Antipsychotic medications, the mainstay of pharmacologic treatment for schizophrenia, are effective for acute positive symptoms and for relapse prevention during maintenance treatment.1 However, they are associated with many potential adverse effects, including extrapyramidal symptoms (EPS), weight gain, metabolic abnormalities, and prolactin elevation. Their relative propensity to induce clinically significant effects in these areas varies considerably across individual agents. Weight gain and metabolic disturbances are of particular concern because individuals with schizophrenia are already at greater risk for cardiovascular disease and diabetes due to a higher prevalence of and lower treatment rates for obesity, dyslipidemia, hyperglycemia, hypertension, and smoking.2-4 In the public sector, people with major mental illnesses such as schizophrenia have reductions in life expectancy of 25-30 years compared with the general population, primarily due to premature cardiovascular disease.5
OBJECTIVE
Weight gain and metabolic abnormalities associated with use of antipsychotic medications increase risk for cardiovascular disease, diabetes mellitus, and obesity-related cancers.1-5 Other problems associated with antipsychotics, such as EPS and prolactin elevation, can interfere with quality of life and ability to function.1 These problems present a dilemma for physicians who commonly face clinical decisions about whether or not to switch a patient from one antipsychotic to another to achieve a potential reduction in these effects. The purpose of this project was to provide evidence-based guidance concerning when and how it may be appropriate to undertake elective changes in antipsychotic medications with the goal of reducing risk of adverse effects, with a particular focus on those adverse effects associated with increased long-term health risks. This project builds on the results of the National Institute of Mental Health (NIMH)-funded 2009 Schizophrenia Patient Outcomes Research Team (PORT) psychopharmacologic treatment recommendations.1
LITERATURE REVIEW METHODOLOGY
To address the question raised by PORT whether there is “a minimal level of evidence that the majority of individuals would experience significant weight loss [or improvement in other adverse event parameters] without clinical deterioration,”1(p88) the literature on switching antipsychotics was reviewed, with a focus on studies published since the 2009 PORT treatment recommendations. PubMed was searched using the terms antipsychotics, switching, randomized, and clinical trials. Initially, 121 articles were identified. Many of these articles were excluded because they were review articles, dealt with changes of antipsychotics for acute symptoms (eg, agitation), or involved disorders other than schizophrenia (eg, bipolar disorder, dementia). Reference lists in the identified articles and unpublished posters were also consulted. Switching studies were limited to those dealing with oral antipsychotics; consideration of issues related to long-acting injectable agents was beyond the scope of this project. This review begins with a discussion of the PORT deliberations on switching antipsychotics,1,6 especially due to weight gain and dyslipidemia. We then present a summary of our literature review results, focusing on studies published since the PORT recommendations were published. Finally, evidence-based recommendations are provided concerning when and how it may be appropriate to undertake elective changes in antipsychotic medications with the goal of reducing problems commonly associated with these agents.
2009 SCHIZOPHRENIA PORT RECOMMENDATIONS
The 2009 PORT project reviewed published research to determine what evidence-based recommendations could be made for the psychopharmacologic treatment of schizophrenia.1 At the time, PORT concluded that the available evidence (eg, number of studies) was insufficient to make a recommendation concerning switching antipsychotics because of persistent problems with weight gain; extrapyramidal symptoms, including tardive dyskinesia; or prolactin elevation. They noted that clinicians need to evaluate the potential benefits of a reduction in these problems against the potential for symptom exacerbation that might accompany a medication change. In their supplemental material, PORT acknowledged that switching antipsychotics can lead to a reduction in a number of adverse effects, including weight gain and elevated prolactin levels.6 However, they concluded that, to develop a treatment recommendation in this area, evidence was required that the majority of individuals would experience significant improvement in these areas (eg, weight gain, lipid abnormalities) without clinical deterioration.6 Clinically significant benefits of a medication change can be estimated most readily for measures such as body weight and plasma lipids, where improvements in modifiable risk factors for cardiovascular disease and diabetes have quantifiable effects on morbidity and mortality risk. Therefore, it is not surprising that the majority of recent switching studies have focused on weight and metabolic outcomes, where the results have the greatest potential to have a significant clinical and public health impact.
When the PORT recommendations were published, only 1 randomized, controlled, double-blind trial had examined switching to address undesirable body weight and lipid profiles. Newcomer et al7 found that switching overweight or obese study participants from olanzapine to aripiprazole was associated with statistically and clinically significant improvement in mean body weight compared to continuing on olanzapine (−1.8 kg vs +1.41 kg, respectively, at week 16, P < .001), as well as clinically and statistically significant differences in percent change in fasting triglycerides and total and high density lipoprotein (HDL) cholesterol. In addition, more subjects who switched had clinically substantial weight loss (11.1% vs 2.6%), while fewer switchers had clinically relevant weight gain. With regard to potential risk associated with switching, mean Clinical Global Impressions-Improvement (CGI-I) scores for both groups were in the range of “no change” to “minimal improvement.” There was a statistically significant advantage in CGI-I endpoint scores for olanzapine (mean ± SE, 3.09 ± 0.16) compared to aripiprazole (3.74 ± 0.15, P < .001), and more subjects randomized to switch to aripiprazole than continue olanzapine discontinued treatment (36% vs 26%). The results indicated that discontinuation of olanzapine and switching to the lower metabolic risk agent aripiprazole was associated with significant improvements in body weight and clinically measured lipid fractions, both relevant to cardiovascular disease and diabetes risk. The observed change in weight was consistent with results of earlier uncontrolled studies in which participants were switched from olanzapine to an antipsychotic with lower liability for weight gain.8-10
While the study did not suggest that switching was associated with clinically significant psychiatric risk, the limited evidence regarding psychiatric risk offered by this single study prompted PORT to want more evidence prior to developing a formal treatment recommendation. PORT indicated that they expected the results of the Comparison of Antipsychotics for Metabolic Problems (CAMP) study,11 ongoing at that time, to help clarify this issue. Similar concerns were raised about switching due to prolactin elevation and tardive dyskinesia, although no studies were ongoing at the time that would clearly address these issues. The schizophrenia PORT publication did not address switching antipsychotics to achieve improvements in other adverse effects (eg, sedation, parkinsonian symptoms).
RESULTS OF THE LITERATURE REVIEW
Criteria for Study Selection
Depending on methodology, studies can be considered either “hypothesis generating” or “hypothesis testing.” Case reports, case series, chart reviews, and open, observational studies provide uncontrolled evidence and are therefore generally useful only for “hypothesis generation.” In contrast, controlled experimental studies, including the gold standard of prospective, randomized, controlled clinical trials, are designed to address specific questions for the purpose of “hypothesis testing.” For this review, the 45 articles that were identified were grouped into 3 categories:
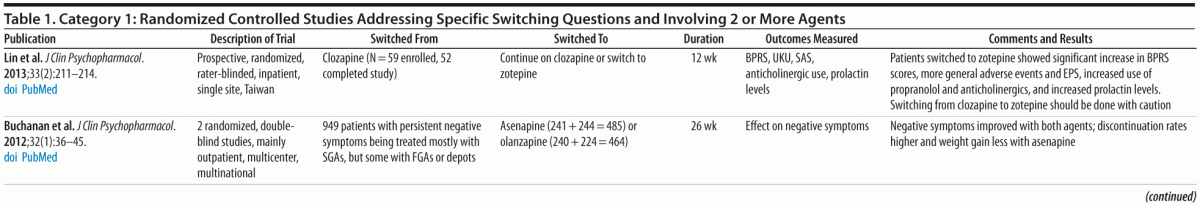
Category 1: Randomized controlled trials (RCTs) (comparing 2 or more agents) designed to answer specific switching questions (20 studies; Table 1)
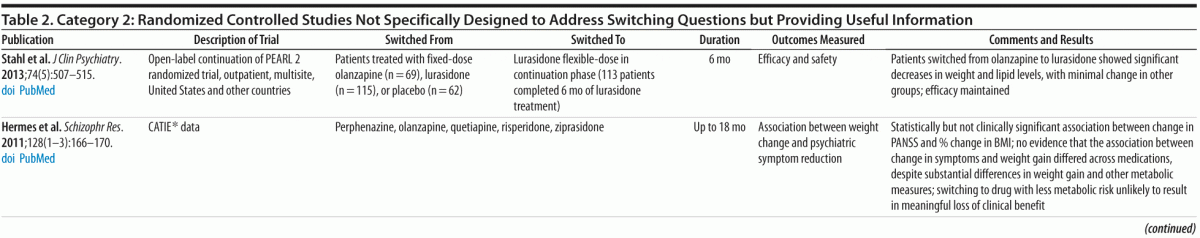
Category 2: Planned or post hoc secondary analyses of data from randomized trials that provided relevant information but were not specifically designed as switching studies (9 studies, 6 of which involved secondary analyses of data from the Clinical Antipsychotic Trials of Intervention Effectiveness [CATIE] study) (Table 2)
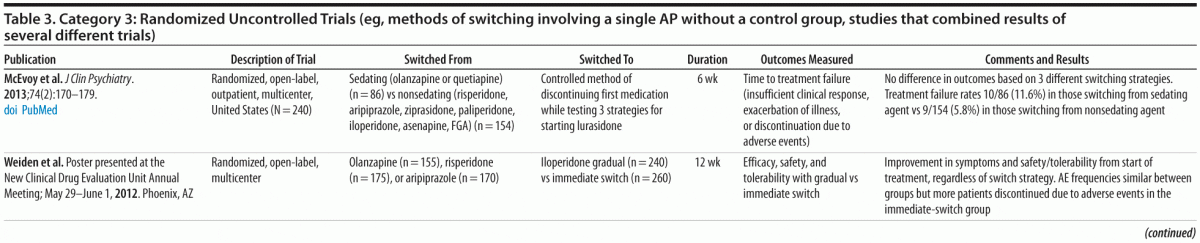
Category 3: Uncontrolled trials of switching (eg, methods of switching involving a single antipsychotic without a control group, studies combining results of several trials) (16 studies; Table 3)
Randomized Controlled Trials (Category 1)
Double-blind studies. Based on available RCTs and the PORT recommendations,1,12 there does not appear to be convincing evidence for preferential efficacy for core positive symptoms among different antipsychotic medications prescribed at optimal therapeutic doses, except for the superior efficacy of clozapine for people with treatment-resistant schizophrenia, aggression/hostility, and suicidality.
Despite initial evidence from the Newcomer et al study,7 PORT concluded that additional evidence was needed before a recommendation for elective switching of antipsychotics could be formulated and expressed interest in the forthcoming results of the CAMP study. The double-blind, randomized CAMP study, conducted by Stroup et al,11 enrolled participants from selected sites used in the CATIE study.12 Entry criteria were a diagnosis of DSM-IV schizophrenia or schizoaffective disorder with a body mass index (BMI) ≥ 27 and a non-HDL cholesterol level ≥ 130 mg/dL. Participants on a stable dose of olanzapine, quetiapine, or risperidone were randomized to switch to aripiprazole (n = 109) or stay on their current medication (n = 106) for 24 weeks.
The primary outcome was change in non-HDL cholesterol. A secondary outcome examined the efficacy of switching to aripiprazole compared to staying on the current agent. In contrast to those who stayed on their original medication, the group switched to aripiprazole had greater reductions in non-HDL cholesterol levels (−20.2 vs −10.8 mg/dL), weight (mean change of −3.6 vs −0.7 kg), and triglyceride levels (−25.7 vs +7.0 mg/dL). The small weight loss in the control group, in contrast to the persistent weight gain seen in the Newcomer et al study,7 may be related to the behavioral exercise and diet intervention all study participants received. In the switching group, beneficial changes in lipids occurred quickly (eg, over the first month), whereas weight changes occurred more gradually, consistent with prior reports.7,10 A significantly higher percentage (n = 47, 44%) of switchers than stayers (n = 26, 25%) discontinued the medication before 24 weeks, which is consistent with previous reports.7,13 However, differences in serious adverse events (SAEs) and number of hospitalizations between the 2 groups were small (18 participants [16.8%] with 21 SAEs in the switchers vs 10 [9.4%] with 14 SAEs in the stayers; 8 [7.5%] hospitalized in the switchers vs 5 [4.7%] in the stayers). A key finding was that rates of efficacy failure (defined as psychiatric hospitalization, 25% increase in Positive and Negative Syndrome Scale total score, or CGI-I ratings of much or very much worse) were very similar in the 2 groups, with 22 (20%) switchers compared with 18 (17%) stayers having a failure in efficacy. The CAMP results make it possible to generalize from the Newcomer et al results,7 since the CAMP study found similar results for people switching from a variety of different agents.
Other randomized studies. Most of the other recent category 1 studies (Table 1), while randomized and controlled, were not double-blind. Thus, while they provide useful confirmatory findings concerning reductions in parameters such as weight, EPS, and lipid and prolactin levels, it is not possible to rule out effects related to investigator bias because of the open-label nature of the studies. In a randomized, open-label study, Kinon et al14 reported a reduction in prolactin levels and improvement in sexual functioning in participants switched from a first-generation antipsychotic (FGA) or risperidone to olanzapine (n = 27) compared with those who stayed on an FGA (n = 9) or risperidone (n = 18). Cortese et al15 found improvement in EPS (ie, parkinsonism, akathisia, and dyskinesia) in participants randomized to switch from olanzapine, risperidone, or an FGA to quetiapine (n = 13) compared to those who continued on the previous antipsychotic (n = 9). Chen et al16 reported statistically significant improvements in weight, BMI, and triglyceride and HDL levels in participants randomized to switch to aripiprazole (n = 24) or ziprasidone (n = 28) from other antipsychotics.
Only 1 recent study has examined elective switches from clozapine.17 A randomized, rater-blinded, inpatient study involving 52 participants treated with clozapine found that participants who discontinued clozapine and switched to zotepine were more likely to experience withdrawal effects and to be at increased risk for destabilization and relapse.
Summary. Evidence from category 1 studies, in particular the Newcomer et al7 and Stroup et al11 studies, indicates that switching to a low metabolic risk antipsychotic under controlled conditions can produce benefits in both lipid profile and weight/BMI without significant risk of clinical deterioration, even when the switch is from olanzapine, which is considered a clearly effective second-generation antipsychotic agent. Randomized controlled open-label studies also suggest benefits when switching medications because of EPS or elevated prolactin levels. One caveat in interpreting these results is that people treated with clozapine were excluded from these studies, so that the relative safety of switching from clozapine for metabolic reasons remains understudied but associated with higher risk in studies to date.
Randomized Trials Not Specifically Designed as Switching Studies (Category 2)
The majority of studies in category 2 (Table 2) involved secondary analyses of data from the NIMH-funded CATIE study,12 which enrolled and randomized approximately 1,500 people with schizophrenia. CATIE provided valuable information about the relative efficacy and safety of first- and second-generation antipsychotics. (Note that the 3 newest antipsychotics, asenapine, iloperidone, and lurasidone, were not included in CATIE or the PORT deliberations.) The CATIE results did not support hypothesized differences in efficacy among non-clozapine antipsychotics. However, they underscored marked variability in the adverse effect profiles of the tested antipsychotics (eg, potential to cause EPS, weight gain, and lipid abnormalities)—ie, these adverse effects do not represent a “class effect.” No large randomized trials examining differences in the mean efficacy of individual antipsychotics have been conducted since CATIE.
Although the primary results of the CATIE study were included in the PORT deliberations, several more recent analyses of the CATIE data address switching questions. Essock et al13 reported the well-replicated observation that study participants who stay on their original antipsychotic are significantly less likely to discontinue that medication than those who switch to a new medication. Citrome18 compared results of the 6 medications in the CATIE study (perphenazine, olanzapine, quetiapine, risperidone, ziprasidone, and clozapine) and reported that risperidone had advantages in tolerability and that ziprasidone had the most benign metabolic profile and greatest likelihood of producing weight loss in participants who had gained significant weight on other antipsychotics. Daumit et al19 reported that change in 10-year coronary heart disease risk (changes in total and HDL cholesterol) differed significantly between treatments, with olanzapine and quetiapine associated with increased risk and perphenazine, risperidone, and ziprasidone associated with reduced risk. Rosenheck et al20 found that stayers versus switchers had no statistically significant differences in psychiatric symptoms, neurocognition, quality of life, neurologic effects, weight change, and health costs, except that participants who stayed on olanzapine showed greater weight gain than those who switched from olanzapine to another antipsychotic. Hermes et al21 found no difference in the association between weight change and psychiatric symptom reduction across medications despite different amounts of weight gain and concluded that switching to a medication with lower liability for weight and metabolic abnormalities was unlikely to result in meaningful loss of clinical benefit.
Three other category 2 studies addressed relevant questions. Faries et al22 evaluated data from a 1-year randomized, open-label, cost-effectiveness study in which participants treated with risperidone, olanzapine, or an FGA could switch to a different agent if clinically indicated. They reported that switchers (n = 191) had poorer clinical and economic outcomes (ie, more frequent and rapid use of acute care services) than stayers (n = 460). A post hoc analysis23 of data found that the nonrandomized subset of participants who switched from risperidone to olanzapine (n = 43) had improvements in clinical outcomes but gained more weight (mean = +2.4 kg after average of 8 months) than when they were treated with risperidone (mean = +0.4 kg after average of < 3 months).
Stahl et al24 described the results of a 6-month open-label extension of the PEARL 2 randomized, placebo-controlled, double-blind study, a large multicenter registration trial that evaluated the efficacy of 40 and 120 mg/d of lurasidone versus placebo or olanzapine 15 mg/d. Participants who completed 6 weeks of double-blind treatment with lurasidone, olanzapine, or placebo were eligible to continue treatment with lurasidone for up to 6 months (44.5% [113/254] completed 6 months). In this continuation phase, participants previously treated with olanzapine (n = 65) experienced decreases in weight (mean = −1.8 kg) and lipid levels while efficacy was maintained. Participants previously treated with placebo or lurasidone had minimal changes in these parameters.
Summary. Evidence from category 2 studies generally supports the observation from category 1 studies7,11 that switching from higher metabolic risk antipsychotics (eg, olanzapine) to lower risk agents (eg, ziprasidone and perhaps risperidone and perphenazine) under controlled conditions can produce metabolic and weight benefits without significant risk of clinical deterioration. However, studies also confirmed that participants switched to a new antipsychotic are more likely to discontinue treatment than those who remain on the same agent, highlighting the need to optimize current treatment and perform a risk/benefit assessment before switching to a new agent.
Uncontrolled and/or Open-Label Trials (Category 3)
We also considered uncontrolled, open-label switching studies (Table 3), which are generally considered hypothesis generating rather than hypothesis testing. The results of these uncontrolled studies were largely consistent with those of the category 1 and 2 studies, but they also provided preliminary data relevant to other questions not addressed in the studies previously noted.
Switching methods. Of the 16 studies in category 3, the majority examined strategies for switching from one medication to another (ie, stopping the first agent before starting the new medication, maintaining a therapeutic dose of the first medication while titrating up to a therapeutic dose of the new medication, gradually discontinuing the first medication while titrating the new medication to a therapeutic dose). In general, these studies found few differences in outcomes among the strategies, although there was some evidence favoring more gradual discontinuation of the first antipsychotic to minimize problems early in the switching process.25-28
As an example, 2 recent studies28,29 provided data in support of this finding. In a 6-week, multicenter, randomized, open-label study in which 240 stable participants were switched from other antipsychotics to lurasidone, because of insufficient efficacy or safety/tolerability concerns, McEvoy et al29 controlled the method of previous medication discontinuation in keeping with the findings that gradual discontinuation is favored. The previous medication was tapered by 50% by day 7 and discontinued by day 14, with participants randomized to 1 of 3 methods of starting lurasidone: 40 mg/d for 2 weeks, 80 mg/d for 2 weeks, and 40 mg/d for week 1 and 80 mg/d for week 2. The investigators reported that, after 6 weeks, participants were able to be successfully switched regardless of method of starting the new medication.
Data from another recent 12-week, randomized, multicenter, open-label switching study,28,30 in which participants were switched from olanzapine (n = 155), risperidone (n = 175), or aripiprazole (n = 170) to iloperidone, found that more participants in the group who were switched abruptly rather than gradually discontinued due to adverse events. The difference in discontinuations was primarily due to dizziness associated with α1 antagonism during the first 1-2 weeks of iloperidone treatment, which decreased over time.
Switching due to adverse effects. Data from open-label uncontrolled studies concerning changes in weight and metabolic parameters after switching antipsychotics are generally consistent with findings from the controlled trials. In a randomized, open-label, multicenter trial, Weiden et al8,9 switched participants from an FGA (n = 108), olanzapine (n = 104), or risperidone (n = 58) to ziprasidone. They reported improved health indices consistent with the pre-switch medication: significant weight loss when participants switched from olanzapine and some weight loss when they switched from risperidone, improved EPS when participants switched from an FGA or risperidone, and decreased prolactin levels when participants switched from an FGA or risperidone. In the following sections, we briefly review findings from more recent studies concerning weight and metabolic abnormalities, prolactin, EPS, and sedation.
Weight gain and/or metabolic abnormalities. In a more recent publication, Weiden et al10 analyzed data from 3 long-term open-label extension studies in which participants who switched from risperidone (n = 43), olanzapine (n = 71), or an FGA (n = 71) to ziprasidone could continue treatment with ziprasidone for up to a total of 58 weeks. Clinically significant sustained reductions in weight and BMI were observed in participants switched from risperidone (mean = −6.9 kg, P < .005) or olanzapine (mean = −9.8 kg, P < .001); participants switched from risperidone or olanzapine to ziprasidone also showed clinically significant and rapid (eg, within 6 weeks) improvements in total cholesterol and triglyceride levels.
In the McEvoy et al29 6-week study discussed earlier, subjects (N = 240) switched to lurasidone showed small mean decreases in weight (−0.3 kg) and improvements in metabolic parameters (eg, mean = −11.3 mg/dL in triglycerides). Among 220 subjects in the safety population, only 2 (0.9%) had weight gain ≥ 7% from baseline and 4 (1.8%) had weight loss ≥ 7% from baseline.
Prolactin elevation. Consistent with the Weiden et al8,9 results, Byerly et al31 found that elevated levels of prolactin in participants treated with risperidone (n = 105) decreased significantly (P < .001) and returned to normal 1 week after switching to aripiprazole, and this change was maintained for the 8 weeks of the study.
Sedation. In the McEvoy et al29 study, the investigators analyzed data separately for the 86 participants switched from an antipsychotic considered sedating (olanzapine or quetiapine) and the 154 participants switched from an agent considered nonsedating (risperidone, aripiprazole, ziprasidone, paliperidone, iloperidone, asenapine, or an FGA) to lurasidone. They found higher treatment failure rates (insufficient clinical response, exacerbation of illness, or discontinuation due to an adverse event) in those switched from a sedating agent (10/86, 11.6%) than in those switched from a nonsedating agent (9/154, 5.8%). Insomnia rates were also higher in those switching from a sedating agent (18.6%) than a nonsedating agent (9.7%), consistent with previous reports of rebound insomnia in people switched from agents with higher to those with lower affinity for H1 receptors.
Summary. Data from category 3 studies generally confirm and extend findings from the category 1 and 2 studies. A majority of the category 3 studies examined methods of switching from one antipsychotic to another, with a number of studies supporting more gradual discontinuation of the first agent to minimize problems early in the switching process, particularly when starting an agent that requires gradual titration (eg, iloperidone, due to hypotension related to α1 antagonism). Studies in all 3 categories suggest that the magnitude of change in parameters such as weight, lipids, prolactin, and EPS can vary substantially depending on the specific agents involved in the switch. Thus, the greatest reduction in weight would be expected when switching from olanzapine to an agent with low liability for weight gain, the greatest reduction in EPS or prolactin would be expected when switching from an FGA or risperidone, and the greatest changes in sedation would be expected when switching from a more sedating (olanzapine or quetiapine) to a less sedating agent.
Switching Antipsychotic MEDICATIONS: Evidence-Based CLINICAL Recommendations
It would be impossible to design a single study to address all the factors clinicians should consider when talking to their patients who have schizophrenia about whether to switch antipsychotics with the goal of reducing specific adverse effects. As recently noted by Murad and Montori,32 clinicians should use the totality of available evidence when considering these issues with patients, preferentially weighing evidence from RCTs as the gold standard, but also incorporating clinical observations from case reports, case series, uncontrolled observational studies, and relevant expert opinion. The following sections present clinical recommendations based on our review of the available evidence, addressing key questions left open by the 2009 PORT publication.1
Can Switching Antipsychotic Medications Produce Improvements in Weight and Lipid Parameters or Prolactin Levels Without Destabilizing Patients?
Results of studies published since the 2009 PORT recommendations strongly support a recommendation that switching from higher to lower metabolic risk antipsychotics can produce weight and lipid benefits without a significant risk of clinical deterioration.7,11 The benefits of switching due to metabolic disturbances are clearest for switches from high metabolic risk agents such as olanzapine to low metabolic risk agents such as aripiprazole, ziprasidone, and lurasidone. Evidence, although not as strong, also exists concerning potential improvements that may be achieved by switching to improve other adverse effects, including EPS and prolactin elevation. Although assessment of the psychiatric risks of switching was not as rigorous in studies that evaluated EPS and prolactin, a number of studies7-11,14-16,20,24-29,31 using different experimental and analytic designs all now support that antipsychotic switches (with the exception of switches from clozapine) are associated with only modest psychiatric risk that is best managed by gradual discontinuation of the prior antipsychotic. It is important to keep in mind that none of these studies switched patients from clozapine because of concern that switching patients from clozapine to non-clozapine antipsychotics is much riskier than switching across first-line agents.
The information on relative risks of common problems associated with antipsychotics in the following sections is based on a recent meta-analysis33 of data from 212 controlled randomized trials concerning 15 antipsychotic medications and other evidence in the literature.
Switching because of weight and/or lipid abnormalities. The greatest amount of evidence supports switching antipsychotic medications to target excessive weight gain and lipid abnormalities. Available studies indicate that such switches can result in improvements in these parameters without a significant risk of destabilization. The relative risk of weight gain is olanzapine > clozapine >> iloperidone > low-potency FGAs > quetiapine > risperidone > paliperidone > asenapine > high-potency FGAs = aripiprazole = lurasidone = ziprasidone.1,33,34
Switching because of EPS. The relative risk of EPS among the antipsychotics is high-potency FGAs > mid-potency FGAs = risperidone > paliperidone > low-potency FGAs > lurasidone = asenapine = ziprasidone > aripiprazole > olanzapine > iloperidone = quetiapine > clozapine.1,33,35,36 Less evidence is available concerning switching in this area, but reductions in EPS have been reported when patients were switched to quetiapine15 or ziprasidone.9
Switching because of elevated prolactin levels. The greatest risk of elevated prolactin levels is associated with paliperidone and risperidone followed by high-potency FGAs; most of the other available antipsychotics are associated with a smaller risk of elevated prolactin levels, while quetiapine and aripiprazole have been found to be associated with less elevation in prolactin levels than placebo.1,33 A randomized, controlled, open-label trial reported reductions in prolactin levels when patients were switched from an FGA or risperidone to olanzapine.14 Two uncontrolled studies also reported reductions in prolactin levels when patients were switched from an FGA or risperidone to ziprasidone9 and from olanzapine or risperidone to aripiprazole.31
Deciding on an Elective Change of Antipsychotic Medication
Physicians, patients, and families may all place different value on various treatment outcomes, highlighting the need for personalized medicine, shared decision-making, and patient-centered care. For example, a reduction in symptom severity may be a critical concern for one patient, while reduced sedation or weight gain may be key for another. In discussing treatment options, clinicians also need to help patients understand the concept of future risk (eg, potential long-term effects of obesity and metabolic abnormalities).
Steps in Deciding to Make an Elective Antipsychotic Switch
1. Identify target symptoms and side effects
2. Translate those therapeutic targets into outcomes that can be tracked
3. Determine if the therapeutic target is amenable to a pharmacologic intervention
4. Optimize current treatment regimen if possible
5. Evaluate appropriateness of adjunctive interventions
6. Conduct risk/benefit assessment with the patient
Data from CATIE13 and other studies7,11,20 indicate that patients who switch to a new medication are more likely to discontinue treatment with the new agent (eg, return to prior medication), presumably due to the challenges of adjusting to a new medication, while those who do not switch medications are more likely to continue treatment with their medications. On the basis of this finding, Essock et al13 recommended first optimizing the current treatment regimen before considering a switch.
Options to consider before switching. If a patient has achieved a satisfactory symptomatic response, but has developed problems (eg, excessive weight gain, dyslipidemia) that make it difficult to continue on the medication or pose long-term health risks, the risk/benefit analysis is complicated. The clinician can (1) monitor but make no change in treatment, based on the judgment that the benefits of ongoing treatment outweigh the risks; (2) adjust the dose to see if efficacy can be maintained while minimizing the problem; (3) add an adjunctive treatment (another medication or a behavioral or psychosocial intervention, such as a weight loss or cognitive-enhancing program); or (4) switch to a different antipsychotic with a lower liability for causing the problem. Because a change of antipsychotic in a relatively stable patient always involves some risk of destabilization, clinicians need to consider other possible interventions before deciding to switch antipsychotics.
Optimization/dose adjustment. When a patient being treated with an antipsychotic medication is experiencing a serious problem that is interfering with quality of life or ability to function or poses long-term health risks, the clinician needs to consider whether a dose adjustment might reduce the problem while maintaining efficacy. Such a dose adjustment will only be helpful if the problem is dose dependent. For example, EPS, sedation, amenorrhea, agitation, and activation may respond to dose adjustment (eg, lowering the dose for early activation with aripiprazole, raising the dose for early activation with ziprasidone), while other effects, such as weight gain, are generally not dose dependent within the usual dose ranges used for treatment of schizophrenia.37
Adjunctive interventions. Adjunctive medications can be considered to treat problems such as early activation or insomnia (eg, benzodiazepines), EPS (eg, anticholinergic agents, β blockers), obesity (eg, weight-loss agents recently approved by the US Food and Drug Administration, although none has been tested or approved in people with schizophrenia or for treatment or prevention of antipsychotic-induced weight gain), dyslipidemia (eg, statins), or elevated prolactin levels (eg, aripiprazole, bromocriptine). Concerns about use of adjunctive agents include adverse effects associated with adding a second medication (eg, cognitive deficits with anticholinergic medications), limited evidence of efficacy, and potential for drug interactions.
Switching to a different antipsychotic medication to reduce or minimize adverse effects. After determining that the adverse effect in question is likely to be amenable to a change in medications, the clinician should conduct a risk/benefit assessment of switching antipsychotic medications with the patient.
Steps in Implementing an Elective Antipsychotic Switch
1. Educate the patient about the benefits and risks of the new medication vis-× -vis current side-effect issues
2. In conjunction with the patient, select the next medication
3. Make a switching plan with attention to the potential sleep-wake effects of both antipsychotics
4. Monitor the patient more closely during the switch
5. Be alert for rebound and new-onset side effects
6. Provide short-term medication to manage sleep disturbance, agitation, and anxiety
7. Evaluate efficacy and safety/tolerability outcomes; note that changes in side effects may appear at different times (eg, shorter period for changes in lipid or prolactin levels, longer period for weight loss)
Implementing the Switch
Selecting the next antipsychotic medication. In consultation with the patient, the clinician needs to select the most appropriate medication to try next, based on evidence in the literature and the pharmacokinetic and pharmacodynamic profiles of both drugs (Table 4).
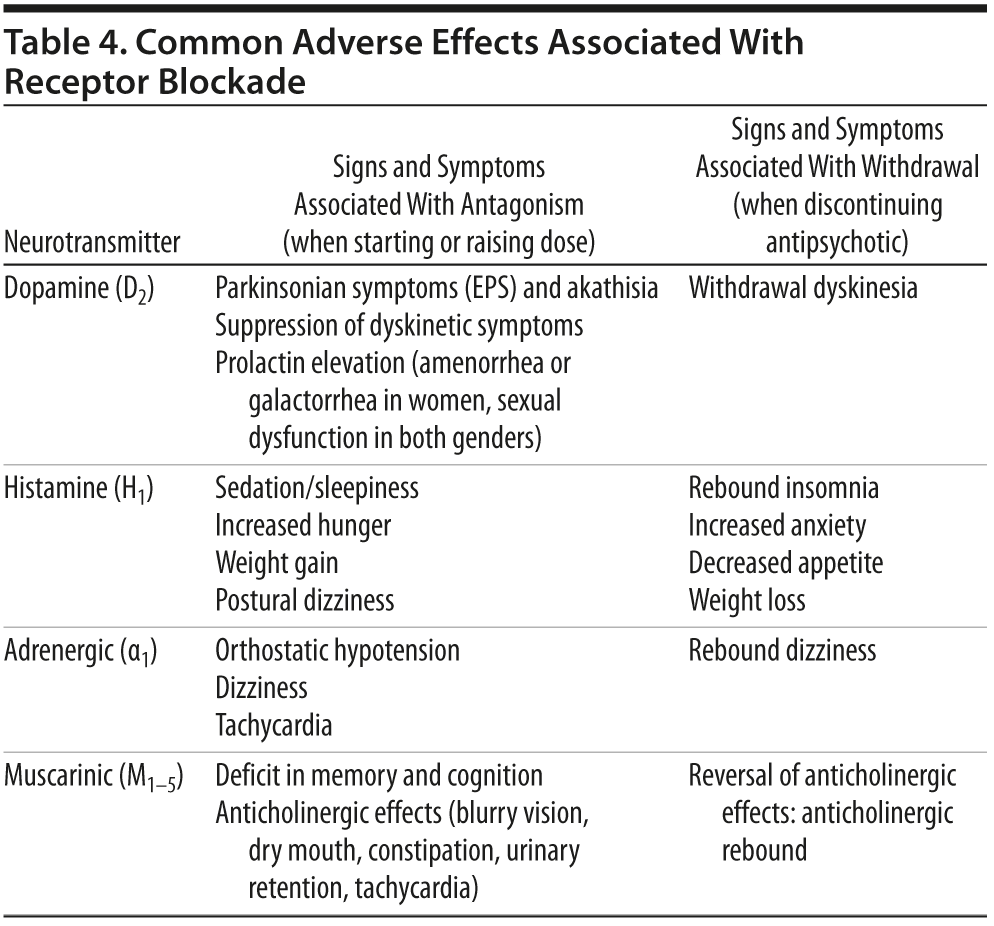
Making a switching plan. Although studies have generally found no ultimate difference in outcomes for different switching strategies, results suggest that more gradual cross-titrations are likely to reduce drop out and rebound adverse effects.25-28 The clinician should have a plan for completely discontinuing the first medication and reaching a therapeutic dose of the new agent and educate patients about potential withdrawal problems.
Monitoring for problems during the switch. The most common problems that complicate switching are insomnia, sedation, and anxiety. Sleep disturbances may represent rebound effects from discontinuing a more sedating drug or a problem due to the new agent. Switches for weight and metabolic problems usually involve changing from a drug with more potent antihistaminic and hence sedating properties (eg, olanzapine) to a less sedating agent, so that rebound insomnia and agitation often occur early during the transition. Thus, McEvoy et al29 found lower rates of completed switches in participants switching from more sedating agents to lurasidone. Although these problems are usually transient (ie, no more than 2 weeks), patients and families need to be educated about the possibility and short-term sedatives provided if needed to facilitate a successful switch. When switching from a drug with more potent to one with less potent D2 blockade (eg, from risperidone to quetiapine or lurasidone), short-term withdrawal dyskinesias may occur that need to be distinguished from effects of the new medication. When switching to a medication with significant α1-adrenergic antagonism (eg, iloperidone), patients should be educated about the potential for early dizziness.30 When switching from more potent anticholinergic regimens (eg, olanzapine, adjunctive benztropine), muscarinic antagonism should be decreased gradually (eg, over 1-2 weeks) to minimize anticholinergic withdrawal symptoms.
Monitoring outcomes. When switching for safety issues, clinicians must continue to focus on efficacy, since even if the safety/tolerability profile of the new drug is more acceptable, a relative loss of efficacy will limit the success of the switch. One cannot be sure about the relative effectiveness of the new medication until the patient has been on a full therapeutic dose of monotherapy for at least 4 weeks, and it may not be possible to evaluate the eventual effectiveness of the new agent until some months have passed.
In monitoring the problems that led to the switch, clinicians should keep in mind that changes occur over varying periods of time after discontinuing the prior medication. Thus, there may be very rapid improvement associated with cessation of pharmacodynamic effects (eg, anticholinergic effects, prolactin elevation). Byerly et al31 found that prolactin levels returned to normal within 1 week after patients were switched from risperidone to aripiprazole. Improvements in lipids and other metabolic parameters are likely to occur relatively rapidly (eg, 4-8 weeks) while changes in body weight are generally more gradual, as seen in the Newcomer et al7 and Stroup et al11 studies. Continued reduction in weight may occur for up to a year or longer.8-10
Special problems in switching from clozapine. The limited research in this area17,38 indicates that switching a patient from clozapine to another antipsychotic should be done very cautiously, because of the risk of withdrawal effects, destabilization, and relapse. However, such a switch may be worth considering for patients who have not achieved a satisfactory response or are experiencing intolerable adverse effects. In light of the risks associated with clozapine treatment when patients are not showing a good response, especially for that subset of patients who may not have presented with an appropriate indication for clozapine treatment in the first place (ie, treatment-resistance, aggression/hostility, or suicidality), one could cautiously consider a switch.
Careful methodology is especially important in switching from clozapine. It has very strong anticholinergic properties, and patients tapered off clozapine often experience anticholinergic rebound phenomena, especially when clozapine is discontinued abruptly. Using an adjunctive anticholinergic agent (eg, benztropine) while slowly lowering the dose of clozapine may help diminish this rebound effect. Antihistaminic and α-adrenergic rebound symptoms may also occur when a patient is tapered off clozapine.
CONCLUSIONS
The goal of this project was to provide updated guidance regarding management of adverse effects via elective changes in oral antipsychotic medications in the treatment of schizophrenia. The 2009 PORT treatment recommendations1 acknowledged that there was evidence that switching antipsychotics can lead to an improvement in a number of parameters, including weight gain and elevated prolactin levels. However, the authors concluded that they could not make a recommendation in these areas without further evidence. The current report examined evidence that is now available concerning switching antipsychotic medications. Two RCTs, one available to the PORT7 and one published since that time,11 support the recommendation that switching from higher to lower metabolic risk antipsychotics can produce lipid and weight benefits without a significant risk of clinical deterioration. Secondary analyses of data from the CATIE study have further established that switching to a medication with lower liability for weight and metabolic abnormalities is unlikely to result in significant loss of clinical benefit.20,21 On the basis of the totality of available evidence, including well-established population-based evidence that reductions in body weight and other metabolic risk factors are strongly associated with reductions in risk for cardiovascular disease and diabetes, clinicians should individually consider patient risk and opportunities for risk reduction that can be afforded by judicious switching from higher risk to lower risk antipsychotics. Evidence also supports the use of switching to address clinical problems such as prolactin elevation with sexual dysfunction and EPS. This topic of adverse event management during chronic antipsychotic therapy continues to be an area where tolerability, personalized medicine, shared decision-making, and patient-centered care will remain crucial.
Potential conflicts of interest: Dr Newcomer has received grant/research support from the National Institutes of Health; honoraria from American Physician Institute, CME Outfitters, CMEology, and American Psychiatric Association; royalties from Jones and Bartlett Publishing; and has served on the Data Safety Monitoring Boards for Bristol-Myers Squibb, Merck, and Vivus. Dr Weiden has received grant/research support from Johnson & Johnson (Janssen), Genentech/Roche, Novartis, Neurocrine, and Sunovion; has served as a consultant for Delpor, Johnson & Johnson (Janssen), Genentech/Roche, Lundbeck, Otsuka, Novartis, Reckitt Benckiser, and Sunovion; and been on the speakers board for Johnson & Johnson (Janssen), Genentech/Roche, Lundbeck, Otsuka, Novartis, and Sunovion. Dr Buchanan has served as a consultant for Abbott, Amgen, Bristol-Myers Squibb, EnVivo, Omeros, and Pfizer; has served on the advisory boards for Abbott, Amgen, Pfizer, and Roche; and has served on the Data Safety Monitoring Board for Pfizer.
REFERENCES
1. Buchanan RW, Kreyenbuhl J, Kelly DL, et al, Schizophrenia Patient Outcomes Research Team (PORT). The 2009 Schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93. PubMed doi:10.1093/schbul/sbp116
2. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298(15):1794-1796. PubMed doi:10.1001/jama.298.15.1794
3. Correll CU, Druss BG, Lombardo I, et al. Findings of a US national cardiometabolic screening program among 10,084 psychiatric outpatients. Psychiatr Serv. 2010;61(9):892-898. PubMed doi:10.1176/appi.ps.61.9.892
4. De Hert M, Detraux J, van Winkel R, et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2012;8(2):114-126. PubMed doi:10.1038/nrendo.2011.156
5. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2). http://www.cdc.gov/pcd/issues/2006/apr/05_0180.htm. Accessed August 18, 2011.
6. Buchanan RW, Kreyenbuhl J, Kelly DL, et al. PORT supplementary material: psychopharmacological summary statements and evidence summaries. 2010. http://schizophreniabulletin.oxfordjournals.org/content/36/1/71/suppl/DC1. Accessed September 26, 2013.
7. Newcomer JW, Campos JA, Marcus RN, et al. A multicenter, randomized, double-blind study of the effects of aripiprazole in overweight subjects with schizophrenia or schizoaffective disorder switched from olanzapine. J Clin Psychiatry. 2008;69(7):1046-1056. PubMed doi:10.4088/JCP.v69n0702
8. Weiden PJ, Simpson GM, Potkin SG, et al. Effectiveness of switching to ziprasidone for stable but symptomatic outpatients with schizophrenia. J Clin Psychiatry. 2003;64(5):580-588. PubMed doi:10.4088/JCP.v64n0514
9. Weiden PJ, Daniel DG, Simpson G, et al. Improvement in indices of health status in outpatients with schizophrenia switched to ziprasidone. J Clin Psychopharmacol. 2003;23(6):595-600. PubMed doi:10.1097/01.jcp.0000095347.32154.08
10. Weiden PJ, Newcomer JW, Loebel AD, et al. Long-term changes in weight and plasma lipids during maintenance treatment with ziprasidone. Neuropsychopharmacology. 2008;33(5):985-994. PubMed doi:10.1038/sj.npp.1301482
11. Stroup TS, McEvoy JP, Ring KD, et al; Schizophrenia Trials Network. A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: Comparison of Antipsychotics for Metabolic Problems (CAMP). Am J Psychiatry. 2011;168(9):947-956. PubMed
12. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223. PubMed doi:10.1056/NEJMoa051688
13. Essock SM, Covell NH, Davis SM, et al. Effectiveness of switching antipsychotic medications. Am J Psychiatry. 2006;163(12):2090-2095. PubMed doi:10.1176/appi.ajp.163.12.2090
14. Kinon BJ, Ahl J, Liu-Seifert H, et al. Improvement in hyperprolactinemia and reproductive comorbidities in patients with schizophrenia switched from conventional antipsychotics or risperidone to olanzapine. Psychoneuroendocrinology. 2006;31(5):577-588. PubMed doi:10.1016/j.psyneuen.2005.12.006
15. Cortese L, Caligiuri MP, Williams R, et al. Reduction in neuroleptic-induced movement disorders after a switch to quetiapine in patients with schizophrenia. J Clin Psychopharmacol. 2008;28(1):69-73. PubMed doi:10.1097/jcp.0b013e318160864f
16. Chen Y, Bobo WV, Watts K, et al. Comparative effectiveness of switching antipsychotic drug treatment to aripiprazole or ziprasidone for improving metabolic profile and atherogenic dyslipidemia: a 12-month, prospective, open-label study. J Psychopharmacol. 2012;26(9):1201-1210. PubMed doi:10.1177/0269881111430748
17. Lin CC, Chiu HJ, Chen JY, et al. Switching from clozapine to zotepine in patients with schizophrenia: a 12-week prospective, randomized, rater blind, and parallel study. J Clin Psychopharmacol. 2013;33(2):211-214. PubMed doi:10.1097/JCP.0b013e31828700c7
18. Citrome L. Interpreting and applying the CATIE results: with CATIE, context is key, when sorting out phases 1, 1A, 1B, 2E, and 2T. Psychiatry (Edgmont). 2007;4(10):23-29. PubMed
19. Daumit GL, Goff DC, Meyer JM, et al. Antipsychotic effects on estimated 10-year coronary heart disease risk in the CATIE schizophrenia study. Schizophr Res. 2008;105(1-3):175-187. PubMed doi:10.1016/j.schres.2008.07.006
20. Rosenheck RA, Davis S, Covell N, et al. Does switching to a new antipsychotic improve outcomes? data from the CATIE trial. Schizophr Res. 2009;107(1):22-29. PubMed doi:10.1016/j.schres.2008.09.031
21. Hermes E, Nasrallah H, Davis V, et al. The association between weight change and symptom reduction in the CATIE schizophrenia trial. Schizophr Res. 2011;128(1-3):166-170. PubMed doi:10.1016/j.schres.2011.01.022
22. Faries DE, Ascher-Svanum H, Nyhuis AW, et al. Clinical and economic ramifications of switching antipsychotics in the treatment of schizophrenia. BMC Psychiatry. 2009;9(1):54. PubMed doi:10.1186/1471-244X-9-54
23. Faries DE, Ascher-Svanum H, Nyhuis AW, et al. Switching from risperidone to olanzapine in a one-year, randomized, open-label effectiveness study of schizophrenia. Curr Med Res Opin. 2008;24(5):1399-1405. PubMed doi:10.1185/030079908X297385
24. Stahl SM, Cucchiaro J, Simonelli D, et al. Effectiveness of lurasidone for patients with schizophrenia following 6 weeks of acute treatment with lurasidone, olanzapine, or placebo: a 6-month, open-label, extension study. J Clin Psychiatry. 2013;74(5):507-515. PubMed doi:10.4088/JCP.12m08084
25. Ganguli R, Brar JS, Mahmoud R, et al. Assessment of strategies for switching patients from olanzapine to risperidone: a randomized, open-label, rater-blinded study. BMC Med. 2008;6(1):17. PubMed doi:10.1186/1741-7015-6-17
26. Pae CU, Serretti A, Chiesa A, et al. Immediate versus gradual suspension of previous treatments during switch to aripiprazole: results of a randomized, open label study. Eur Neuropsychopharmacol. 2009;19(8):562-570. PubMed doi:10.1016/j.euroneuro.2009.04.002
27. Stip E, Zhornitsky S, Potvin S, et al. Switching from conventional antipsychotics to ziprasidone: a randomized, open-label comparison of regimen strategies. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(6):997-1000. PubMed doi:10.1016/j.pnpbp.2010.05.010
28. Weiden PJ, Alva G, Brams M, et al. Efficacy and safety/tolerability of two approaches for switching to iloperidone in patients with schizophrenia. Poster presented at the New Clinical Drug Evaluation Unit Annual Meeting. Phoenix, AZ, May 29-June 1, 2012.
29. McEvoy JP, Citrome L, Hernandez D, et al. Effectiveness of lurasidone in patients with schizophrenia or schizoaffective disorder switched from other antipsychotics: a randomized, 6-week, open-label study. J Clin Psychiatry. 2013;74(2):170-179. PubMed doi:10.4088/JCP.12m07992
30. Citrome L, Kianifard F, Meng X, et al. Discontinuations following a switch from risperidone, olanzapine, or aripiprazole to iloperidone in patients with schizophrenia: the i-FANS study. Poster presented at American College of Neuropsychopharmacology Annual Meeting. Hollywood, FL, December 2-6, 2012.
31. Byerly MJ, Marcus RN, Tran QV, et al. Effects of aripiprazole on prolactin levels in subjects with schizophrenia during cross-titration with risperidone or olanzapine: analysis of a randomized, open-label study. Schizophr Res. 2009;107(2-3):218-222. PubMed doi:10.1016/j.schres.2008.09.019
32. Murad MH, Montori VM. Synthesizing evidence: shifting the focus from individual studies to the body of evidence. JAMA. 2013;309(21):2217-2218. PubMed doi:10.1001/jama.2013.5616
33. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962. PubMed doi:10.1016/S0140-6736(13)60733-3
34. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(suppl 1):1-93. PubMed doi:10.2165/00023210-200519001-00001
35. Weiden PJ. Iloperidone for the treatment of schizophrenia: an updated clinical review. Clin Schizophr Relat Psychoses. 2012;6(1):34-44. PubMed doi:10.3371/CSRP.6.1.5
36. Citrome L, Cucchiaro J, Sarma K, et al. Long-term safety and tolerability of lurasidone in schizophrenia: a 12-month, double-blind, active-controlled study. Int Clin Psychopharmacol. 2012;27(3):165-176. PubMed doi:10.1097/YIC.0b013e32835281ef
37. Weiden PJ, Preskorn SH, Fahnestock PA, et al; Roadmap Survey. Translating the psychopharmacology of antipsychotics to individualized treatment for severe mental illness: a Roadmap. J Clin Psychiatry. 2007;68(suppl 7):1-48. PubMed
38. Tollefson GD, Dellva MA, Mattler CA, et al; The Collaborative Crossover Study Group. Controlled, double-blind investigation of the clozapine discontinuation symptoms with conversion to either olanzapine or placebo. J Clin Psychopharmacol. 1999;19(5):435-443. PubMed doi:10.1097/00004714-199910000-00007