ABSTRACT
Selective serotonin reuptake inhibitors (SSRIs) may predispose to postpartum hemorrhage (PPH) by interfering with platelet-mediated hemostasis and serotonin-mediated myometrial contractility. A meta-analysis of 8 observational studies found that, regardless of drug class, gestational exposure to antidepressants was associated with a small (odds ratio, 1.25) but statistically significantly increased risk of PPH; however, this finding was true only when antidepressant exposure was proximal to the date of delivery. A recent, moderately large, nationally representative, Swedish observational study also found that gestational exposure to SSRIs was associated with a significantly increased risk of PPH; the crude number needed to harm was 48. For reasons related to the methodology employed, it is possible that the risk was underestimated in this study. The findings of the meta-analysis and of the observational study are examined with a view to help readers understand how to critically read and interpret the research literature in the field. A reasonable viewpoint is that the increase in risk of PPH associated with gestational exposure to SSRIs is smaller than the increase in risk associated with obstetric risk factors for PPH; nevertheless, following precautionary measures would be wise. Such measures would include the routine administration of a uterotonic agent immediately after delivery to all women who have received serotonin reuptake inhibitor treatment during the month preceding delivery; the choice of uterotonic agent would depend on local hospital protocols. Women at risk should also be closely monitored for continued blood loss during the first 24 hours after delivery.
J Clin Psychiatry 2022;83(2):22f14455
To cite: Andrade C. Selective serotonin reuptake inhibitor use in pregnancy and risk of postpartum hemorrhage. J Clin Psychiatry. 2022;83(2):22f14455.
To share: https://doi.org/10.4088/JCP.22f14455
© Copyright 2022 Physicians Postgraduate Press, Inc.
Serotonin plays an important role in hemostatic mechanisms: when released by platelets after vascular injury, it causes platelet aggregation and vasoconstriction, resulting in hemostasis. Platelets do not synthesize serotonin, and serotonin reuptake inhibitor (SRI) drugs, such as the selective serotonin reuptake inhibitors (SSRIs), inhibit the uptake of serotonin into platelets much as they inhibit the reuptake of serotonin into neurons. So, in persons who receive SRIs, platelet and hence hemostatic mechanisms are potentially impaired, resulting in an increased risk of bleeding at various sites such as the gastrointestinal tract, and in various contexts such as in association with surgery.1,2 Separation of the placenta from the uterus during childbirth is one context in which bleeding occurs. Usually, once the placenta separates, the uterus contracts; the interlacing myometrial fibers retract, compressing the vasculature that had supplied the placenta, resulting in mechanically driven hemostasis and restriction of blood loss to < 500 mL. When blood loss is greater, it is known as postpartum hemorrhage (PPH). More recent definitions of PPH set the threshold for blood loss at 1,000 mL (especially in the context of cesarean section) or grade the severity of PPH based on the quantity of blood lost.3–5
The association between gestational exposure to SRIs and PPH has only become apparent in the last decade. Besides the platelet-mediated mechanisms described, a potential effect of these drugs on myometrial contraction and hence atonic PPH has also been speculated.6 This article examines one meta-analysis7 and a recent observational study8 to illustrate the risk of PPH in association with selective serotonin reuptake inhibitor (SSRI) use. Readers are also assisted in the critical examination of these studies.
Antidepressant Use in Pregnancy and Postpartum Hemorrhage: Meta-analysis
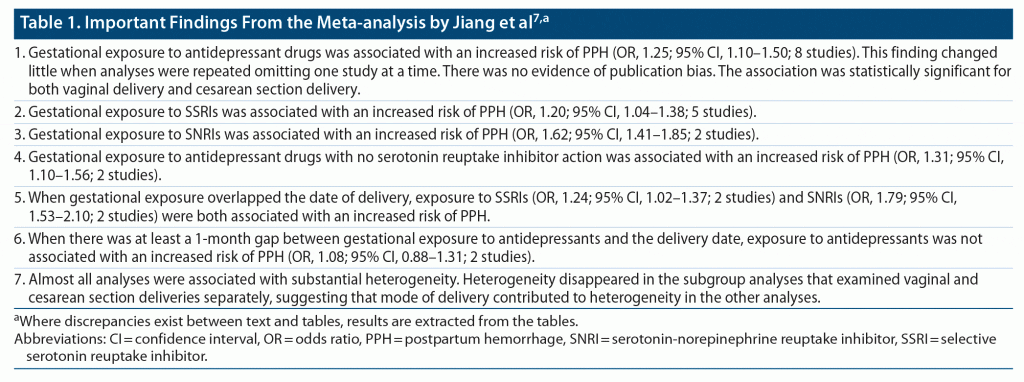
Jiang et al7 described a systematic review and meta-analysis of the association between antidepressant drug use during pregnancy and the risk of PPH. They searched electronic databases and reference lists and identified 6 cohort and 2 case-control studies that compared pregnancies that were versus were not exposed to antidepressants. These studies had been conducted in the US, Canada, Australia, Norway, and Sweden. Study sample sizes ranged from 367 to 318,840 (total, N = 572,686), and PPH events in the studies ranged from 23 to 22,509 (total, N = 48,784). Six studies were rated to be of high quality.
Important findings from the meta-analysis are presented in Table 1. In summary, exposure to antidepressant drugs during pregnancy was associated with an increased risk of PPH; this was true for SSRIs and serotonin-norepinephrine reuptake inhibitors, as well as antidepressants with no serotonin reuptake inhibitor property. Whereas the association between antidepressant exposure and PPH was significant when exposure overlapped the date of delivery, it was not significant when exposure ended 1 month or more before the date of delivery.
Limitations of the Meta-analysis
The meta-analysis7 had several limitations. The authors did not provide an idea of whether the unexposed comparison groups in the included studies comprised women with psychiatric illnesses or without psychiatric illnesses; in the latter situation, the risk of confounding, associated with maternal mental illness, is higher. The authors pooled data for up to 6 comparisons from the same study in the same forest plot without indicating what the different comparisons were or whether exposed or control groups were repeated (counted twice or more often) in these different comparisons. Many of the subgroup analyses were based on very few studies, such as just 2 studies. Summary statistics were reported as relative risks (RRs) in the text, and, in many cases, values identical to the RRs were reported as odds ratios (ORs) in a table.
Antidepressant Use in Pregnancy and Postpartum Hemorrhage: Cohort Study
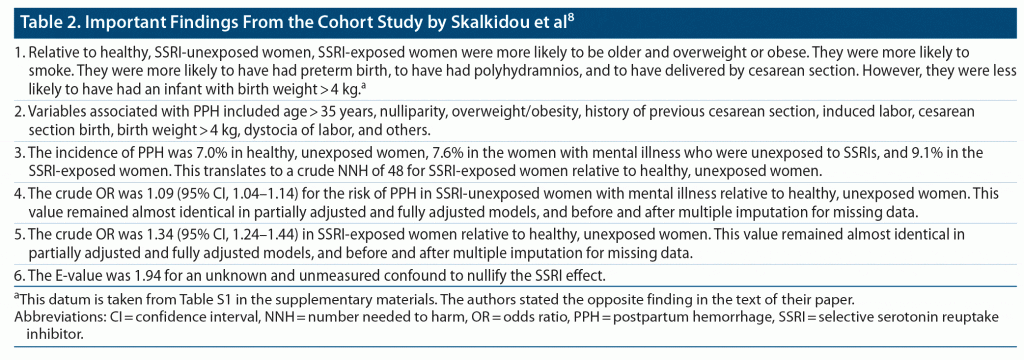
Skalkidou et al8 described a retrospective cohort study with prospective data ascertainment, based on data from the Swedish Pregnancy Register; this register covers 90% of the deliveries in Sweden, making it reasonably nationally representative. There were 8,643 (2.8% of the total sample) women who had received an SSRI (fluoxetine, sertraline, paroxetine, fluvoxamine, citalopram, escitalopram) at some time during pregnancy, 28,672 women with past or current major mental illness but no gestational exposure to SSRIs, and 268,006 “healthy” women with neither past nor current history of major mental illness, nor gestational exposure to SSRIs. All women had singleton pregnancies, only the first pregnancy was included if women had more than one pregnancy, and no woman had placental abnormalities or gestational anticoagulant exposure that could predispose to PPH.
All women received prophylactic oxytocin after delivery, administered parenterally in the dose of 5 IU after cesarean section or 10 IU after vaginal delivery. PPH was defined as the loss of > 1,000 mL of blood within the first 2 hours postpartum, determined from absorption in cloth or swabs and visually estimated at low volumes but weighed at high volumes. In case of cesarean section, surgical suction volume was noted after deducting amniotic fluid volume.
The occurrence of PPH was compared between groups using logistic regression. The analyses were adjusted for potential confounding variables such as maternal age, maternal body mass index, parity, history of previous cesarean section, maternal smoking, and others. Analyses were also adjusted for variables that could independently influence PPH; these variables included mode of delivery, labor dystocia, and delivery of large babies (birth weight > 4 kg).
Important findings from the study8 are presented in Table 2. In summary, relative to healthy, SSRI-unexposed women, SSRI-exposed women were at a slightly but significantly higher risk of experiencing PPH (OR, 1.34; crude number needed to harm [NNH], 48).
Strengths and Limitations of the Cohort Study
Strengths of the study8 were the large number of women exposed to SSRIs during pregnancy and the nationally representative sample.
Limitations of the study8 were many. Medication exposure and past and current mental health were recorded based on patient self-report. Data were unavailable for individual SSRIs and for doses of SSRIs used, so analyses could not be conducted for individual SSRIs, and dose-dependent associations could not be examined. Drugs such as venlafaxine and clomipramine, both of which potently inhibit serotonin reuptake, were not included for study, so women receiving these drugs may have been included in the group of women with a history of mental illness but no exposure to SSRIs. The implication is that if the serotonin reuptake inhibition mechanism predisposes to PPH, then the group of previously/currently ill women with no SSRI exposure was improperly constituted. Last but not least, SSRI exposure in the concluding weeks of gestation and especially overlapping the date of delivery was not studied; serotonin reuptake inhibition cannot predispose to PPH if the SSRI has been washed out of the body and platelet serotonin stores have been replenished before delivery.
Other Comments on the Cohort Study
In the Statistics subsection of Methods, the authors8 stated that they compared women exposed to SSRIs with women in the other 2 groups; however, what they actually did and presented in Table 1 in their paper was compare healthy, SSRI-unexposed women with women in the other 2 groups. Regardless of this startling discrepancy, the findings of their study do lend themselves to interpretation.
There were several important variables that distinguished SSRI-exposed women from healthy, SSRI-unexposed women (Table 2); many of these variables are risk factors for PPH. Nevertheless, and very unexpectedly, the ORs were almost identical in crude, partially adjusted, and fully adjusted analyses. This suggests possible errors in analysis. However, if there were no mistakes, the crude NNH presented in Table 2 is likely to be a good approximation of an adjusted NNH.
The E-value for an unknown and unmeasured confound to nullify the SSRI effect was 1.94 (Table 2). This means that the OR for the confound would need to be 1.94 for the risk of PPH to be the same in the SSRI-exposed and the healthy, SSRI-unexposed women.9 Given that the OR for the SSRI effect was itself in the 1.31–1.34 range in crude and adjusted analyses, it seems unlikely that an unknown confound could have a larger effect. This conclusion comes with the caveat that there could be more than one confound with additive smaller effects.
Women were considered exposed to SSRIs if they had received SSRIs at any time during pregnancy. However, serotonin depletion in platelets as a predisposition to PPH would be clinically relevant only if SSRI exposure occurred proximal to the date of delivery. This means that the SSRI-exposed group was diluted by the inclusion of women with no SSRI-related risk of PPH, and so the findings in this study could have underestimated the true magnitude of the SSRI-related risk of PPH.
As a final note: the findings of the study8 apply only to more severe grades of PPH, that is, with blood loss > 1,000 mL. Furthermore, this definition was applied at 2 hours postpartum rather than within the first 24 hours postpartum, as is more usual.3–5 On both counts, the findings in this study could have underestimated the true magnitude of PPH as more conventionally or more broadly defined.
Summary and Implications
There is at least one plausible pathway through which gestational exposure to SSRIs proximal to the date of delivery may increase the risk of PPH: impairment of platelet-mediated vasoconstriction and coagulatory mechanisms.1,2 It has also been suggested that SSRIs may interfere with serotonin-mediated myometrial contractility postpartum,6 but there is no evidence for this conjecture, so far.
It is possible that the recent observational study by Skalkidou et al8 underestimated the risk of SSRI-associated PPH for reasons outlined in the previous section; so, the true NNH may be lower than 48 (Table 2), indicating higher risk. These findings suggest that a uterotonic agent should be routinely administered to SSRI-exposed women immediately after delivery, and that such women should be closely monitored for subsequent bleeding during the 24 hours after delivery; the choice of the uterotonic so administered would depend on local hospital protocols.
The above notwithstanding, it must be recognized that obstetric risk factors for PPH have a far greater impact than that detected for SSRIs by Skalkidou et al8 or estimated in the earlier meta-analysis.7 Whereas it has been suggested that the SSRI-associated increased risk is so low as to be reassuring and to not require additional precautionary steps,10 the wise clinician will err on the side of caution and treat with a prophylactic uterotonic drug and closely monitor, as recommended in the previous paragraph.
Published online: April 4, 2022.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
References (10)

- Andrade C, Sandarsh S, Chethan KB, et al. Serotonin reuptake inhibitor antidepressants and abnormal bleeding: a review for clinicians and a reconsideration of mechanisms. J Clin Psychiatry. 2010;71(12):1565–1575. PubMed CrossRef
- Andrade C, Sharma E. Serotonin reuptake inhibitors and risk of abnormal bleeding. Psychiatr Clin North Am. 2016;39(3):413–426. PubMed CrossRef
- El-Refaey H, Rodeck C. Post-partum haemorrhage: definitions, medical and surgical management. a time for change. Br Med Bull. 2003;67(1):205–217. PubMed CrossRef
- Evensen A, Anderson JM, Fontaine P. Postpartum hemorrhage: prevention and treatment. Am Fam Physician. 2017;95(7):442–449. PubMed
- No authors listed. Prevention and management of postpartum haemorrhage: green-top guideline no. 52. BJOG. 2017;124(5):e106–e149. PubMed CrossRef
- Palmsten K, Hernández-Díaz S, Huybrechts KF, et al. Use of antidepressants near delivery and risk of postpartum hemorrhage: cohort study of low income women in the United States. BMJ. 2013;347:f4877. PubMed CrossRef
- Jiang HY, Xu LL, Li YC, et al. Antidepressant use during pregnancy and risk of postpartum hemorrhage: a systematic review and meta-analysis. J Psychiatr Res. 2016;83:160–167. PubMed CrossRef
- Skalkidou A, Sundström-Poromaa I, Wikman A, et al. SSRI use during pregnancy and risk for postpartum haemorrhage: a national register-based cohort study in Sweden. BJOG. 2020;127(11):1366–1373. PubMed CrossRef
- Haneuse S, VanderWeele TJ, Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA. 2019;321(6):602–603. PubMed CrossRef
- Sholapurkar SL. Re: SSRI use during pregnancy and risk for postpartum haemorrhage: a national register-based cohort study in Sweden: this registry-based large study of postpartum haemorrhage with SSRI usage, despite crucial limitations, shows any increased risk to be reassuringly low and clinically non-significant. BJOG. 2021;128(3):620. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite