 2016.
9. American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
10. Unützer J, Park M. Strategies to improve the management of depression in primary care. Prim Care. 2012;39(2):415-431. PubMed CrossRef
11. American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
12. Bennik EC, Nederhof E, Ormel J, et al. Anhedonia and depressed mood in adolescence: course, stability, and reciprocal relation in the TRAILS study. Eur Child Adolesc Psychiatry. 2014;23(7):579-586. PubMed CrossRef
13. Wagner KD, Hirschfeld RM, Emslie GJ, et al. Validation of the Mood Disorder Questionnaire for bipolar disorders in adolescents. J Clin Psychiatry. 2006;67(5):827-830. PubMed CrossRef
14. Knight JR, Shrier LA, Bravender TD, et al. A new brief screen for adolescent substance abuse. Arch Pediatr Adolesc Med. 1999;153(6):591-596. PubMed CrossRef
15. Johnson JG, Harris ES, Spitzer RL, et al. The Patient Health Questionnaire for Adolescents: validation of an instrument for the assessment of mental disorders among adolescent primary care patients. J Adolesc Health. 2002;30(3):196-204. PubMed CrossRef
16. Richardson LP, McCauley E, Grossman DC, et al. Evaluation of the Patient Health Questionnaire-9 Item for detecting major depression among adolescents. Pediatrics. 2010;126(6):1117-1123. PubMed CrossRef
17. Shaffer D, Fisher P, Lucas CP, et al. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39(1):28-38. PubMed CrossRef
18. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737-1744. PubMed
19. Spence SH. A measure of anxiety symptoms among children. Behav Res Ther. 1998;36(5):545-566. PubMed CrossRef
20. Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann. 2002;32(9).
21. Garrison GM, Angstman KB, O’ Connor SS, et al. Time to remission for depression with Collaborative Care Management (CCM) in primary care. J Am Board Fam Med. 2016;29(1):10-17. PubMed CrossRef
22. Angstman KB, Rasmussen NH, MacLaughlin KL, et al. Inter-relationship of the functional status question of the PHQ-9 and depression remission after six months of collaborative care management. J Psychiatr Res. 2013;47(3):418-422. PubMed CrossRef
23. Huijbregts KM, de Jong FJ, van Marwijk HW, et al. A target-driven collaborative care model for major depressive disorder is effective in primary care in the Netherlands: a randomized clinical trial from the depression initiative. J Affect Disord. 2013;146(3):328-337. PubMed CrossRef
24. National Committee for Quality Assurance. HEDIS depression measures specified for electronic clinical data systems. NCQA website. http://www.ncqa.org/hedis-quality-measurement/hedis-learning-collaborative/hedis-depression-measures. Accessed February 17, 2017.
25. Spence Children’s Anxiety Scale website. http://www.scaswebsite.com/. Accessed February 17, 2017.
26. Huffman JC, Mastromauro CA, Beach SR, et al. Collaborative care for depression and anxiety disorders in patients with recent cardiac events: the Management of Sadness and Anxiety in Cardiology (MOSAIC) randomized clinical trial. JAMA Intern Med. 2014;174(6):927-935. PubMed CrossRef
27. Katon W, Russo J, Lin EH, et al. Cost-effectiveness of a multicondition collaborative care intervention: a randomized controlled trial. Arch Gen Psychiatry. 2012;69(5):506-514. PubMed CrossRef
28. Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363(27):2611-2620. PubMed CrossRef
29. Katon WJ, Von Korff M, Lin EH, et al. The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry. 2004;61(10):1042-1049. PubMed CrossRef
30. Richards DA, Hill JJ, Gask L, et al. Clinical effectiveness of collaborative care for depression in UK primary care (CADET): cluster randomised controlled trial. BMJ. 2013;347:f4913. PubMed CrossRef
31. Unützer J, Katon W, Callahan CM, et al; IMPACT Investigators. Improving Mood-Promoting Access to Collaborative Treatment. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288(22):2836-2845. PubMed CrossRef
32. Asarnow JR, Rozenman M, Wiblin J, et al. Integrated medical-behavioral care compared with usual primary care for child and adolescent behavioral health: a meta-analysis. JAMA Pediatr. 2015;169(10):929-937. PubMed CrossRef
33. Asarnow JR, Jaycox LH, Duan N, et al. Effectiveness of a quality improvement intervention for adolescent depression in primary care clinics: a randomized controlled trial. JAMA. 2005;293(3):311-319. PubMed CrossRef
34. Clarke G, Debar L, Lynch F, et al. A randomized effectiveness trial of brief cognitive-behavioral therapy for depressed adolescents receiving antidepressant medication. J Am Acad Child Adolesc Psychiatry. 2005;44(9):888-898. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite