Objective: Young adults attempt suicide at disproportionately high rates relative to other groups and demonstrate high rates of sleep disturbance. No study has yet prospectively evaluated disturbed sleep as an acute indicator of risk using an objective index of sleep. We investigated objective and subjective parameters of disturbed sleep as a warning sign of suicidal ideation among young adults over an acute period.
Methods: A longitudinal study across a 21-day observation period and 3 time points. Fifty of 4,847 participants (aged 18-23 years) were prescreened from a university undergraduate research pool (February 2007-June 2008) on the basis of suicide attempt history and recent suicidal ideation. Actigraphic and subjective sleep parameters were evaluated as acute predictors of suicidal ideation (Beck Scale for Suicide Ideation), with adjustment for baseline symptoms. Hierarchical regression analyses were employed to predict residual change scores.
Results: Ninety-six percent of participants (n = 48) endorsed a suicide attempt history. Mean actigraphy values revealed objectively disturbed sleep parameters; 78% (n = 39) and 36% (n = 18) endorsed clinically significant insomnia and nightmares, respectively. When results were controlled for baseline suicidal and depressive symptoms, actigraphic and subjective sleep parameters predicted suicidal ideation residual change scores at 7- and 21-day follow-ups (P < .001). Specifically, actigraphy-defined variability in sleep timing, insomnia, and nightmares predicted increases in suicidal ideation (P < .05). In a test of competing risk factors, sleep variability outperformed depressive symptoms in the longitudinal prediction of suicidal ideation across time points (P < .05).
Conclusions: Objectively and subjectively measured sleep disturbances predicted acute suicidal ideation increases in this population, independent of depressed mood. Self-reported insomnia and nightmares and actigraphically assessed sleep variability emerged as acute warning signs of suicidal ideation. These findings highlight the potential utility of sleep as a proposed biomarker of suicide risk and a therapeutic target.

Objectively Assessed Sleep Variability as an Acute Warning Sign of Suicidal Ideation in a Longitudinal Evaluation of Young Adults at High Suicide Risk

ABSTRACT
Objective: Young adults attempt suicide at disproportionately high rates relative to other groups and demonstrate high rates of sleep disturbance. No study has yet prospectively evaluated disturbed sleep as an acute indicator of risk using an objective index of sleep. We investigated objective and subjective parameters of disturbed sleep as a warning sign of suicidal ideation among young adults over an acute period.
Methods: A longitudinal study across a 21-day observation period and 3 time points. Fifty of 4,847 participants (aged 18-23 years) were prescreened from a university undergraduate research pool (February 2007-June 2008) on the basis of suicide attempt history and recent suicidal ideation. Actigraphic and subjective sleep parameters were evaluated as acute predictors of suicidal ideation (Beck Scale for Suicide Ideation), with adjustment for baseline symptoms. Hierarchical regression analyses were employed to predict residual change scores.
Results: Ninety-six percent of participants (n = 48) endorsed a suicide attempt history. Mean actigraphy values revealed objectively disturbed sleep parameters; 78% (n = 39) and 36% (n = 18) endorsed clinically significant insomnia and nightmares, respectively. When results were controlled for baseline suicidal and depressive symptoms, actigraphic and subjective sleep parameters predicted suicidal ideation residual change scores at 7- and 21-day follow-ups (P < .001). Specifically, actigraphy-defined variability in sleep timing, insomnia, and nightmares predicted increases in suicidal ideation (P < .05). In a test of competing risk factors, sleep variability outperformed depressive symptoms in the longitudinal prediction of suicidal ideation across time points (P < .05).
Conclusions: Objectively and subjectively measured sleep disturbances predicted acute suicidal ideation increases in this population, independent of depressed mood. Self-reported insomnia and nightmares and actigraphically assessed sleep variability emerged as acute warning signs of suicidal ideation. These findings highlight the potential utility of sleep as a proposed biomarker of suicide risk and a therapeutic target.
J Clin Psychiatry 2017;78(6):e678-e687
https://doi.org/10.4088/JCP.16m11193
© Copyright 2017 Physicians Postgraduate Press, Inc.
aStanford Mood Disorders Center, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Stanford, California
bDepartment of Psychology, Florida State University, Tallahassee
*Corresponding author: Rebecca Bernert, PhD, Suicide Prevention Research Laboratory, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, 401 Quarry Rd, Stanford, CA 94304-5797 ([email protected]).
Suicide represents a preventable public health problem and global disease burden, accounting for nearly 1 million deaths annually worldwide.1 The Institute of Medicine furthermore estimates an additional 25 attempts (100-200 for youth) for every suicide death.2 Calls to action by the US Surgeon General consistently highlight the need to identify risk factors to enhance surveillance and treatment development to prevent suicide, particularly among youth.3 In general, selective interventions for suicide as an indication remain alarmingly scarce, unacceptable (ie, based on attrition), or inaccessible to those highest in need.
Sleep disturbances are among the top warning signs of suicide according to the Substance Abuse and Mental Health Services Administration,4 and preliminary research suggests they may confer risk for suicidal behaviors.5 Even so, numerous methodological limitations, often inherent to the study of suicide, constrain the impact of such findings.6,7 These include the frequent use of retrospective and cross-sectional study designs, chart review or historical assessment of suicidal symptoms, and reliance on single-item evaluations of suicidal symptoms (eg, from depression inventories) versus validated instruments.7 Finally, investigations often fail to control for the confounding influence of existing psychopathology, particularly depression severity.7 Because sleep and suicidal symptoms are diagnostic criteria for depression,8 and among the strongest predictors of suicide risk,6,9 controlling for depression severity is crucial to delineating independent risk for suicidal behaviors (ie, versus representing a mere correlate of depression).
Evaluation among young adults is motivated by the shared prevalence of sleep disturbance and suicide risk during this developmental period. Sleep disturbances are overrepresented among young adults,10,11 and suicide is the second leading cause of death among those aged 19-24 years.12 Relative to other risk factors, sleep appears visible as a warning sign. In a psychological autopsy study13 of 140 adolescent suicide decedents and 131 community-matched controls, sleep problems were visible to friends and family in the weeks and months prior to death. Even after adjustment for depression, disturbed sleep predicted up to a 10-fold greater risk for suicide compared to controls. Population-based studies of adolescents also identify poor sleep in association with suicidal behavior,14 controlling for depressive symptoms.15
According to a systematic review7 of studies addressing the previously mentioned methodological issues, research supports sleep disturbances (eg, insomnia, poor sleep quality, nightmares) as an independent risk factor for suicidal ideation, suicide attempts, and suicide fatalities—with adjustment for depression—across investigations diverse in design, samples, and assessment techniques. This evidence includes self-reported insomnia and fatigue as correlates and risk factors for suicidal ideation and suicide attempts and poor subjective sleep quality as a risk factor for suicide death in a longitudinal, epidemiologic study.16 Given the inherent costs and methodological challenges to assessing sleep objectively within large-scale studies of suicide, reports have primarily relied on surveyed sleep complaints versus objective sleep parameters.5,7 By comparison, objective assessment of sleep in association with suicidal behaviors has received less attention in suicide research. Indeed, of 10 total studies7 conducted evaluating electroencephalographic (EEG)-assessed sleep in association with suicide risk, only 1 study17 included depression as a covariate. Nonetheless, this study18 reported significant relationships between EEG parameters and past suicidal ideation and suicide attempts, with depression severity as a covariate.
To address these gaps in the literature, we sought to confirm that disturbed sleep confers independent risk for suicidal ideation symptoms in young adults (ie, beyond known covariates, depression6,9 and alcohol use19,20) using a longitudinal design, validated symptom measures, and an acute time frame (aim 1) and to evaluate whether this relationship emerges using objective and subjective sleep measures (aim 2). Because of the invasiveness of EEG and its time-limited nature in the context of suicidal symptom change (ie, single-night vs ongoing assessments), actigraphy was selected as our primary sleep measure. Last, on the basis of research suggesting that emotion regulation deficits are associated with sleep disruption21 and suicidal behaviors,22-24 we explored whether intraindividual variation in mood was related to suicidal ideation and sleep parameters (aim 3).

- Self-reported sleep disturbances confer risk for suicide; however, little is known about objective sleep parameters as a warning sign for suicidal behaviors.
- Assessment and management of suicide risk are indicated among patients with disturbed sleep, particularly variability in sleep timing, insomnia, and nightmares.
- Poor sleep is proposed as a biomarker of risk and a potential therapeutic target for suicide prevention.
METHODS
Participants and Procedures
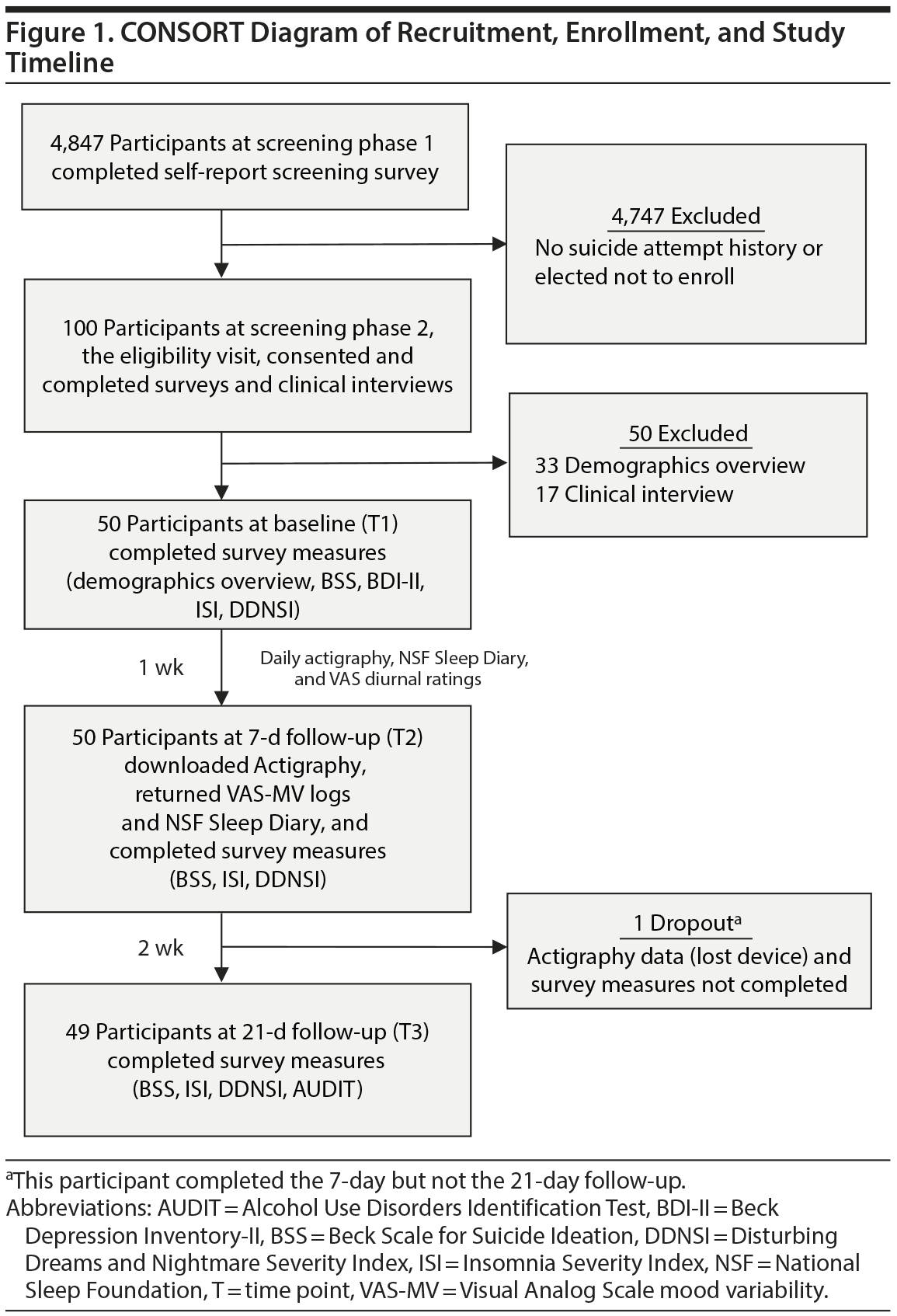
Participants aged 18-23 years were recruited from a university undergraduate research pool for high suicide risk through a multiphase screening process (February 2007-June 2008) described in a previous report,25 which is a secondary analysis of the data reported in this prospective primary analysis. Participants were university undergraduates (N = 4,847) screened for high suicide risk for inclusion in the present study (recruitment and enrollment procedures are shown in Figure 1 and Supplementary eTable 1). Inclusion criteria for the current study were (1) age ≥ 18 years and (2) endorsement of either ≥ 1 past suicide attempt, verified by clinical interview,26 and recent (≤ 6 months) suicidal ideation or no suicide attempt history but current (≤ 1 month) and recent (≤ 6 months) suicidal ideation. Suicide attempt history was used as a proxy for current risk based on past research,27,28 and study criteria and recruitment procedures were modeled after large-scale suicide prevention trials.29 The National Institutes of Health human subjects and Florida State University institutional review boards approved all study procedures.
Materials
Demographic characteristics. This survey assessed baseline demographic information, personal and family suicide attempt history, medication use, medical history, diagnostic characteristics, shiftwork status, and nonsuicidal self-injury.
Suicide risk measures. Beck Scale for Suicide Ideation (BSS). This 21-item self-report instrument30 assesses suicidal symptom severity. Higher total scores (0-38) indicate greater levels of suicidal ideation. This scale shows strong reliability and validity and has been normed among inpatient and outpatient samples.30
Pierce Suicidal Intent Scale. This 12-item, clinician-administered interview26 documents the severity and intent of past suicide attempts. Total scores (0-25) reflect summed subscales (circumstances, self-report, medical risk). Research demonstrates good psychometric properties for this measure26 used to verify inclusion criteria.
Objective sleep measures. Actigraphy. Actigraphy (Actiwatch; Respironics) provided an objective measurement of sleep. Actigraphs are small, watch-like devices (37 ×— 29 ×— 9 mm; 17 g) worn on the nondominant wrist. An electronic accelerometer records movement over preprogrammed 30-second intervals. The actigraph wearer inserts event markers by pressing a button on the actigraph to demarcate, in real time, his or her attempted sleep onsets and offsets, which define a given sleep interval. Data were verified for adherence and correspondence with sleep diaries and analyzed by the first author using commercially available software algorithms (Actiware), which automatically generate sleep-wake statistics. Actigraphy data reliably discriminate sleep-wake patterns,31 which show good correspondence with EEG data.32 For each sleep interval, derived variables included sleep-onset latency (minutes elapsed between attempting and achieving sleep onset), total sleep time (sleep duration, minus awakenings), wake after sleep onset (minutes awake following sleep onset), sleep efficiency (percentage of time asleep to time in bed), and sleep variability (standard deviations of daily sleep onsets [ie, time when sleep was initiated] and sleep offsets [ie, time of final wakening]; these indices were summed to provide an overall index of sleep variability, consistent with past reports33), identified using Actiware algorithms. Additional variables included average bedtime and wake time, time in bed (hours in bed, regardless of time asleep), and nap and sleep interval frequency. For post hoc analyses, standard deviations of sleep-onset latency, total sleep time, wake after sleep onset, and sleep efficiency were also calculated. Actigraphy sleep data were computed based on a 7-day period for each participant.
Daily Sleep Diary. Actigraphy data were verified using the National Sleep Foundation diary (https://sleepfoundation.org/sleep-diary/SleepDiaryv6.pdf), adapted for study use. Diaries provide reliable comparison with actigraphy32 and were inspected for missing event markers, consistent with past studies.31,34,35
Subjective sleep measures. Insomnia Severity Index (ISI). This 7-item self-report measure36 assesses the frequency and severity of insomnia symptoms. Higher total scores indicate greater insomnia severity. This measure is a gold standard insomnia instrument,37 widely used in clinical trials. Scores ≥ 10 appear to provide optimal sensitivity and specificity.37
Disturbing Dreams and Nightmare Severity Index (DDNSI). This 5-item self-report scale38 assesses the intensity, frequency, and severity of disturbing dreams and nightmares. It is a revised version of the Nightmare Frequency Questionnaire39 and is used commonly in nightmare assessment. Scores > 10 suggest clinically significant nightmares.
Covariate measures. Beck Depression Inventory-II (BDI-II). This 21-item self-report inventory40 assesses depressive symptomatology, with higher scores reflecting greater symptom severity. It yields adequate reliability estimates and psychometrics.40 To prevent collinearity, overlapping items (9, 16, 20) were removed, consistent with previous reports.15,16
Alcohol Use Disorders Identification Test (AUDIT). This 10-item self-report questionnaire41 screens for hazardous alcohol use. Items address consumption, alcohol-related problems, and dependence symptoms. Total scores ≥ 8 generally signal harmful drinking.41
Exploratory measures: Visual Analog Scale (VAS) mood ratings. Self-ratings of depressed mood were administered alongside daily sleep diaries diurnally (ie, once in the morning, once at night). Participants used a vertical line to indicate ratings (anchors: "not at all depressed" to "very depressed"). To index intraindividual mood variation, the standard deviation of VAS (VAS mood variability [VAS-MV]) was calculated as a proxy for mood lability.
Sequence of Investigation
Those invited from screening phase 1 scheduled in-person eligibility visits for screening phase 2 using a secure, fully encrypted online network. Participants provided written informed consent after thorough explanation of study procedures. Visits occurred at 3 study time points: T1 (baseline), T2 (7-day follow-up), and T3 (21-day follow-up).
Statistical Analyses
Descriptive statistics were planned for all key variables, including intercorrelations between measures. Factors significantly associated with baseline BSS score were planned for inclusion as covariates.
Linear hierarchical multiple regression analyses were employed to test hypotheses. To assess causal risk and temporal ordering, symptom relationships were assessed longitudinally, with adjustment for baseline symptoms in each model. BSS residual change scores were created to evaluate actigraphic and subjective sleep parameters in association with suicidal symptom increases at 7- and 21-day follow-ups (T2, T3). To create residual change scores, T1 BSS total scores were entered into block 1 of each regression; planned covariates were entered into block 2. For actigraphy analyses, lower sleep efficiency and higher total sleep time, sleep-onset latency, wake after sleep onset, and sleep variability were hypothesized to predict T2 and T3 BSS increases. This approach was repeated for subjective sleep analyses, wherein higher ISI/DDNSI scores were hypothesized to predict T2 and T3 BSS increases. For exploratory analyses, higher VAS-MV was expected to predict BSS change and actigraphic and subjective sleep parameters.
Analyses were conducted using SPSS Software, Version 23.00 (IBM Inc).
Power
Based on similarly designed studies,16,42 proposed analyses had adequate power (1 − β > .85; α < .05) to detect a medium effect size (d of approximately 0.5) or higher.
RESULTS
Descriptive Statistics
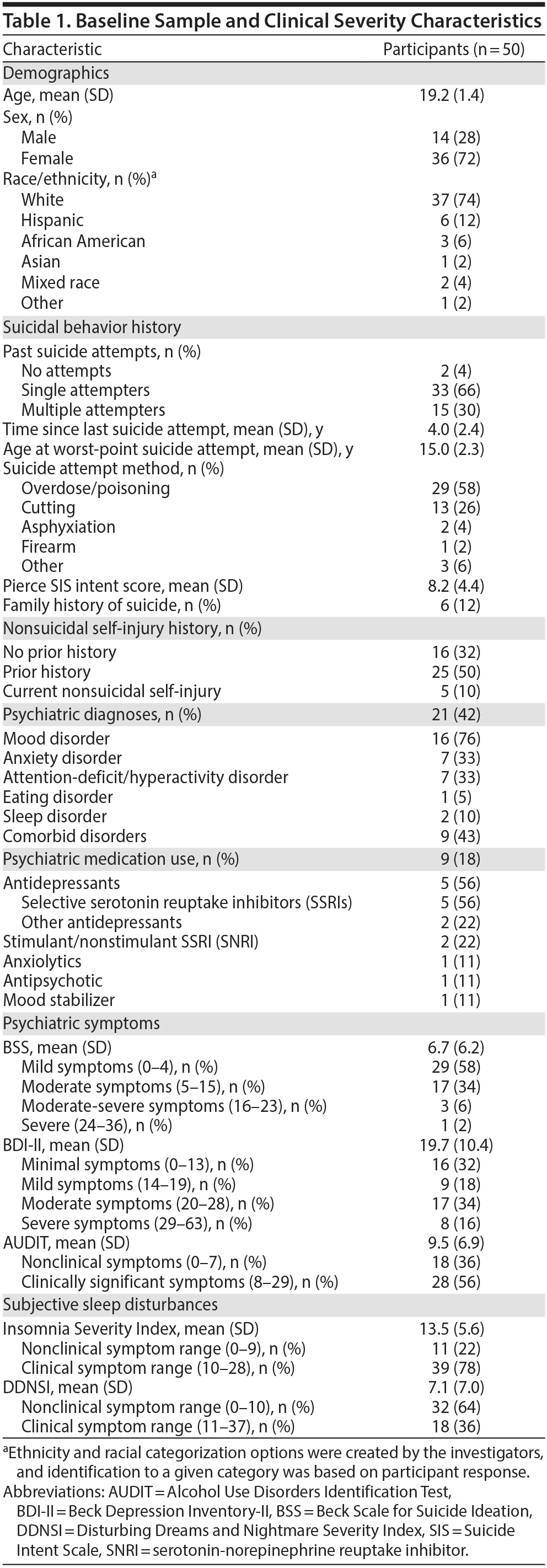
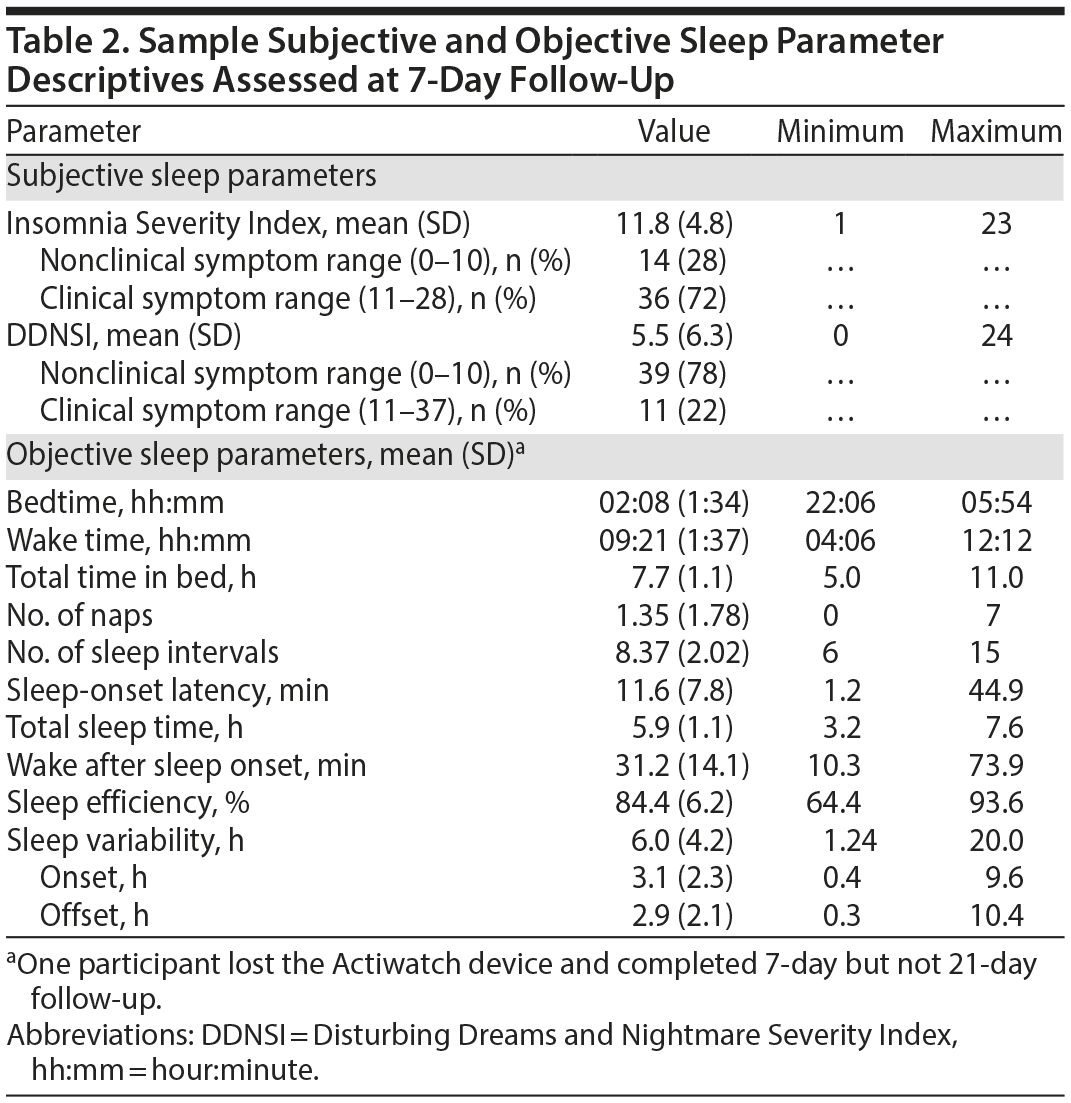
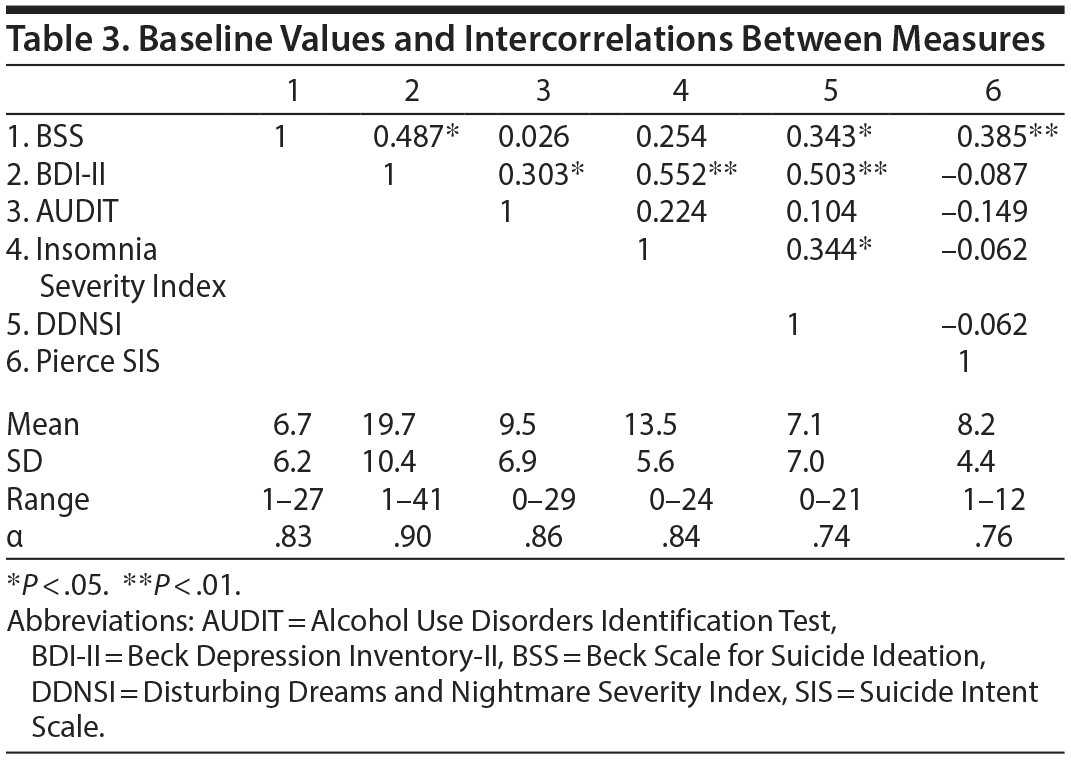
Sample characteristics and descriptive statistics are presented in Tables 1, 2, and 3.
Covariates. Alcohol-related problems as measured by AUDIT were common, and participants reported moderate to severe depressive symptoms as assessed by BDI-II.
Suicidal symptoms. Mean BSS scores indicated moderate suicidal symptoms, with maximum scores in the severe range for suicidal ideation.
Actigraphic sleep parameters. Sleep diaries were complete in 100% of cases, which paralleled actigraphy use compliance (> 98%). Event marker adherence was also high (2.67 event markers missing of 14.77 mean total). Independent of shiftwork status (3 participants recorded shiftwork schedules from 1 to 3 days; ie, schedules overlapping with midnight), 3 other participants demonstrated extended wakefulness (ie, 24-hour periods without initiating sleep). Both shiftwork and extended wakefulness appeared associated with greater sleep variability, according to mean actigraphy metrics and their intercorrelations. For example, sleep variability correlated with delayed bedtime (r = 0.46, P = .001), wake time (r = 0.45, P = .001), nap frequency (r = 0.42, P = .003), and sleep interval number (r = 0.27, P = .052).
Subjective sleep parameters. Mean ISI scores were consistent with an insomnia diagnosis37; one-third of participants reported clinically significant nightmares.
Covariate Analyses
Demographic variables were not significantly correlated with BSS (age: r = -0.08, P = .57; sex: r = -0.05, P = .74). Total BDI-II scores correlated with BSS across time points (r = 0.40 to 0.50, P < .01), whereas AUDIT scores did not (r = -0.05 to 0.10, P > .05). The BDI-II was thus retained as a covariate.
Regression Analyses
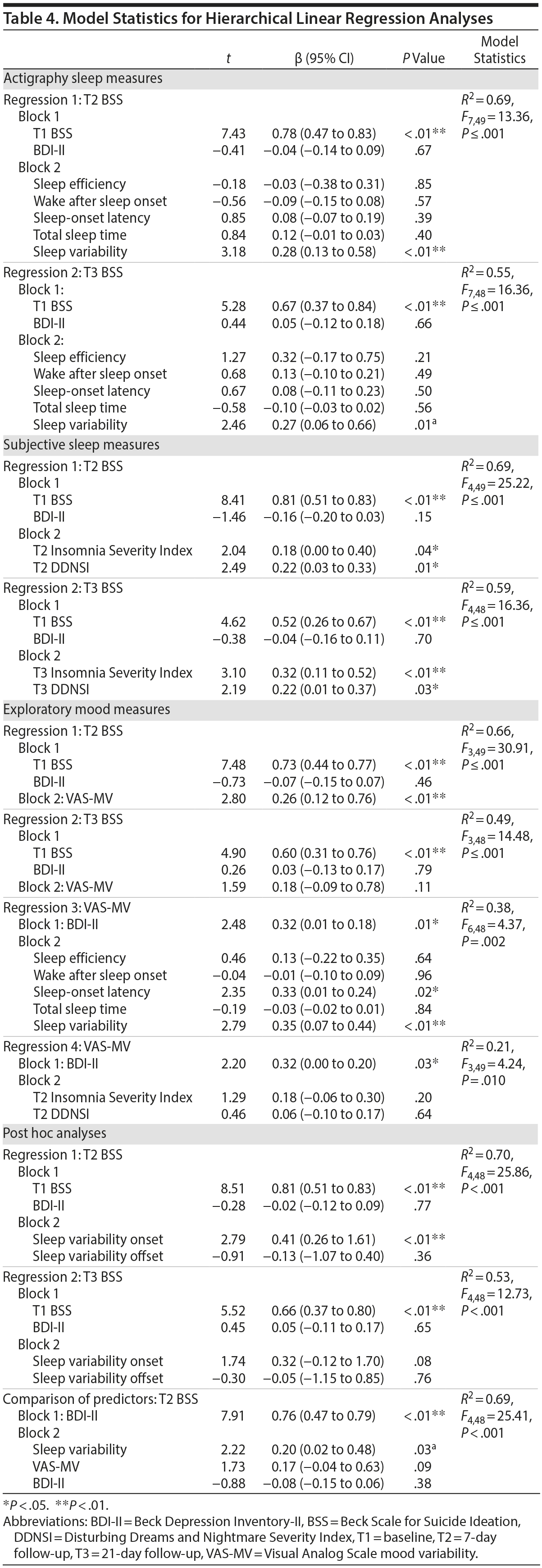
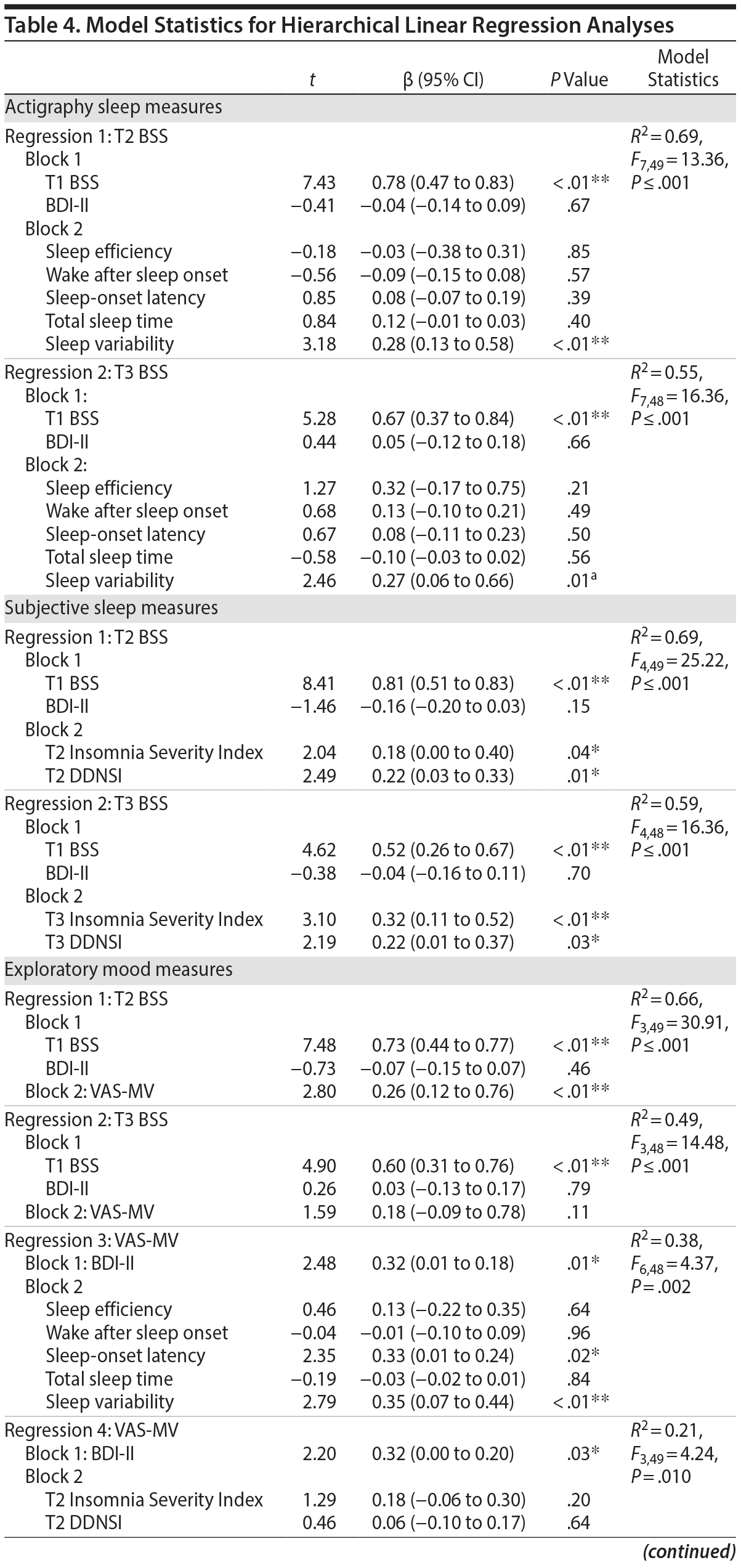
Model statistics are shown in Table 4.
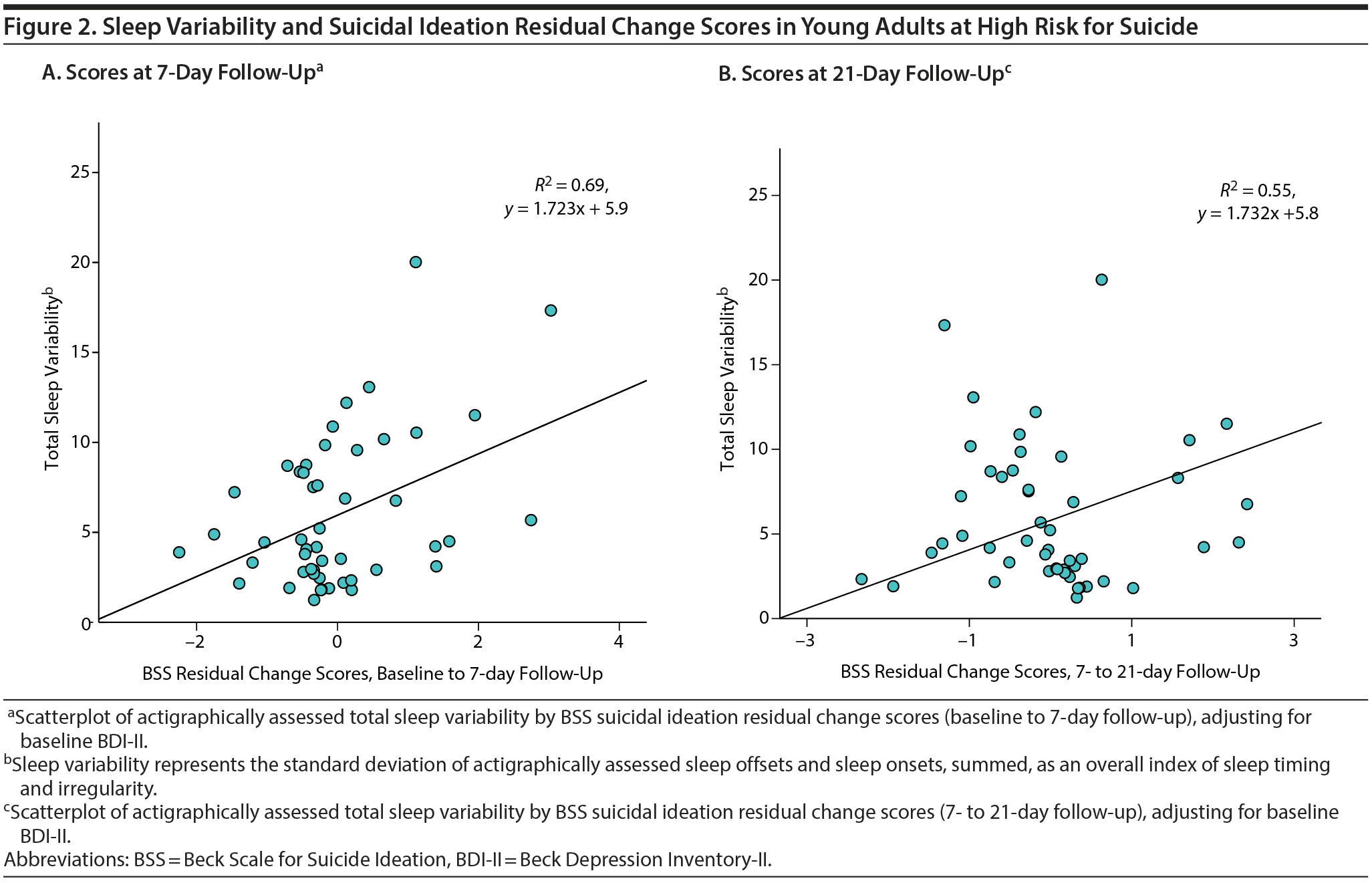
Actigraphic sleep parameters. For regressions 1 and 2, actigraphy variables, as a set, significantly predicted T2 and T3 BSS residual change scores when controlled for BDI-II score. However, only sleep variability predicted such change relative to other actigraphic parameters (Figure 2).
Subjective sleep parameters. For regressions 1 and 2, higher scores on the ISI and DDNSI were significantly associated with T2 and T3 BSS residual change scores when adjusted for BDI-II score. Each emerged as individual predictors of symptom increases.
Exploratory mood analyses. For regressions 1 and 2, main effects were significant for model statistics. However, this was a nonsignificant trend for T3 BSS. For regression 3, whereas actigraphy variables jointly predicted VAS-MV when controlled for BDI-II score, only sleep-onset latency and sleep variability individually contributed to the model variance. For regression 4, neither ISI nor DDNSI individually predicted VAS-MV.
Post Hoc Analyses
Based on observed findings for sleep variability as a significant predictor of risk, post hoc analyses were conducted to evaluate whether a specific component of the sleep-wake cycle (ie, sleep onsets vs offsets) differentially contributed to risk and, by extension, might serve as a treatment target. Specifically, sleep-wake timing variability (ie, sleep variability onset vs sleep variability offset) was simultaneously compared in the model. Including BDI-II and baseline BSS as covariates, sleep variability onset accounted for a significant portion of the variance in T2 and T3 BSS change scores relative to sleep variability offset. Variability of other sleep parameters (ie, variations in sleep efficiency, wake after sleep onset, sleep-onset latency, and total sleep time) was also examined as predictors of risk within a single model with sleep variability, including BDI-II and baseline BSS as covariates. Only greater sleep variability and less variability in total sleep time were significant (P ≤ .02) predictors of T2 BSS change scores (Supplementary eTable 2).
Correlations were employed to elucidate sleep variability in relationship to other sleep parameters. Beyond delayed bedtime/wake time and nap frequency, sleep variability was not significantly correlated with other actigraphy variables (total sleep time, sleep-onset latency, wake after sleep onset, sleep efficiency; P > .05), although a nonsignificant trend emerged for total sleep time (r = -0.26, P = .07). Significant intercorrelations were, however, observed for sleep variability and both T2 ISI (r = 0.35, P = .02) and DDNSI (r = 0.02, P = .04).
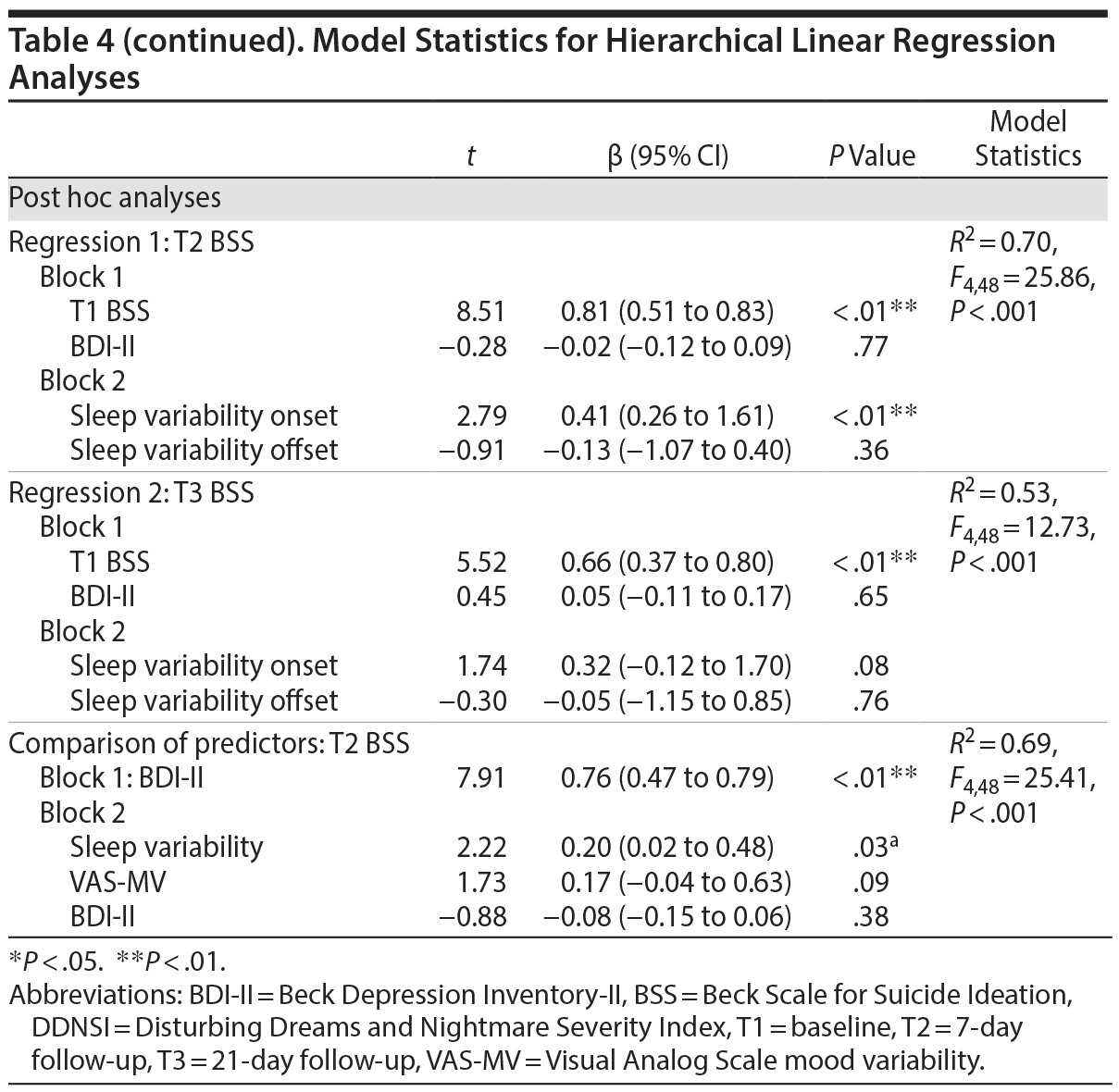
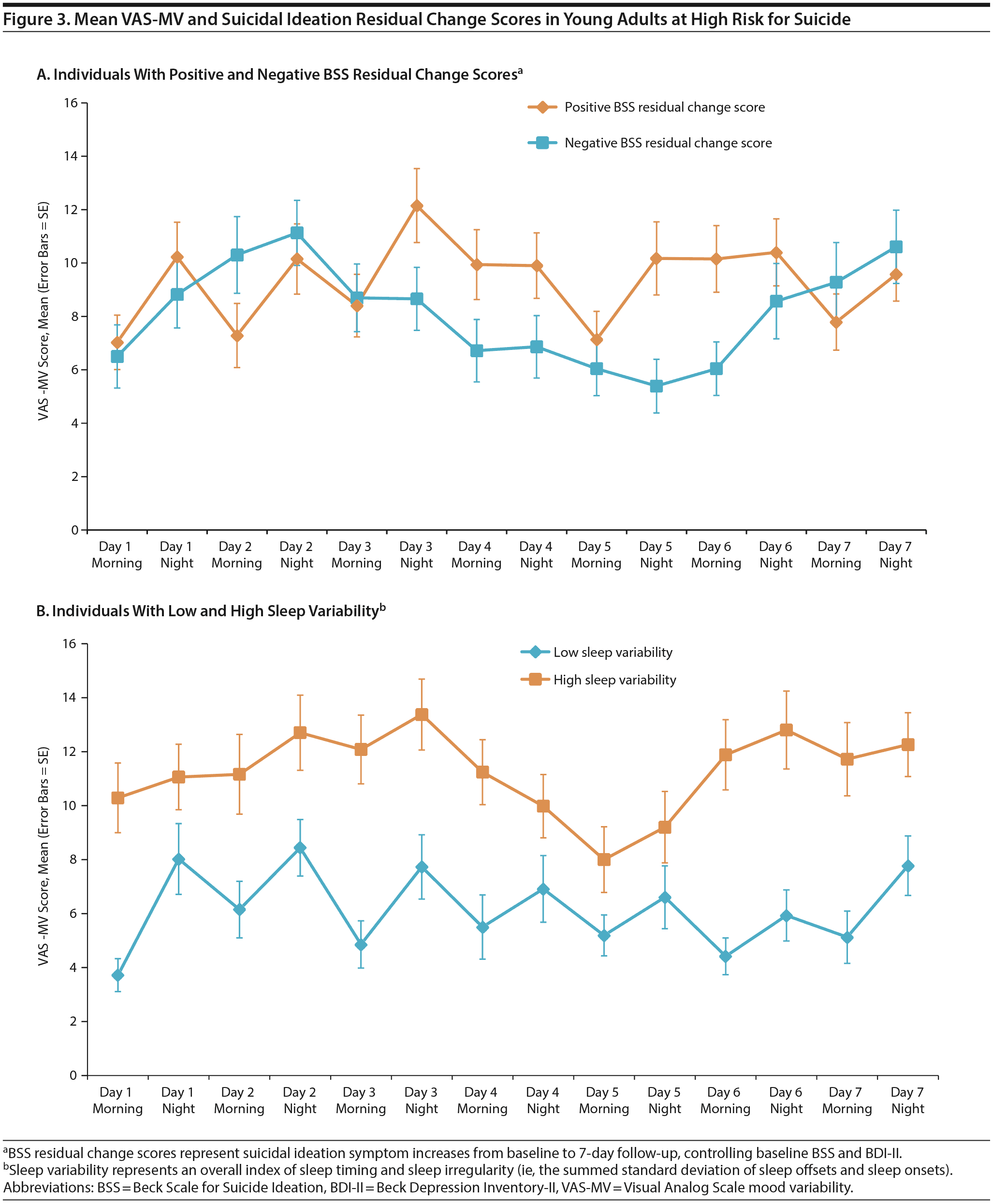
Finally, given that sleep variability and VAS-MV significantly predicted T2 BSS symptom change when the results were controlled for BDI-II, a follow-up test was conducted to identify the most robust predictor of risk. All 3 variables were included in a competing model for simultaneous comparison as predictors (regression 3) and to replicate past findings,16,43 using an objective sleep index. Surprisingly, sleep variability and VAS-MV accounted for more unique variance in prediction of BSS symptom change compared to BDI-II (Figure 3).
DISCUSSION
Findings revealed that actigraphic and self-reported sleep disturbances (ie, sleep variability, insomnia, and nightmares) predicted acute suicidal ideation symptom changes, an effect that occurred independent of depressive symptoms at 7- and 21-day follow-ups. This study converges with past reports7,13,16 demonstrating associations between subjective sleep problems and suicidal behaviors—when data were controlled for depression severity and affective disorder—and with studies44,45 identifying nocturnal wakefulness as a predictor of suicidal ideation and suicide deaths. Such effects were observed despite clinical levels of covariates and sleep problems commensurate with a diagnosis. Likewise, mean actigraphy indices exceeded cut points distinguishing participants with insomnia from controls,34,35 which revealed significant variability in the timing of sleep onsets and offsets (≥ 2.9 hours). Such patterns strongly conflict with an increased developmental need for sleep among this age group.10,46
Although actigraphic parameters together predicted suicidal ideation increases, only sleep variability—in particular, sleep-onset variability—individually predicted such symptom change. Greater variability of other objective sleep parameters (ie, sleep efficiency, wake after sleep onset, sleep-onset latency) did not predict suicidal ideation changes, whereas less variability in total sleep time (ie, consistent lack of sleep) significantly predicted risk, alongside irregularity in the overall timing of sleep. Our findings build upon an emerging literature tying intraindividual variability in sleep timing to an array of adverse outcomes, including stressful life events, negative affect, insomnia, and depression.33,47 Similar to studies among youth,48 our study found significant correlations between sleep variability and subjective sleep complaints, delayed bedtimes, and nap frequency. This link aligns with conceptual models of insomnia development, underscoring the unpredictability of sleep as a cause and a consequence of insomnia and its maintenance.49,50 Such findings suggest that sleep variability may be an important feature—alongside insomnia, nightmares, and nocturnal wakefulness44,45—to assess in the presence of other well-established suicide risk factors. These findings may also guide application to risk assessment, given findings that combination of actigraphy and self-reported sleep may significantly aid prediction of risk and specific mood states.51,52
Our sample reflected a group at heightened risk for suicide based on history of suicidal behaviors28 and nonsuicidal self-injury.53,54 The majority of participants endorsed a past suicide attempt, and one-third reported multiple attempts. In most cases, these occurred by 15 years of age. This finding corresponds with a recent epidemiologic study55 among adolescents (n = 12,395) in which a newly identified "invisible" risk group (ie, with reduced sleep, high media use, and sedentary behaviors) showed prevalence of suicidal ideation comparable to those classified by more traditional risk factors. Given escalation of suicide risk from adolescence to adulthood among those with past attempts,56,57 sleep may warrant investigation as a risk factor and intervention target for repeat suicidal behaviors.
Regarding clinical rationale, sleep disturbances are arguably less stigmatized compared to other risk factors yet modifiable and highly treatable. Gold standard insomnia interventions indicate preliminary efficacy in as few as 1-4 treatment sessions.58 Such treatments reduce sleep variability, which otherwise predicts treatment resistance.47,59 Insomnia and nightmare treatments furthermore demonstrate improvements in nonsleep outcomes, including depression and suicidal ideation.39,60 Additionally, the brevity of this approach appears well matched to the acute nature of a suicidal crisis and treatment engagement difficulties following a suicide attempt (ie, where up to half will refuse treatment61 and 75% may drop out in ≤ 1 year62). Taken together, sleep treatment may represent a low-risk intervention strategy for suicidal behaviors.
As a proposed exploratory factor, intraindividual mood variation predicted suicidal symptom increases, independent of depressive symptoms. Although main effects were observed for objective and subjective sleep parameters, only sleep variability and sleep-onset latency were individually related to mood fluctuations, which may be unsurprising given the degree of symptom overlap. Finally, in a competing risk model, we compared sleep variability, depressive symptoms, and mood variability as predictors of suicidal ideation. Remarkably, sleep variability—and to a lesser extent, mood variability—outperformed depression severity in the prediction of acute suicidal symptom change. While unexpected, this finding converges with at least 2 reports, including a study43 among military personnel referred for imminent suicide risk and a population-based investigation16 of late-life suicide. In both cases, poor subjective sleep quality outperformed depressive symptoms and hopelessness in the prediction of incident risk for suicide attempts (ie, ≤ 1 month) and fatalities (ie, ≤ 10 years). To our knowledge, this is the first study yielding similar results utilizing an actigraphic sleep measure and acute time frame.
Sleep disturbances and suicidal behaviors cut across psychiatric and medical illness,63,64 suggesting a shared underlying neurobiology. On the basis of the role of sleep in emotion21 and its association to suicide risk,24 we evaluated mood variability as an exploratory risk factor. Our findings broadly converge with experimental and nonexperimental studies of sleep deprivation, which show effects on emotional reactivity,65 amygdala activation,66 a blunting of positive affect,65 and depression-like changes in serotonergic function.64 Interestingly, a number of biomarkers (eg, inflammatory cytokines, brain-derived neurotrophic factor, serotonin transporter expression, etc) exist at the intersection of sleep and suicidal behaviors,48,67,68 suggesting that sleep loss may induce vulnerability at the molecular level. Given the neurocognitive deficits observed in suicidal behaviors,69 and the degree to which poor sleep and suicide cut across psychiatric illness,8 we recommend further study of sleep using a systems neuroscience approach, which would be commensurate with initiatives emphasizing the importance of transdiagnostic risk factors70 and development of a biosignature for suicide.67,68
Several limitations should be noted. First, actigraphy served as our objective measure of sleep versus polysomnography. Future studies evaluating correspondence to sleep architecture are needed. Next, although research identifies poor sleep as a risk factor across diagnostically diverse samples, our study was limited to self-reported diagnostic histories. Replication is thus warranted using clinician-administered diagnostic interviews and to evaluate generalizability among those without a suicide attempt history. Finally, suicidal behaviors range in severity from suicidal ideation to suicide attempts to suicide fatalities. While suicidal thoughts are well established as a suicide risk factor,71 especially in the presence of past attempts,28,72 investigation of actigraphically assessed sleep and for suicide attempt risk is strongly recommended.
CONCLUSIONS
Research suggests that less than 1% of studies examining suicide risk reflect the weeks prior to suicidal behaviors,6 elevating the need for methodologically rigorous screening and evaluation of warning signs. Study strengths include its highly selective sample, longitudinal design, and acute time frame. To our knowledge, this is the first longitudinal report indicating that objectively and subjectively measured sleep disturbance confers risk for suicidal ideation independent of depression severity. Given the ease of sleep disturbance assessment and treatment, and its unique visibility as a warning sign, we propose poor sleep as a potential biomarker and therapeutic target for suicide prevention.
Submitted: September 3, 2016; accepted January 5, 2017.
Potential conflicts of interest: The authors have no conflicts of interest to report.
Funding/support: This work was supported in part by the John Simon Guggenheim Foundation (Dr Joiner) and National Institutes of Health (NIH): F31MH080470-01 (Dr Bernert), NIH K23MH093490 (Dr Bernert).
Role of the sponsor: The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Supplementary material: See accompanying pages.
REFERENCES
1. World Health Organization (WHO). Preventing Suicide: A Global Imperative. Geneva, Switzerland: WHO Press; 2014.
2. Goldsmith SK, Pellmar RC, Kleinman AM, Bunney WE, eds. Reducing Suicide: A National Imperative. Washington, DC: National Academies Press; 2002.
3. Office of the Surgeon General (US); National Action Alliance for Suicide Prevention (US). 2012 National Strategy for Suicide Prevention: Goals and Objectives for Action, a Report of the US Surgeon General and of the National Action Alliance for Suicide Prevention. Washington, DC; US Department of Health & Human Services; 2012.
4. National Mental Health Information Center. Suicide Warning Signs. SAMHSA website. http://store.samhsa.gov/shin/content//SVP11-0126/SVP11-0126.pdf. 2005.
5. Pigeon WR, Pinquart M, Conner K. Meta-analysis of sleep disturbance and suicidal thoughts and behaviors. J Clin Psychiatry. 2012;73(9):e1160-e1167. PubMed doi:10.4088/JCP.11r07586
6. Franklin JC, Ribeiro JD, Fox KR, et al. Risk factors for suicidal thoughts and behaviors: a meta-analysis of 50 years of research. Psychol Bull. 2017;143(2):187-232. PubMed doi:10.1037/bul0000084
7. Bernert RA, Kim JS, Iwata NG, et al. Sleep disturbances as an evidence-based suicide risk factor. Curr Psychiatry Rep. 2015;17(3):554. PubMed doi:10.1007/s11920-015-0554-4
8. American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
9. Brown GK, Beck AT, Steer RA, et al. Risk factors for suicide in psychiatric outpatients: a 20-year prospective study. J Consult Clin Psychol. 2000;68(3):371-377. PubMed doi:10.1037/0022-006X.68.3.371
10. Owens J; Adolescent Sleep Working Group; Committee on Adolescence. Insufficient sleep in adolescents and young adults: an update on causes and consequences. Pediatrics. 2014;134(3):e921-e932. PubMed doi:10.1542/peds.2014-1696
11. Buysse DJ, Angst J, Gamma A, et al. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008;31(4):473-480. PubMed doi:10.1093/sleep/31.4.473
12. WISQARS: Cost of Injury Reports. CDC website. https://wisqars.cdc.gov:8443/cost. Published 2015.
13. Goldstein TR, Bridge JA, Brent DA. Sleep disturbance preceding completed suicide in adolescents. J Consult Clin Psychol. 2008;76(1):84-91. PubMed doi:10.1037/0022-006X.76.1.84
14. Roane BM, Taylor DJ. Adolescent insomnia as a risk factor for early adult depression and substance abuse. Sleep. 2008;31(10):1351-1356. PubMed
15. Wong MM, Brower KJ. The prospective relationship between sleep problems and suicidal behavior in the National Longitudinal Study of Adolescent Health. J Psychiatr Res. 2012;46(7):953-959. PubMed doi:10.1016/j.jpsychires.2012.04.008
16. Bernert RA, Turvey CL, Conwell Y, et al. Association of poor subjective sleep quality with risk for death by suicide during a 10-year period: a longitudinal, population-based study of late life. JAMA Psychiatry. 2014;71(10):1129-1137. PubMed doi:10.1001/jamapsychiatry.2014.1126
17. Keshavan MS, Reynolds CF, Montrose D, et al. Sleep and suicidality in psychotic patients. Acta Psychiatr Scand. 1994;89(2):122-125. PubMed doi:10.1111/j.1600-0447.1994.tb01498.x
18. Sabo E, Reynolds CF 3rd, Kupfer DJ, et al. Sleep, depression, and suicide. Psychiatry Res. 1991;36(3):265-277. PubMed doi:10.1016/0165-1781(91)90025-K
19. Wilcox HC, Conner KR, Caine ED. Association of alcohol and drug use disorders and completed suicide: an empirical review of cohort studies. Drug Alcohol Depend. 2004;76(suppl):S11-S19. PubMed doi:10.1016/j.drugalcdep.2004.08.003
20. Bagge CL, Lee HJ, Schumacher JA, et al. Alcohol as an acute risk factor for recent suicide attempts: a case-crossover analysis. J Stud Alcohol Drugs. 2013;74(4):552-558. PubMed doi:10.15288/jsad.2013.74.552
21. Walker MP. The role of sleep in cognition and emotion. Ann N Y Acad Sci. 2009;1156:168-197. PubMed doi:10.1111/j.1749-6632.2009.04416.x
22. Minkel JD, Banks S, Htaik O, et al. Sleep deprivation and stressors: evidence for elevated negative affect in response to mild stressors when sleep deprived. Emotion. 2012;12(5):1015-1020. PubMed doi:10.1037/a0026871
23. Akiskal HS, Benazzi F. Psychopathologic correlates of suicidal ideation in major depressive outpatients: is it all due to unrecognized (bipolar) depressive mixed states? Psychopathology. 2005;38(5):273-280. PubMed doi:10.1159/000088445
24. Zlotnick C, Donaldson D, Spirito A, et al. Affect regulation and suicide attempts in adolescent inpatients. J Am Acad Child Adolesc Psychiatry. 1997;36(6):793-798. PubMed doi:10.1097/00004583-199706000-00016
25. Hom MA, Joiner TE, Bernert RA. Limitations of a single-item assessment of suicide attempt history: implications for standardized suicide risk assessment. Psychol Assess. 2016;28(8):1026-1030. PubMed doi:10.1037/pas0000241
26. Pierce DW. Suicidal intent in self-injury. Br J Psychiatry. 1977;130:377-385. PubMed doi:10.1192/bjp.130.4.377
27. Forman EM, Berk MS, Henriques GR, et al. History of multiple suicide attempts as a behavioral marker of severe psychopathology. Am J Psychiatry. 2004;161(3):437-443. PubMed doi:10.1176/appi.ajp.161.3.437
28. Bostwick JM, Pabbati C, Geske JR, et al. Suicide attempt as a risk factor for completed suicide: even more lethal than we knew. Am J Psychiatry. 2016;173(11):1094-1100. PubMed doi:10.1176/appi.ajp.2016.15070854
29. Brown GK, Ten Have T, Henriques GR, et al. Cognitive therapy for the prevention of suicide attempts: a randomized controlled trial. JAMA. 2005;294(5):563-570. PubMed doi:10.1001/jama.294.5.563
30. Beck AT, Steer RA, Ranieri WF. Scale for suicide ideation: psychometric properties of a self-report version. J Clin Psychol. 1988;44(4):499-505. PubMed doi:10.1002/1097-4679(198807)44:4<499::AID-JCLP2270440404>3.0.CO;2-6
31. Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev. 2011;15(4):259-267. PubMed doi:10.1016/j.smrv.2010.10.001
32. Kushida CA, Chang A, Gadkary C, et al. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2(5):389-396. PubMed doi:10.1016/S1389-9457(00)00098-8
33. Bei B, Wiley JF, Trinder J, et al. Beyond the mean: a systematic review on the correlates of daily intraindividual variability of sleep/wake patterns. Sleep Med Rev. 2016;28:108-124. PubMed doi:10.1016/j.smrv.2015.06.003
34. Natale V, Léger D, Bayon V, et al. The consensus sleep diary: quantitative criteria for primary insomnia diagnosis. Psychosom Med. 2015;77(4):413-418. PubMed doi:10.1097/PSY.0000000000000177
35. Natale V, Léger D, Martoni M, et al. The role of actigraphy in the assessment of primary insomnia: a retrospective study. Sleep Med. 2014;15(1):111-115. PubMed doi:10.1016/j.sleep.2013.08.792
36. Bastien CH, Valliרres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297-307. PubMed doi:10.1016/S1389-9457(00)00065-4
37. Morin CM, Belleville G, Bélanger L, et al. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601-608. PubMed doi:10.1093/sleep/34.5.601
38. Krakow BJ, Melendrez DC, Johnston LG, et al. Sleep Dynamic Therapy for Cerro Grande Fire evacuees with posttraumatic stress symptoms: a preliminary report. J Clin Psychiatry. 2002;63(8):673-684. PubMed doi:10.4088/JCP.v63n0804
39. Krakow B, Hollifield M, Johnston L, et al. Imagery rehearsal therapy for chronic nightmares in sexual assault survivors with posttraumatic stress disorder: a randomized controlled trial. JAMA. 2001;286(5):537-545. PubMed doi:10.1001/jama.286.5.537
40. Beck AT, Steer RA. Manual for the Revised Beck Depression Inventory. San Antonio, TX: Psychological Corporation; 1987.
41. Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption—II. Addiction. 1993;88(6):791-804. PubMed doi:10.1111/j.1360-0443.1993.tb02093.x
42. Bernert RA, Joiner TE Jr, Cukrowicz KC, et al. Suicidality and sleep disturbances. Sleep. 2005;28(9):1135-1141. PubMed doi:10.1093/sleep/28.9.1135
43. Ribeiro JD, Pease JL, Gutierrez PM, et al. Sleep problems outperform depression and hopelessness as cross-sectional and longitudinal predictors of suicidal ideation and behavior in young adults in the military. J Affect Disord. 2012;136(3):743-750. PubMed doi:10.1016/j.jad.2011.09.049
44. Perlis ML, Grandner MA, Brown GK, et al. Nocturnal wakefulness as a previously unrecognized risk factor for suicide. J Clin Psychiatry. 2016;77(6):e726-e733. PubMed doi:10.4088/JCP.15m10131
45. Ballard ED, Vande Voort JL, Bernert RA, et al. Nocturnal wakefulness is associated with next-day suicidal ideation in major depressive disorder and bipolar disorder. J Clin Psychiatry. 2016;77(6):825-831. PubMed doi:10.4088/JCP.15m09943
46. Carskadon MA. Sleep in adolescents: the perfect storm. Pediatr Clin North Am. 2011;58(3):637-647. PubMed doi:10.1016/j.pcl.2011.03.003
47. Suh S, Nowakowski S, Bernert RA, et al. Clinical significance of night-to-night sleep variability in insomnia. Sleep Med. 2012;13(5):469-475. PubMed doi:10.1016/j.sleep.2011.10.034
48. Carskadon MA, Sharkey KM, Knopik VS, et al. Short sleep as an environmental exposure: a preliminary study associating 5-HTTLPR genotype to self-reported sleep duration and depressed mood in first-year university students. Sleep. 2012;35(6):791-796. PubMed doi:10.5665/sleep.1876
49. Manber R, Chambers AS. Insomnia and depression: a multifaceted interplay. Curr Psychiatry Rep. 2009;11(6):437-442. PubMed doi:10.1007/s11920-009-0066-1
50. Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004). Sleep. 2006;29(11):1398-1414. PubMed doi:10.1093/sleep/29.11.1398
51. Geoffroy PA, Boudebesse C, Bellivier F, et al. Sleep in remitted bipolar disorder: a naturalistic case-control study using actigraphy. J Affect Disord. 2014;158:1-7. PubMed doi:10.1016/j.jad.2014.01.012
52. Thompson WK, Gershon A, O’ Hara R, et al. The prediction of study-emergent suicidal ideation in bipolar disorder: a pilot study using ecological momentary assessment data. Bipolar Disord. 2014;16(7):669-677. PubMed doi:10.1111/bdi.12218
53. Ribeiro JD, Franklin JC, Fox KR, et al. Self-injurious thoughts and behaviors as risk factors for future suicide ideation, attempts, and death: a meta-analysis of longitudinal studies. Psychol Med. 2016;46(2):225-236. PubMed doi:10.1017/S0033291715001804
54. Hawton K, Harriss L, Zahl D. Deaths from all causes in a long-term follow-up study of 11,583 deliberate self-harm patients. Psychol Med. 2006;36(3):397-405. PubMed doi:10.1017/S0033291705006914
55. Carli V, Hoven CW, Wasserman C, et al. A newly identified group of adolescents at "invisible" risk for psychopathology and suicidal behavior: findings from the SEYLE study. World Psychiatry. 2014;13(1):78-86. PubMed doi:10.1002/wps.20088
56. Goldston DB, Erkanli A, Daniel SS, et al. Developmental trajectories of suicidal thoughts and behaviors from adolescence through adulthood. J Am Acad Child Adolesc Psychiatry. 2016;55(5):400-407.e1. PubMed doi:10.1016/j.jaac.2016.02.010
57. Goldman-Mellor SJ, Caspi A, Harrington H, et al. Suicide attempt in young people: a signal for long-term health care and social needs. JAMA Psychiatry. 2014;71(2):119-127. PubMed doi:10.1001/jamapsychiatry.2013.2803
58. Ellis JG, Cushing T, Germain A. Treating acute insomnia: a randomized controlled trial of a "single-shot" of cognitive behavioral therapy for insomnia. Sleep. 2015;38(6):971-978. PubMed
59. Sánchez-Ortu×±o MM, Edinger JD. Internight sleep variability: its clinical significance and responsiveness to treatment in primary and comorbid insomnia. J Sleep Res. 2012;21(5):527-534. PubMed doi:10.1111/j.1365-2869.2012.01010.x
60. Manber R, Edinger JD, Gress JL, et al. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31(4):489-495. PubMed doi:10.1093/sleep/31.4.489
61. Kurz A, Möller HJ. Help-seeking behavior and compliance of suicidal patients [in German]. Psychiatr Prax. 1984;11(1):6-13. PubMed
62. Rudd MD, Joiner TE Jr, Rajab MH. Help negation after acute suicidal crisis. J Consult Clin Psychol. 1995;63(3):499-503. PubMed doi:10.1037/0022-006X.63.3.499
63. Mann JJ, Brent DA, Arango V. The neurobiology and genetics of suicide and attempted suicide: a focus on the serotonergic system. Neuropsychopharmacology. 2001;24(5):467-477. PubMed doi:10.1016/S0893-133X(00)00228-1
64. Roman V, Walstra I, Luiten PG, et al. Too little sleep gradually desensitizes the serotonin 1A receptor system. Sleep. 2005;28(12):1505-1510. PubMed
65. Zohar D, Tzischinsky O, Epstein R, et al. The effects of sleep loss on medical residents’ emotional reactions to work events: a cognitive-energy model. Sleep. 2005;28(1):47-54. PubMed doi:10.1093/sleep/28.1.47
66. Gujar N, Yoo SS, Hu P, et al. Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. J Neurosci. 2011;31(12):4466-4474. PubMed doi:10.1523/JNEUROSCI.3220-10.2011
67. Oquendo MA. Subtyping suicidal behavior: a strategy for identifying phenotypes that may more tractably yield molecular underpinnings. J Clin Psychiatry. 2016;77(6):813-814. PubMed doi:10.4088/JCP.16f10933
68. Oquendo MA, Sullivan GM, Sudol K, et al. Toward a biosignature for suicide. Am J Psychiatry. 2014;171(12):1259-1277. PubMed doi:10.1176/appi.ajp.2014.14020194
69. Keilp JG, Sackeim HA, Brodsky BS, et al. Neuropsychological dysfunction in depressed suicide attempters. Am J Psychiatry. 2001;158(5):735-741. PubMed doi:10.1176/appi.ajp.158.5.735
70. Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751. PubMed doi:10.1176/appi.ajp.2010.09091379
71. Rudd MD, Berman AL, Joiner TE Jr, et al. Warning signs for suicide: theory, research, and clinical applications. Suicide Life Threat Behav. 2006;36(3):255-262. PubMed doi:10.1521/suli.2006.36.3.255
72. Chu C, Klein KM, Buchman-Schmitt JM, et al. Routinized assessment of suicide risk in clinical practice: an empirically informed update. J Clin Psychol. 2015;71(12):1186-1200. PubMed doi:10.1002/jclp.22210
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Suicide section. Please contact Philippe Courtet, MD, PhD, at [email protected].
Please sign in or purchase this PDF for $40.00.
Save
Cite