ABSTRACT
Objective: Fatty acids (FAs) are involved in the functioning of biological systems previously associated with suicidal behavior (eg, monoamine signaling and the immune system). We sought to determine (1) whether observed FA levels in a sample of military suicide decedents and living matched controls were consistent with latent classes having distinctive FA profiles and (2) whether those latent classes were associated with suicide and mental health diagnoses.
Methods: Serum samples from 800 US military suicide decedents who died between 2002 and 2008 and 800 demographically matched living controls were selected at random from a large military serum repository and assayed for 22 different FAs. A latent class cluster analysis was performed using values of 6 FAs previously individually associated with suicide. Once the latent classes were identified, they were compared in terms of suicide decedent proportion, demographic variables, estimated FA enzyme activity, diagnoses, and mental health care usage.
Results: A 6–latent class solution best characterized the dataset. Suicide decedents were less likely to belong to 2 of the classes and more likely to belong to 3 of the classes. The low-decedent classes differed from the high-decedent classes on 9 FAs and on estimated indices of activity for 3 FA enzymes: 14:0, 24:0, 18:1 n-9, 24:1 n-9, 22:5 n-3, 22:6 n-3, 20:2 n-6, 20:4 n-6, 22:5 n-6, elongation of very long chain fatty acids protein 1 (ELOVL1), ELOVL6, and Δ9 desaturase. The FA profiles of the latent classes were consistent with biological abnormalities previously associated with suicidal behavior.
Conclusions: This study suggests the utility of methods that simultaneously examine multiple FAs when trying to understand their relationship with suicide and psychiatric illness.
J Clin Psychiatry 2021;82(2):20m13275
To cite: Ryan AT, Postolache TT, Taub DD, et al. Serum fatty acid latent classes are associated with suicide in a large military personnel sample. J Clin Psychiatry. 2021;82(2):20m13275.
To share: https://doi.org/10.4088/JCP.20m13275
© Copyright 2021 Physicians Postgraduate Press, Inc.
aVeterans Affairs VISN 5 Mental Illness Research, Education, and Clinical Center (MIRECC), Baltimore, Maryland
bDepartment of Psychiatry, University of Maryland School of Medicine, Baltimore, Maryland
cNow with Rocky Mountain Mental Illness Research, Education, and Clinical Center (MIRECC), Rocky Mountain Regional Veterans Affairs (VA) Medical Center, Aurora, Colorado; Department of Psychiatry, University of Coloraso Anschutz School of Medicine, Aurora, Colorado; and Washington DC VA Medical Center, Washington, DC
dRocky Mountain MIRECC for Suicide Prevention, Aurora, Colorado
eWashington DC VA Medical Center, Washington, DC
fDepartment of Mental Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland
gDepartment of Psychiatry and Behavioral Sciences, Johns Hopkins School of Medicine, Baltimore, Maryland
hDepartment of Medical and Clinical Psychology, Uniformed Services University of the Health Sciences, Bethesda, Maryland
iOffice of New Drugs, Division of Psychiatry Products Center for Drug Evaluation and Research, Food and Drug Administration, Silver Spring, Maryland
jFort Belvoir Community Hospital, Fort Belvoir, Maryland
kConsortium for Health and Military Performance, Department of Military and Emergency Medicine, Uniformed Services University of the Health Sciences, Bethesda, Maryland
*Corresponding author: Arthur Thomas Ryan, PhD, Mental Illness Research, Education, and Clinical Center (MIRECC), Baltimore VA Annex, 7th Floor, 209 West Fayette St, Baltimore, MD 21201 ([email protected]).
Suicide is now the leading cause of death among members of the US military.1 Individuals who die from suicide have been shown to differ from other clinical populations and healthy controls on immune, endocrine, oxidative stress, and metabolic biomarkers.2–4
Based on their number of carbon double bonds, fatty acids (FAs) are classified into saturated FAs (SFAs), monounsaturated FAs (MUFAs), and polyunsaturated FAs (PUFAs); PUFAs include omega-3 (n-3) FAs and omega-6 (n-6) FAs. One FA may be converted to another via the activity of elongation of very long chain fatty acid proteins 1–7 (ELOVL1-7) as well as by delta (Δ) FA desaturases.5 Both de novo FAs produced by the body and FAs consumed in the diet can be elongated by these enzymes.6 These enzymes play an important role in circulating levels of FAs.
FAs are implicated in biological systems associated with depression and suicide, including neurotransmitters, cell membranes, and the immune system.7,8 Individuals with mental illness have altered levels of various FAs as compared with healthy controls.9 The levels of several FAs, particularly n-3 FAs, have also been associated with suicidal behavior; however, the relationship between various FAs and suicidal behavior has been somewhat inconsistent across studies.10
The current study analyzed serum FA levels in a sample of 800 military suicide decedents and 800 matched living military controls. We employed latent class cluster analysis (LCCA)11 to search for multi-FA profiles rather than examining the effects of individual FAs. We proposed the following 4 hypotheses: (1) LCCA would find that 2 or more latent classes better described serum FA concentrations than a single underlying class. This hypothesis stemmed from the documented heterogeneity of clinical and biological markers of suicidal behavior, which suggests the possibility that more homogenous subgroups might exist.12 (2) The proportion of suicide decedents would be greater in some of the latent classes. This hypothesis was based on existing literature showing the association of some FAs with suicidal behavior.10 (3) The latent classes would differ in mental health care utilization and psychiatric diagnoses. This hypothesis was based on the fact that risk for suicide has been linked to psychiatric illnesses such as depression and that FA levels have been linked to psychiatric illnesses.13,14 (4) FA elongase and desaturase activity (as estimated by ratios of measured FAs) would significantly differ between the latent classes. This hypothesis was based on existing research showing that differences in elongase and desaturase activity may be associated with mental health outcomes.15
METHODS
Sample and Procedures
A detailed description of the experimental and data collection procedures has been published in a previous study16 that employed the same participant sample. In brief, serum samples used in this analysis were provided by the Armed Forces Health Surveillance Center (AFHSC), which hosts a repository of fasting serum samples drawn regularly from US military personnel with matched health data. Eight hundred active duty US military personnel who died by suicide between 2002 and 2008 were randomly selected for inclusion in the study, as were 800 living matched controls (total N = 1,600). Controls were randomly selected by the AFHSC and matched by age, sex, and rank; they were not matched on any other variables. Each suicide decedent’s index serum sample was the serum sample collected closest to the decedent’s date of death. For the living matched controls, the serum sample selected was the one collected most proximally in time to when the corresponding suicide decedent’s sample was collected. Controls were required to have a sample collected within 1 year of their matched decedent’s sample. Controls, but not decedents, were also required to have an available DD 2679 form: the DD 2679 form is required to be completed by a US service member after a military deployment and collects information about exposure to environmental hazards and potentially traumatic events (eg, seeing a fellow service member killed). Given the requirement that controls have a DD 2679 form, all of them had been deployed at some point during their military service; in contrast, 62% of suicide decedents were deployed at some point during their service. Matched living controls were not necessarily “healthy” controls and could have diagnoses of any mental or physical health condition so long as they were alive at the time of data collection and met the other specified enrollment criteria.
The institutional review board of The Uniformed Services University of the Health Sciences (FWA00001628; DOD assurance P60001) granted approval for this study’s research protocol (HU873B-01). A waiver was granted to analyze already collected health care data and blood serum samples without seeking individual consent from each of the individuals whose data were used.
Serum Analysis
All serum samples were assayed for total FA composition at a single laboratory utilizing a high throughput robotic direct methylation coupled with fast gas-liquid chromatography.17,18 The case status of all samples was masked until all FA analyses were completed. All samples were collected while individuals were fasting, and all FAs were expressed as percent of total FAs.19 Values for 22 separate FAs were measured in each of these samples, and the activity of a further 5 FA elongases and 4 FA desaturases was estimated based on the ratios of measured FAs.
Clinical Data
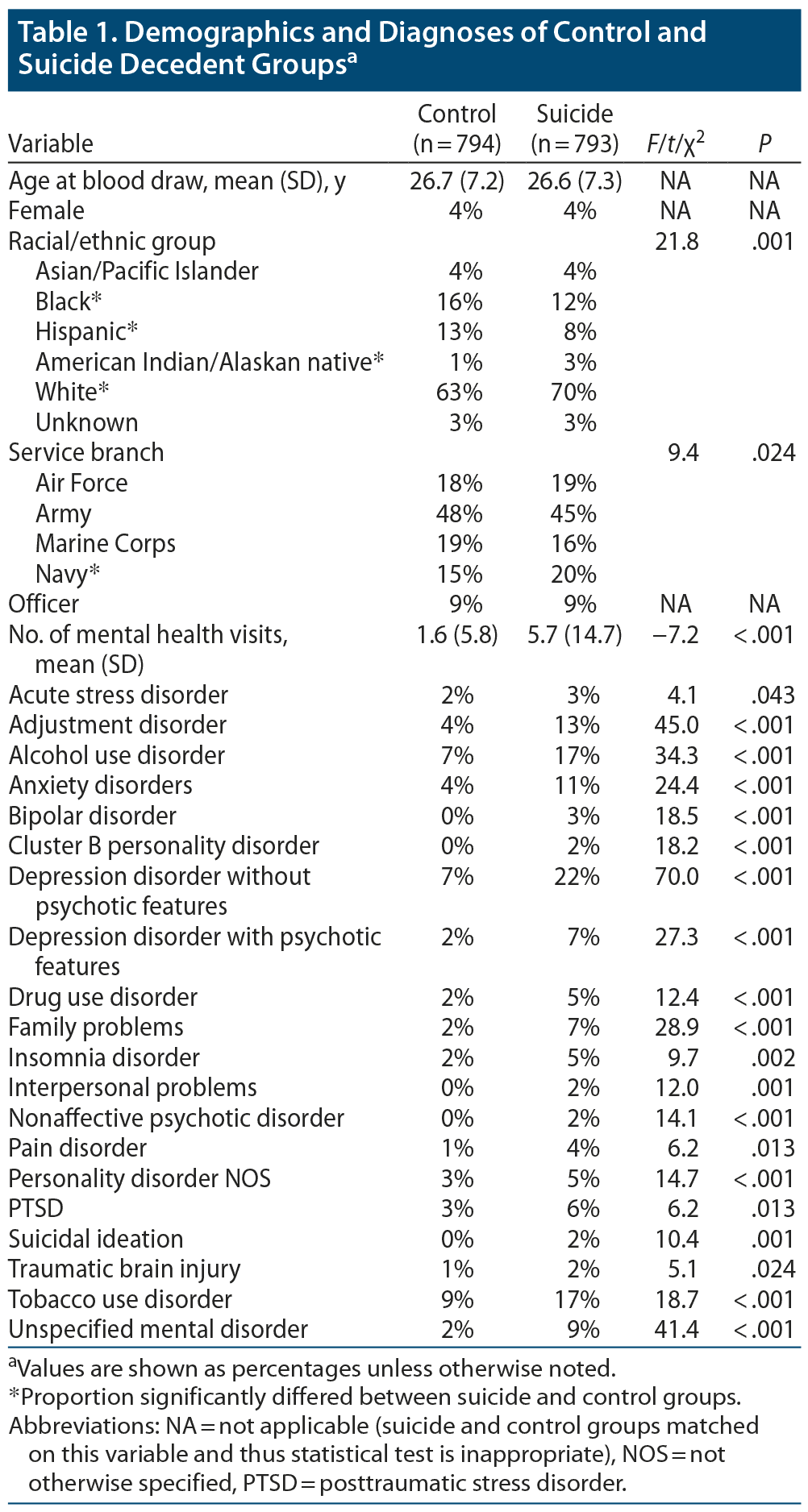
ICD-9 discharge diagnosis codes from inpatient and ambulatory/outpatient care data reports were provided by AFHCS. A mental health visit was operationalized as any health care visit that included an ICD-9 mental health code (ICD-9 290–219) or substance abuse codes (ICD-9 292, 303–305). An initial listing of the frequency of all ICD-9 diagnoses from mental health visits was reviewed. Based on this review, diagnoses for similar conditions were combined into summary categories. Demographics and diagnosis rates for the suicide decedent and living control groups are shown in Table 1.
Statistical Analyses
FAs from 13 individuals (7 cases and 6 controls) were not successfully assayed, and thus data for these individuals were not included in the analysis. FA data were assessed for normality of distribution and population skewing: no outliers were excluded. All case and control participants with valid FA data (n = 1,587) were included in the LCCA. FA data (expressed as percent of total FAs) were converted to z-scores and entered as continuous variables into the LCCA.
LCCA is an empirically driven method for determining whether the observations in a dataset appear to reflect either (a) a single underlying population with a mean and standard deviation for each variable or (b) several underlying populations (ie, latent classes), each with its own mean and standard deviation on each of the observed variables. Several previous studies12,20,21 of suicidal behavior have used LCCA and related techniques to identify latent classes that differ on important outcomes, eg, the likelihood of later suicide. We used R version 3.4.3 supplemented with the mclust package to conduct the LCCA.22–24 The mclust package implements LCCA by attempting to identify a best fitting Gaussian finite mixture model—ie, the one with the lowest Bayesian information criterion (BIC) value—using an expectation-maximization (EM) algorithm. In our LCCA, we included values for 5 FAs that a previously published study16 employing the same dataset had identified as being individually associated with the likelihood of being a decedent rather than a control: (1) 18:0, (2) 16:1 n-7, (3) 18:1 n-7, (4) 22:6 n-3, and (5) 20:3 n-6. We additionally included a sixth FA, 20:5 n-3 EPA, in our LCCA analysis because its association with decedent status reached near significance in the original study’s analysis and because previous studies by other researchers have found associations between EPA and suicide.10 To be clear, only the values for these 6 FAs were included during computation of the LCCA model: the data for the remaining 16 FAs and 9 enzymes were set aside and analyzed only after the latent classes were identified. We used the subset of 6 FAs rather than including all observed FAs in the LCCA because we were most interested in discovering latent classes of fatty acids that were associated with suicidal behavior. As these fatty acids had already been shown to be associated individually with suicidal behavior, we believed that profiles derived from them would be most likely to identify latent classes relevant to suicidal behavior. In contrast, if all fatty acids were included, the identified FA profiles might well reflect, for example, general truths about latent classes of FA profiles generally (eg, that there is a high saturated FA profile and low saturated FA profile and that these are associated with obesity and healthy body weight), but these profiles would be less likely to convey information about suicide-relevant biology.
Once the LCCA determined the number of classes that best described the underlying dataset and assigned individuals to each of the identified classes, those class assignments served as the grouping variables for all subsequent analyses.
Chi-square (χ2) and analysis of variance (ANOVA) tests were used to compare the classes on nominal and numeric variables, respectively. If a χ2 or ANOVA test was significant, individual comparisons were run between each of the classes to determine which classes significantly differed from one another on that variable.
Indices of elongase and desaturase enzyme activity can be calculated using the ratio of a FA product to its FA precursor.25–27 For each participant, we calculated this ratio for each relevant FA pair, yielding a single raw ratio for each pair that we then converted to a z-score; we then calculated the mean of the z-scores for the ratios associated with each enzyme to create an enzyme’s index score. We conducted 1-way ANOVAs to compare the classes on the enzyme index scores and performed pairwise comparisons for significant ANOVAs.
RESULTS
Latent Class Cluster Analysis
The LCCA analysis of the 6 selected FAs identified an ellipsoidal, equal shape and orientation Gaussian distribution with 6 classes as the best fitting model (log likelihood = 39,184, n = 1,587, df = 63, BIC = 77,874, clustering table = 170/183/947/88/160/39). Bootstrap likelihood ratio testing confirmed that the 6-class solution represented a significant improvement over the 5- and 7-class solutions. A normalized entropy value provides an overall estimate of how confidently a LCCA model predicts the class membership of each individual. A value of ≥ 0.80 is considered to indicate that a model is a good fit for the data.28,29 The normalized entropy value for our model was 0.85, suggesting the model was a good fit for the data.
Case Prevalence and Serum Fatty Acid Comparisons Across Classes
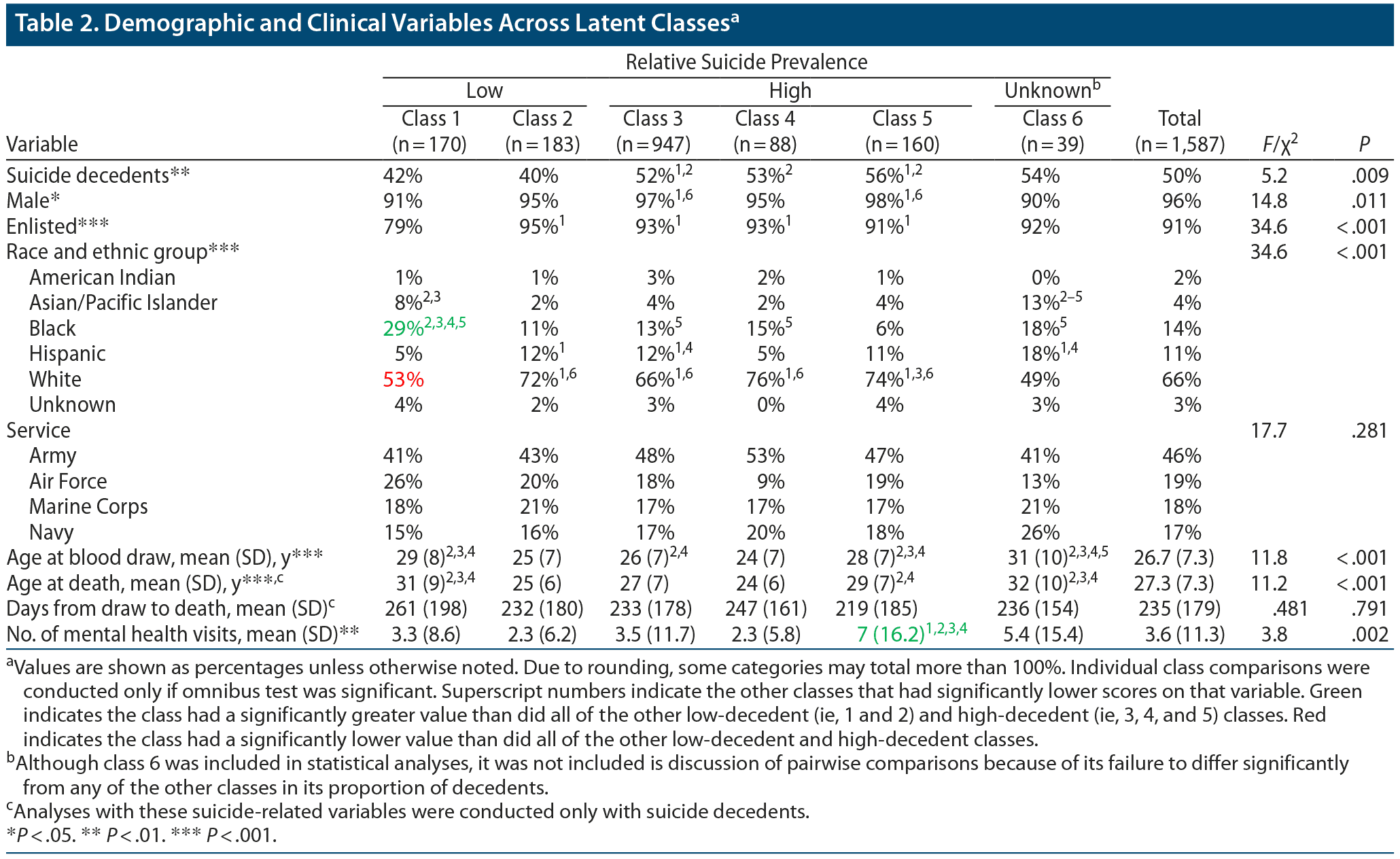
A χ2 test showed that suicide decedents were unequally distributed among the classes, χ25 = 5.19, P = .009; N = 1,587). Between-group comparisons showed that classes 1 and 2 had significantly lower proportions of decedents than classes 3 and 5. In addition, class 2 had a significantly lower proportion of decedents than class 4. We thus refer to classes 1 and 2 as “protective” classes given their relatively lower proportion of decedents and classes 3, 4, and 5 as “risky” classes given their relatively higher proportion of decedents. The proportion of decedents in class 6 did not significantly differ from that in any of the other classes, most likely due to its small sample size. We included class 6 in all subsequent statistical analyses. However, class 6 is not included in descriptions of pairwise comparisons because of its failure to differ significantly from any of the other classes in its proportion of decedents. The odds ratio of being a suicide decedent for members of the risky classes (3, 4, and 5) as compared with members of the protective classes (1 and 2) was 1.61 (95% CI, 1.27–2.05; P < .001). Details regarding the individual classes can be found in Table 2.
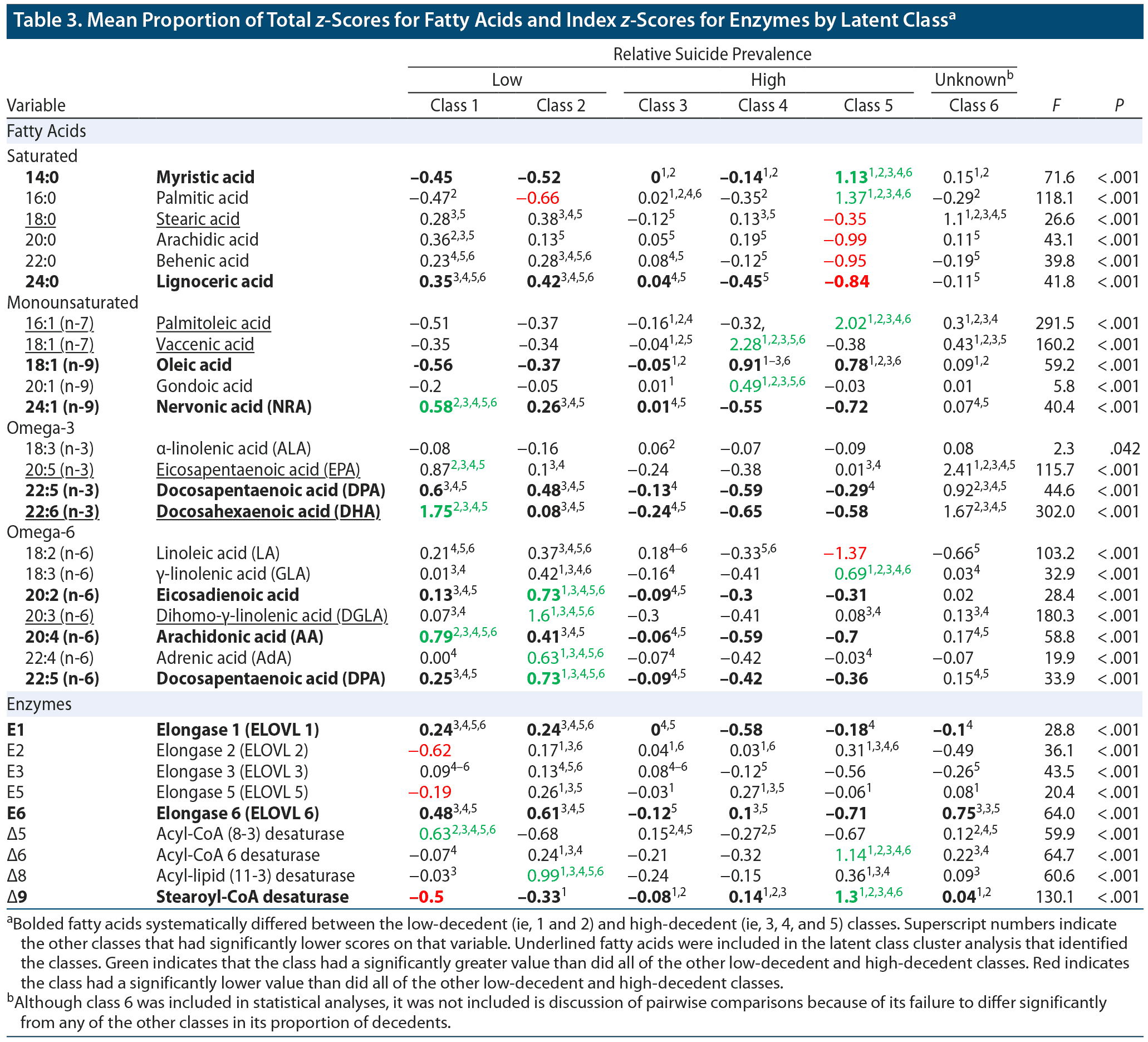
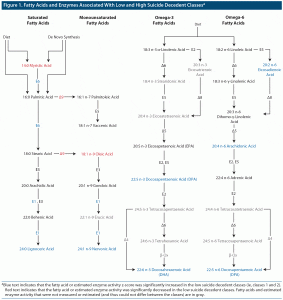
We analyzed how the latent classes differed on the 22 measured FAs (ie, the 6 FAs employed in the LCCA that identified the latent classes as well as the additional 16 FAs that had been set aside and not used by the LCCA) as well as on estimated activity for 5 FA elongases and 4 FA desaturases. Table 3 shows the proportion of total FA z-scores and estimated enzyme activity index z-scores for each of the classes. The results are also summarized in the context of a fatty acid metabolism diagram in Figure 1. As can be seen in Table 3, 9 FAs and 3 enzymes significantly differed between the protective and risky classes (ie, were higher or lower in both protective classes as compared with the 3 risky classes). Those FAs and enzymes were (1) 14:0 myristic acid, (2) 24:0 lignoceric acid, (3) 18:1 n-9 oleic acid, (4) 24:1 n-9 nervonic acid, (5) 22:5 n-3 docosapentaenoic acid (DPA n-3), (6) 22:6 n-3 docosahexaenoic acid (DHA), (7) 20:2 n-6 eicosadienoic acid, (8) 20:4 n-6 arachidonic acid (AA), (9) 22:5 n-6 docosapentaenoic acid (DPA n-6), (10) ELOVL1, (11) ELOVL6, and (12) Δ9 desaturase.
Demographic and Clinical Variables
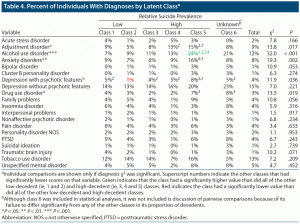
Demographics and number of mental health visits for the classes are summarized in Table 2. The classes differed significantly on sex, military grade, race/ethnicity, and mean number of mental health visits. Results of pairwise comparisons for these variables are also shown in Table 2. The proportions of individuals in each class with various psychiatric diagnoses are shown in Table 4. The classes significantly differed in their rates of adjustment disorders, alcohol use disorders, anxiety disorders, depression with psychotic features, and drug use disorders.
Age at Suicide and Days Between Blood Draw and Suicide
Age at suicide significantly differed between the classes (F5, 787 = 11.174, P < .001). Days between serum blood draw and suicide did not differ significantly between the classes (F5, 787 = 0.48, P = .79), suggesting that time between the serum draw and death did not account for the differences between classes. These results are shown in Table 2.
DISCUSSION
General Discussion
In this study, we performed a LCCA on serum concentrations of 6 FAs in a sample of US military suicide decedents and living matched controls. A 6-class model best represented the underlying data. Two “protective” classes had significantly lower proportions of suicide decedents than 3 “risky” classes. The proportion of suicide decedents in 1 class did not significantly differ from any of the other classes, most likely due to its small size. The protective and risky classes showed systematic differences on levels of 9 FAs, including 8 FAs that were not analyzed by the initial LCCA. Estimates of the activity of 2 FA elongases and 1 FA desaturase also differed systematically between the protective and risky classes.
Constellations of Fatty Acids in the Protective and Risky Classes Are Consistent With Biological Abnormalities Previously Associated With Suicidal Behavior
The protective classes had increased levels of the very long chain FAs 22:5 n-3 (DPA), 22:6 n-3 (DHA), 24:0, and 24:1 n-9 as well as increased ELOVL1 activity. ELOVL1 activity produces 24:0 and 24:1 n-9 FAs (collectively referred to as C24 FAs). 22:5 n-3 (DPA), 22:6 n-3 (DHA), and C24 FAs play key roles in the creation and regulations of lipid rafts, a form of lipid microdomain.30–33 In neurons, lipid rafts modulate monoaminergic (ie, serotonergic, dopaminergic, and adrenergic) neurotransmission by altering the spatial organization of neurotransmitter receptors and transporters, the endocytosis of neurotransmitter receptors, agonist and antagonist binding, and downstream secondary-messenger effects.31,32 For example, the disruption of lipid rafts via cholesterol depletion has been shown to decrease serotonin transporter activity by 50%.34,35 Modification of lipid raft functioning by antidepressants and mood stabilizers has been hypothesized to contribute to their therapeutic modulation of neurotransmitter signaling.32 Abnormalities of dopamine, norepinephrine, and especially serotonin signaling are well established in mood disorders and suicidal behavior.36–38 Thus, the different profiles of FAs between the risky and protective clusters may reflect differences in lipid raft functioning, which in turn might reflect differences in monoaminergic neurotransmission that predispose some individuals toward suicidal behavior.
The protective clusters’ increased levels of 22:6 n-3 (DHA), 20:4 n-6 (AA), and C24 FAs and ELOVL1 may also be related to suicidal behavior via their relationship to myelin. These FAs and ELOVL1 appear to be essential for the creation and maintenance of myelin.33,39–42 Reduced white matter volume and integrity is a well-established finding in individuals who have attempted or died by suicide.43
Our finding of increased Δ9 desaturate activity in the risky classes is consistent with a study44 that found increased expression of Δ9 desaturase activity in the prefrontal cortex of individuals with major depressive disorder who died of suicide compared with those who died of other causes. In a previous study26 of individuals with recurrent depression and healthy controls, the individuals with recurrent depression also shared the risky classes’ pattern of increased levels of 16:1 n-7, 18:1 n-9, and Δ9 desaturase.
Protective class 1 evinced increased Δ5 desaturase activity and increased levels of its downstream FA products, including 20:5 n-3 EPA, 22:6 n-3 DHA, and 20:4 n-6 AA. Class 1’s increased Δ5 desaturase activity is consistent with a postmortem gene expression study45 that found decreased expression of the Δ5 desaturase gene (FADS1) in the prefrontal cortex of suicide decedents. Class 1’s increased levels of 20:5 n-3 EPA and 22:6 n-3 DHA are also consistent with previous studies, which have often, though not always, found that elevated levels of these FAs are associated with reduced risk of suicide.10 The difference in EPA and DHA levels between the two protective classes might help to explain the sometimes inconsistent findings regarding whether increased EPA and DHA levels are associated with reduced risk of suicide; our findings suggest that one potentially protective lipid profile (ie, that of class 2) does not involve marked elevations of EPA and DHA.
De novo FA lipogenesis primarily produces 14:0 and 16:0.6 The risky classes showed marked elevations of 14:0 and 16:0. Alterations in de novo lipogenesis are associated with obesity, insulin resistance, and inflammatory disease, all of which have been linked with depressive illness and suicide.46 All of the risky classes also had particularly low levels of 22:5 n-3 DPA and 22:6 n-3 DHA. One method by which low levels of DPA and DHA might be associated with suicide is via increased inflammation. Along with 22:5 n-6 and 20:4 n-6, 22:5 n-3 DPA is an endogenous ligand for the anti-inflammatory retinoid X receptor (RXR).47 DHA inhibits the inclusion of toll-like receptor (TLR) 4 into the cell membrane: TLR4 has been found to be altered in the postmortem brain tissue of suicide decedants.48 DPA and DHA also serve as the precursors to anti-inflammatory and neuroprotective molecules, including resolvins and protectins.47 Considered as a whole, the low levels of DHA and DPA in the risky classes might be associated with increased inflammation and atrophy of brain tissue, both of which have been associated with suicidal behavior.43,49
Strengths
Strengths of our study include a large cohort of suicide decedents, the collection of serum samples prior to death by suicide, and a carefully demographically matched control group.
Our study provides novel findings that go beyond those found in the original analysis of this dataset by Lewis et al16 in the following ways: (a) While our LCCA identified latent classes using 20:5 n-3 and the 5 FAs that Lewis et al identified as being individually associated with suicidal behavior, the latent classes identified by our LCCA did not simply represent high and low values on the suicide-related FAs from that study. Indeed, 7 of the 8 FAs that differed systematically between the protective and risky classes in our analysis were not at all associated with suicidal behavior in the original analysis by Lewis et al. Thus at a basic level, we did not simply replicate the findings of Lewis et al; instead, we found a very different set of FAs that were associated with suicidal behavior using LCCA. (b) Our analysis allows for the specification of multi-FA profiles that were associated with suicidal behavior rather than a list of individual FAs that were associated with suicidal behavior. As a specific example of this, Lewis et al reported that an increased level of DHA was associated with decreased risk of being a suicide decedent. In contrast, our analysis allows us to state that increased levels of DHA characterize one of the protective profiles (class 1) but not the other (class 2). Instead, class 2’s profile was associated with elevations in a host of n-6 fatty acids that were not found significant in Lewis and colleagues’ analyses. (c) The increased level of specificity in our analysis may allow for the identification of biological mechanisms that may be relevant to suicidal behavior in subgroups of individuals with particular FA profiles (eg, de novo FA synthesis in risky class 5) but not for individuals with other FA profiles. The relevant biological mechanism thus could be studied in individuals who display that particular FA profile but not in individuals who have other profiles, whose suicidal behavior might be related to other biological mechanisms. (d) Similarly, the biological mechanisms suggested by particular FA profiles could suggest which interventions might be particularly relevant to particular individuals, eg, interventions targeting mechanisms related to de novo FA synthesis might be more likely to help individuals in risky class 5 but not for individuals with other FA profiles.
Limitations
A primary limitation of this observational study is the inability to examine the causal underpinnings of the particular constellations of FA levels observed in the latent classes and those FAs’ association with later suicide. Differences in FA levels across the classes may stem from a variety of causes. Dietary intake of FAs and genetic polymorphisms conferring increased or decreased FA enzyme activity can alter FA serum levels.25 Dietary intake of FAs can also lead to changes in the methylation of FA enzyme genes and thereby increase or decrease enzyme transcription.50 We also lacked information on diet and medications that could have affected FA levels.51 The activation of biological systems known to be independently associated with mental illness and suicide, eg, the hypothalamic-pituitary-adrenal axis and immune system, can also directly alter FA metabolism, which further complicates the question of causality.52,53 Another limitation to note is that the “risky” class FA profiles should not be thought of as providing information that can be used in a clinical setting to identify patients at particularly high risk for suicide; like all other known biological markers for suicide, the low base rate of suicide means that much greater specificity is required for any biological test to be clinically useful.54 Instead, the potential utility of these FA profiles is that they may suggest biological factors that contribute to suicidal behavior in some individuals and perhaps also suggest the possibility of public health level interventions that might have some impact on the population-level prevalence of suicidal behavior (eg, replacement of some dietary saturated and monounsaturated fats with polyunsaturated fats). Finally, as our sample was active duty military and mostly male, the applicability of our findings to more general populations needs to be verified.
Conclusion and Future Research
A LCCA of 6 FAs found evidence for 6 latent classes in a sample of military suicide decedents and living matched controls, including 2 “protective” classes with a lower proportion of suicide decedents and 3 “risky” classes with a higher proportion of suicide decedents. Levels of 9 individual FAs and indices of 3 FA enzymes differed between the protective and risky classes. The constellations of FA levels and enzyme indices in the risky classes were plausibly associated with alterations in biological systems that have been previously associated with suicidal behavior (eg, inflammation). These findings suggest the possibility of FA-related biotypes with suicide-relevant biological alterations. The existence of research showing that the effects of FA supplementation may depend on the genetic makeup of an individual further suggests the possibility that some individuals might be more or less likely to benefit from FA supplementation.25 Future research is needed to replicate our findings and simultaneously measure biological alterations (eg, inflammation) that might be associated with the FA profiles of our LCCA identified classes.
Submitted: February 11, 2020; accepted August 17, 2020.
Published online: February 23, 2021.
Author contributions: Dr Ryan conceived of and designed the analysis, conducted the analysis, and wrote the primary draft of the manuscript. Drs Postolache, Taub, Wilcox, Ghahramanlou-Holloway, Umhau, and Deuster contributed to the interpretation of the data analysis and contributed significantly to the drafting of the final manuscript. All authors approved of the final version of the manuscript to be published.
Potential conflicts of interest: None.
Funding/support: Support was provided by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment to Dr Ryan during preparation of this manuscript. Dr Ryan was a postdoctoral fellow at VA Capitol Health Care Network (VISN 5) MIRECC at the Veterans Affairs Maryland Health Care System, Baltimore, Maryland, during the preparation and submission of this article, and his work was supported with resources and the use of its facilities. Dr Ryan was also a visiting postdoctoral fellow in the Division of Psychiatric Services Research at the University of Maryland School of Medicine Department of Psychiatry. Dr Postolache’s contribution was supported by VISN 5 MIRECC and by the Rocky Mountain MIRECC for Suicide Prevention. Original data collection was supported by grants from the Defense Advanced Research Projects Agency, Arlington, Virginia, and by the Division of Intramural Basic and Clinical Research, National Institute on Alcohol Abuse and Alcohol, National Institutes of Health, Bethesda, Maryland.
Role of the sponsor: The funding agencies had no role in decisions regarding the conduct or publication of the study.
Disclaimer: This article reflects the authors’ personal views and in no way represents the official view of any branch of the Department of Veterans Affairs, the Uniformed Services University of the Health Sciences, the Department of Defense, or the US Government.
Previous presentation: A preliminary version of this analysis and study were presented in the form of an invited oral presentation at the International Academy of Suicide Research and American Foundation for Suicide Prevention International Summit on Suicide Research; October 2019; Miami Beach, Florida.
Acknowledgments: Michael D. Lewis, MD (Brain Health and Research Institute, Potomac, MD, USA), Joseph R. Hibbeln, MD (National Institute of Alcohol Abuse and Alcoholism, Bethesda, MD), Jeremiah E. Johnson, RD (affiliation unknown); Yu Hong Lin, PhD (National Institute of Alcohol Abuse and Alcoholism, Bethesda, MD), Duk Y. Hyun (affiliation unknown), and James D. Loewke (affiliation unknown) contributed to the original creation of the dataset analyzed for this study. They have no conflicts of interest.
Additional information: The Armed Forces Health Surveillance Center dataset is held by Dr Patricia A. Deuster, Professor at the Uniformed Services University of the Health Sciences Department of Military and Emergency Medicine. Those with questions regarding the data may contact her at [email protected].
Clinical Points
- Fatty acids may play a role in biological systems relevant to psychopathology and suicide, but existing research has often focused on the effects of individual fatty acids and findings have been confusing at times.
- Encouraging patients to follow established health recommendations to increase the consumption of foods rich in polyunsaturated fats might have a positive effect on suicide risk, in addition to well-established cardiovascular benefits.
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Early Career Psychiatrists section. Please contact Joseph F. Goldberg, MD, at [email protected].
References (54)

- Armed Forces Health Surveillance Center (AFHSC). Surveillance snapshot: manner and cause of death, active component, US Armed Forces, 1998–2013. MSMR. 2014;21(10):21. PubMed NLM
- Pandey GN. Biological basis of suicide and suicidal behavior. Bipolar Disord. 2013;15(5):524–541. PubMed CrossRef NLM
- Black C, Miller BJ. Meta-analysis of cytokines and chemokines in suicidality: distinguishing suicidal versus nonsuicidal patients. Biol Psychiatry. 2015;78(1):28–37. PubMed CrossRef NLM
- Sudol K, Mann JJ. Biomarkers of suicide attempt behavior: towards a biological model of risk. Curr Psychiatry Rep. 2017;19(6):31. PubMed CrossRef NLM
- Kihara A. Very long-chain fatty acids: elongation, physiology and related disorders. J Biochem. 2012;152(5):387–395. PubMed CrossRef NLM
- Jump DB. Mammalian fatty acid elongases. Methods Mol Biol. 2009;579:375–389. PubMed CrossRef NLM
- Daray FM, Mann JJ, Sublette ME. How lipids may affect risk for suicidal behavior. J Psychiatr Res. 2018;104:16–23. PubMed CrossRef NLM
- Müller CP, Reichel M, Mühle C, et al. Brain membrane lipids in major depression and anxiety disorders. Biochim Biophys Acta. 2015;1851(8):1052–1065. PubMed CrossRef NLM
- Schneider M, Levant B, Reichel M, et al. Lipids in psychiatric disorders and preventive medicine. Neurosci Biobehav Rev. 2017;76(pt B):336–362. PubMed CrossRef NLM
- Pompili M, Longo L, Dominici G, et al. Polyunsaturated fatty acids and suicide risk in mood disorders: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2017;74:43–56. PubMed CrossRef NLM
- Vermunt JK, Magidson J. Latent class cluster analysis. In: Hagenaars J, McCutcheon A, eds. Applied Latent Class Analysis. 2002;89–106.
- Ginley MK, Bagge CL. Psychiatric heterogeneity of recent suicide attempters: a latent class analysis. Psychiatry Res. 2017;251:1–7. PubMed CrossRef NLM
- Hyman J, Ireland R, Frost L, et al. Suicide incidence and risk factors in an active duty US military population. Am J Public Health. 2012;102(suppl 1):S138–S146. PubMed CrossRef NLM
- Liao Y, Xie B, Zhang H, et al. Efficacy of omega-3 PUFAs in depression: a meta-analysis. Transl Psychiatry. 2019;9(1):190. PubMed CrossRef NLM
- Haghighi F, Galfalvy H, Chen S, et al. DNA methylation perturbations in genes involved in polyunsaturated fatty acid biosynthesis associated with depression and suicide risk. Front Neurol. 2015;6:92. PubMed CrossRef NLM
- Lewis MD, Hibbeln JR, Johnson JE, et al. Suicide deaths of active-duty US military and omega-3 fatty-acid status: a case-control comparison. J Clin Psychiatry. 2011;72(12):1585–1590. PubMed CrossRef NLM
- Masood A, Stark KD, Salem N Jr. A simplified and efficient method for the analysis of fatty acid methyl esters suitable for large clinical studies. J Lipid Res. 2005;46(10):2299–2305. PubMed CrossRef NLM
- Masood MA, Salem N Jr. High-throughput analysis of plasma fatty acid methyl esters employing robotic transesterification and fast gas chromatography. Lipids. 2008;43(2):171–180. PubMed CrossRef NLM
- Bradbury KE, Skeaff CM, Green TJ, et al. The serum fatty acids myristic acid and linoleic acid are better predictors of serum cholesterol concentrations when measured as molecular percentages rather than as absolute concentrations. Am J Clin Nutr. 2010;91(2):398–405. PubMed CrossRef NLM
- Kim H, Kim B, Kim SH, et al. Classification of attempted suicide by cluster analysis: a study of 888 suicide attempters presenting to the emergency department. J Affect Disord. 2018;235:184–190. PubMed CrossRef NLM
- Lopez-Castroman J, Nogue E, Guillaume S, et al. Clustering suicide attempters: impulsive-ambivalent, well-planned, or frequent. J Clin Psychiatry. 2016;77(6):e711–e718. PubMed CrossRef NLM
- R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2012.
- Fraley C, Raftery AE. Model-based clustering, discriminant analysis, and density estimation. J Am Stat Assoc. 2002;97(458):611–631. CrossRef
- Fraley C, Raftery AE, Murphy TB, Scrucca L. Mclust Version 4 for R: Normal Mixture Modeling for Model-Based Clustering, Classification, and Density Estimation. Department of Statistics, University of Washington website. https://stat.uw.edu/sites/default/files/files/reports/2012/tr597.pdf. 2012. Accessed January 1, 2020.
- Cormier H, Rudkowska I, Lemieux S, et al. Effects of FADS and ELOVL polymorphisms on indexes of desaturase and elongase activities: results from a pre-post fish oil supplementation. Genes Nutr. 2014;9(6):437. PubMed CrossRef NLM
- Assies J, Pouwer F, Lok A, et al. Plasma and erythrocyte fatty acid patterns in patients with recurrent depression: a matched case-control study. PLoS One. 2010;5(5):e10635. PubMed CrossRef NLM
- Mocking RJT, Assies J, Koeter MWJ, et al. Bimodal distribution of fatty acids in recurrent major depressive disorder. Biol Psychiatry. 2012;71(1):e3–e5. PubMed CrossRef NLM
- Muthén L, Muthén B. Re: What is a good value of entropy. Mplus website. http://www.statmodel.com/discussion/messages/13/2562.html?1237580237. Published 2007. Accessed March 8, 2017.
- Scrucca L, Raftery AE. Improved initialisation of model-based clustering using Gaussian hierarchical partitions. Adv Data Anal Classif. 2015;9(4):447–460. PubMed CrossRef NLM
- Ohno Y, Suto S, Yamanaka M, et al. ELOVL1 production of C24 acyl-CoAs is linked to C24 sphingolipid synthesis. Proc Natl Acad Sci U S A. 2010;107(43):18439–18444. PubMed CrossRef NLM
- Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci. 2007;8(2):128–140. PubMed CrossRef NLM
- Liu JJ, Hezghia A, Shaikh SR, et al. Regulation of monoamine transporters and receptors by lipid microdomains: implications for depression. Neuropsychopharmacology. 2018;43(11):2165–2179. PubMed CrossRef NLM
- Sassa T, Kihara A. Metabolism of very long-chain fatty acids: genes and pathophysiology. Biomol Ther (Seoul). 2014;22(2):83–92. PubMed CrossRef NLM
- Magnani F, Tate CG, Wynne S, et al. Partitioning of the serotonin transporter into lipid microdomains modulates transport of serotonin. J Biol Chem. 2004;279(37):38770–38778. PubMed CrossRef NLM
- Scanlon SM, Williams DC, Schloss P. Membrane cholesterol modulates serotonin transporter activity. Biochemistry. 2001;40(35):10507–10513. PubMed CrossRef NLM
- Donati RJ, Dwivedi Y, Roberts RC, et al. Postmortem brain tissue of depressed suicides reveals increased Gsα localization in lipid raft domains where it is less likely to activate adenylyl cyclase. J Neurosci. 2008;28(12):3042–3050. PubMed CrossRef NLM
- van Heeringen K, Mann JJ. The neurobiology of suicide. Lancet Psychiatry. 2014;1(1):63–72. PubMed CrossRef NLM
- Fitzgerald ML, Kassir SA, Underwood MD, et al. Dysregulation of striatal dopamine receptor binding in suicide. Neuropsychopharmacology. 2017;42(4):974–982. PubMed CrossRef NLM
- Peters BD, Voineskos AN, Szeszko PR, et al. Brain white matter development is associated with a human-specific haplotype increasing the synthesis of long chain fatty acids. J Neurosci. 2014;34(18):6367–6376. PubMed CrossRef NLM
- Kutkowska-Kaźmierczak A, Rydzanicz M, Chlebowski A, et al. Dominant ELOVL1 mutation causes neurological disorder with ichthyotic keratoderma, spasticity, hypomyelination and dysmorphic features. J Med Genet. 2018;55(6):408–414. PubMed CrossRef NLM
- Bourre J-M. Developmental Synthesis of Myelin Lipids: Origin of Fatty Acids—Specific Role of Nutrition. In: Evrard P, Minkowski A, eds. Developmental Neurobiology. New York, NY: Raven Press; 1989:111–154.
- Isokawa M, Sassa T, Hattori S, et al. Reduced chain length in myelin sphingolipids and poorer motor coordination in mice deficient in the fatty acid elongase Elovl1. FASEB Bioadv. 2019;1(12):747–759. PubMed CrossRef NLM
- Bani-Fatemi A, Tasmim S, Graff-Guerrero A, et al. Structural and functional alterations of the suicidal brain: an updated review of neuroimaging studies. Psychiatry Res Neuroimaging. 2018;278:77–91. PubMed CrossRef NLM
- McNamara RK, Liu Y. Reduced expression of fatty acid biosynthesis genes in the prefrontal cortex of patients with major depressive disorder. J Affect Disord. 2011;129(1–3):359–363. PubMed CrossRef NLM
- Lalovic A, Klempan T, Sequeira A, et al. Altered expression of lipid metabolism and immune response genes in the frontal cortex of suicide completers. J Affect Disord. 2010;120(1–3):24–31. PubMed CrossRef NLM
- de Sousa Rodrigues ME, Bekhbat M, Houser MC, et al. Chronic psychological stress and high-fat high-fructose diet disrupt metabolic and inflammatory gene networks in the brain, liver, and gut and promote behavioral deficits in mice. Brain Behav Immun. 2017;59:158–172. PubMed CrossRef NLM
- Dyall SC. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci. 2015;7:52. PubMed CrossRef NLM
- Pandey GN, Rizavi HS, Bhaumik R, et al. Innate immunity in the postmortem brain of depressed and suicide subjects: role of toll-like receptors. Brain Behav Immun. 2019;75:101–111. PubMed CrossRef NLM
- Brundin L, Erhardt S, Bryleva EY, et al. The role of inflammation in suicidal behaviour. Acta Psychiatr Scand. 2015;132(3):192–203. PubMed CrossRef NLM
- Hoile SP, Clarke-Harris R, Huang R-C, et al. Supplementation with N-3 long-chain polyunsaturated fatty acids or olive oil in men and women with renal disease induces differential changes in the DNA methylation of FADS2 and ELOVL5 in peripheral blood mononuclear cells. PLoS One. 2014;9(10):e109896. PubMed CrossRef NLM
- Anguizola JA, Basiaga SBG, Hage DS. Effects of fatty acids and glycation on drug interactions with human serum albumin. Curr Metabolomics. 2013;1(3):239–250. PubMed CrossRef NLM
- Bhathena SJ. Relationship between fatty acids and the endocrine system. Biofactors. 2000;13(1–4):35–39. PubMed CrossRef NLM
- Khovidhunkit W, Kim M-S, Memon RA, et al. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004;45(7):1169–1196. PubMed CrossRef NLM
- Chang BP, Franklin JC, Ribeiro JD, et al. Biological risk factors for suicidal behaviors: a meta-analysis. Transl Psychiatry. 2016;6(9):e887. PubMed CrossRef NLM
Please sign in or purchase this PDF for $40.
Save
Cite