Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
To the Editor: Bipolar depression is a leading cause of disability in the United States. Recently, N-methyl-d-asparate glutamate-receptor (NMDAR) antagonists, such as ketamine, have been shown to induce remission in bipolar depression. Nevertheless, ketamine use is limited by transient effects and psychogenic potential during repeated administration. d-Cycloserine is a US Food and Drug Administration (FDA)-approved antituberculosis drug that acts as an NMDAR antagonist when used at high doses (> 750 mg).
See commentary by Iosifescu
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
To the Editor: Bipolar depression is a leading cause of disability in the United States. Recently, N-methyl-d-asparate glutamate-receptor (NMDAR) antagonists, such as ketamine, have been shown to induce remission in bipolar depression.1,2 Nevertheless, ketamine use is limited by transient effects and psychogenic potential during repeated administration.3 d-Cycloserine is a US Food and Drug Administration (FDA)-approved antituberculosis drug that acts as an NMDAR antagonist when used at high doses (> 750 mg). d-Cycloserine targets the glycine coreceptor of the NMDAR and may have improved safety relative to ketamine (analogous to use of benzodiazepines vs barbiturates at γ-aminobutyric acid-A receptors). Antidepressant effects of d-cycloserine were first noted in the 1950s.4-6 However, double-blind, placebo-controlled studies at high doses were not conducted until recently.7 A large, between-group, large effect-size (d = 0.91, P = .005) difference was seen in unipolar depression, corresponding to a mean 48% reduction in symptoms. We report the first study of acute ketamine followed by daily d-cycloserine in bipolar depression (ClinicalTrials.gov identifier NCT01833897).
Method. Enrollment criteria included current bipolar disorder I or II (DSM-IV-TR), a Montgomery-Asberg Depression Rating Scale8 (MADRS) score > 20, and no current or chronic psychosis or substance dependence. Twelve subjects consented (Supplementary eFigure 1), and 8 received ketamine and d-cycloserine (mean age = 37 ± 16 years, 5 women). Subjects were treatment-resistant to a clinically determined regimen for a mean 3.3 ± 4 months prior to enrollment. Patients remained on previously prescribed mood stabilizers or benzodiazepines, but antidepressants and antipsychotics not FDA-approved for bipolar depression were withdrawn.
After consent, subjects were prospectively treated with medications FDA-approved for bipolar depression (olanzapine/fluoxetine [n = 2], lurasidone [n = 4], or quetiapine [n = 2]), with treatment resistance further demonstrated by a nonsignificant, mean 0.2 ± 1 point change from screening MADRS of 29.0 ± 6 over a mean 4.2 ± 1 weeks.
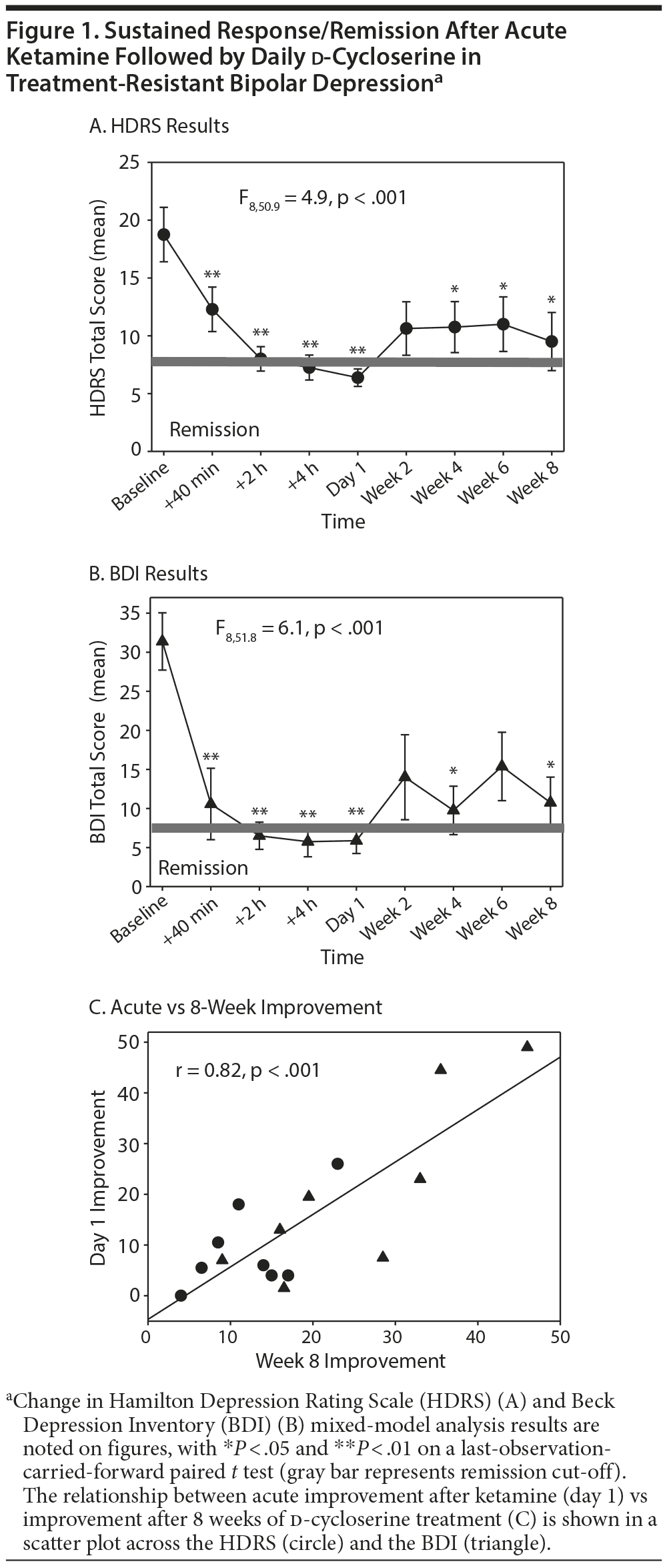
Subjects then received open-label ketamine hydrochloride (0.5 mg/kg administered intravenously over 60 minutes) followed by 8 weeks of d-cycloserine (titrated to 1,000 mg/d from a starting dose of 250 mg over 3 weeks) and pyridoxine, adjunctive to continued FDA-approved treatment and mood stabilizers. This slower rate of ketamine infusion was anticipated to increase tolerability. To minimize baseline MADRS inflation bias, primary outcomes were the Hamilton Depression Rating Scale (HDRS)9 and Beck Depression Inventory.10 Statistical analysis was performed by linear, mixed-effects model regression, with follow-up paired t tests as required.
Results. Seven subjects completed the study, and 4 met remission criteria at 8 weeks (HDRS score < 7). On mixed-model analysis, a significant overall response over time was seen (F1,6.4 = 161.8, P < .001). Follow-up paired t test analysis suggested significant improvement from baseline at all rating points except at 2 weeks, with a large effect size seen at day 1 (Cohen d = 2.0 SD) and 8 weeks (Cohen d = 1.1 SD), Figure 1A and 1B). One subject was withdrawn after 1 week of d-cycloserine treatment for relapse after 1 day of ketamine treatment.
Acute response was predictive of response at 8 weeks (r = 0.82, Figure 1C). Study treatments were well tolerated, with mild sedation reported by 3 subjects, headaches by 2, and phosphenes by 1. One subject required a d-cycloserine dose reduction to 500 mg at 6 weeks for mild sedation, and 1 subject had hypomanic symptoms 2 weeks after stopping FDA-approved treatment for lack of efficacy. All other subjects remained on stable concomitant medications during the study. Five subjects requested to continue d-cycloserine after the study, with 3 successfully obtaining insurance coverage, further suggesting tolerability by the patients taking d-cycloserine.
Discussion. These findings provide proof-of-concept for further study of combined treatment with NMDAR antagonists and FDA-approved medications for bipolar depression. Although d-cycloserine is generally well tolerated by psychiatric populations,7 majors risks include psychosis11 and seizures, a risk we minimized by concomitant treatment with antipsychotics and pyridoxine.
Recent publications12–14 of large cohorts of clinically treated patients with multidrug-resistant tuberculosis have been reassuring about the safety of d-cycloserine. Approximately 1,100 patients are included in these case series of patients treated with 500-1,000 mg of d-cycloserine daily for 6 to 24 months. Analysis of these cases is complicated by their uncontrolled nature and the fact that most patients were receiving multiple medications (about 5) and were often seriously ill. Despite this, almost 95% of subjects completed treatment without incident across cohorts. Reported adverse effects of d-cycloserine included dizziness/vertigo (14.3%), headache (11.7%), sleep disturbances (11.5%), peripheral neuropathy (7.9%), depression (6.2%), tinnitus (5.1%), visual disturbances (4.4%), seizures (4%), and psychosis (3.4%).13 Only 2.1% of subjects stopped treatment because of adverse events.13 A recent meta-analysis14 was also reassuring on the relative safety of d-cycloserine. The pooled estimate for the frequencies of psychiatric adverse drug reactions was 5.7% (95% CI, 3.7-7.6) and 1.1% (95% CI, 0.2-2.1) for central nervous system-related adverse reactions. Overdose can result in coma; alcohol consumption may increase the risk of seizures.16
Limitations include the small sample and a non-placebo-controlled, open-label design. Consistent with prior ketamine-only studies,2 partial relapse was seen at 2 weeks. Further mood improvement after 2 weeks, however, is consistent with time-course of improvement seen in previous d-cycloserine-only studies.7 Since all subjects received both NMDAR antagonists, and had already shown highly significant improvement with ketamine, an assessment of d-cycloserine’s independent efficacy and safety in bipolar depression will require future study.
Author affiliations: Psychiatry, Columbia University, New York (all authors); and Schizophrenia Research Center, Nathan Kline Institute, Orangeburg (Dr Kantrowitz), New York.
Potential conflicts of interest: Dr Kantrowitz reports having received consulting payments within the last 36 months from Otsuka Pharmaceuticals, Vindico Medical Education, Health Advances, LLC, Strategic Edge Communications, and Cowen and Company. He has conducted clinical research supported by the National Institute of Mental Healthppp, the Stanley Foundation, Roche-Genentech, Forum, Psychogenics, Sunovion, Novartis, Pfizer, Lilly, and GlaxoSmithKline. He owns a small number of shares of common stock in GlaxoSmithKline. Drs Halberstam and Gangwisch report no financial relationships with commercial interests.
Funding/support: Supported in part by the 2011 ASPIRE Major Depression Disorder Competitive Research Grant program (Pfizer) to Dr Kantrowitz.
Role of the sponsor: The funding agency had no role in the design, preparation, or decision to submit this letter for publication.
Previous presentation: Presented in part at the American College of Neuropsychopharmacology Annual Meetings; December 8-12, 2013; Hollywood, Florida; and December 7-11, 2014; Phoenix, Arizona.
Supplementary material: Available at PSYCHIATRIST.COM.
J Clin Psychiatry 2015;76(6):737-738 (doi:10.4088/JCP.14l09527).
© Copyright 2015 Physicians Postgraduate Press, Inc.
References
1. Zarate CA Jr, Brutsche NE, Ibrahim L, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71(11):939-946. PubMed doi:10.1016/j.biopsych.2011.12.010
2. Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of an N-methyl-d-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67(8):793-802. PubMed doi:10.1001/archgenpsychiatry.2010.90
3. Murrough JW, Perez AM, Pillemer S, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013;74(4):250-256. PubMed doi:10.1016/j.biopsych.2012.06.022
4. Epstein IG, Nair KG, Boyd LJ. The treatment of human pulmonary tuberculosis with cycloserine: progress report. Dis Chest. 1956;29(3):241-257. doi:10.1378/chest.29.3.241PubMed
5. Crane GE. Cycloserine as an antidepressant agent. Am J Psychiatry. 1959;115(11):1025-1026. PubMed doi:10.1176/ajp.115.11.1025
6. Crane GE. The psychotropic effects of cycloserine: a new use for an antibiotic. Compr Psychiatry. 1961;2(1):51-59. doi:10.1016/S0010-440X(61)80007-2
7. Heresco-Levy U, Gelfin G, Bloch B, et al. A randomized add-on trial of high-dose d-cycloserine for treatment-resistant depression. Int J Neuropsychopharmacol. 2013;16(3):501-506. PubMed doi:10.1017/S1461145712000910
8. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382-389. PubMed doi:10.1192/bjp.134.4.382
9. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56-62. PubMed doi:10.1136/jnnp.23.1.56
10. Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. Mod Probl Pharmacopsychiatry. 1974;7(0):151-169. PubMed
11. Kantrowitz JT, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res Bull. 2010;83(3-4):108-121. doi:10.1016/j.brainresbull.2010.04.006PubMed
12. Goble M, Iseman MD, Madsen LA, et al. Treatment of 171 patients with pulmonary tuberculosis resistant to isoniazid and rifampin. N Engl J Med. 1993;328(8):527-532. doi:10.1056/NEJM199302253280802PubMed
13. Nathanson E, Gupta R, Huamani P, et al. Adverse events in the treatment of multidrug-resistant tuberculosis: results from the DOTS-Plus initiative. Int J Tuberc Lung Dis. 2004;8(11):1382-1384. PubMed
14. Törün T, Güngör G, Ozmen I, et al. Side effects associated with the treatment of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2005;9(12):1373-1377.PubMed
15. Hwang TJ, Wares DF, Jafarov A, et al. Safety of cycloserine and terizidone for the treatment of drug-resistant tuberculosis: a meta-analysis. Int J Tuberc Lung Dis. 2013;17(10):1257-1266. PubMed doi:10.5588/ijtld.12.0863
16. Seromycin (cycloserine) [package insert]. West Lafayette, IN: Chao Center; 2009.
This PDF is free for all visitors!
Save
Cite