Early Career Psychiatrists
ABSTRACT
Objective: Extensive combination pharmacotherapy regimens for bipolar disorder have gained increasing use in routine practice in ways that outpace data from evidence-based clinical trials. The present review examined the prevalence of complex pharmacotherapy regimens in bipolar disorder patients and sought to characterize factors that most influence polypharmacy prescribing patterns.
Data Sources: The authors independently systematically searched the MEDLINE, PsycINFO, and Embase databases for English-language observational/naturalistic or randomized controlled polypharmacy trials, using the keywords bipolar and polypharmacy or bipolar and combination treatment and pharmacotherapy.
Study Selection: From among 3,566 publications, 49 ultimately met study inclusion criteria.
Data Extraction: Information was obtained regarding prevalence rates of extensive polypharmacy as well as clinical characteristics and naturalistic outcomes for patients with simple (≤ 2) or complex (≥ 3) regimens of psychotropic agents.
Results: A weighted mean percentage of 32.7% of bipolar outpatients (4,535/13,863) taking ≥ 3 psychotropic medications was identified. Factors associated with complex polypharmacy use include female sex, White race, age > 50 years, history of psychosis, greater burden of depressive illness, subtherapeutic dosing, lower treatment adherence, more extensive psychiatric comorbidity, and a greater history of suicide attempts.
Conclusions: Extensive or complex combination pharmacotherapy regimens are common in many patients with bipolar disorder and often reflect greater overall illness severity. Naturalistic studies do not point to better outcomes for patients receiving more complex drug regimens, suggesting likely confounding by indication, high severity, or comorbid conditions. Formal clinical trials are needed to identify optimal drug combinations and durations when using ≥ 3 psychotropic medications to treat patients with bipolar disorder.
J Clin Psychiatry 2021;82(3):20r13263
To cite: Kim AM, Salstein L, Goldberg JF. A systematic review of complex polypharmacy in bipolar disorder: prevalence, clinical features, adherence, and preliminary recommendations for practitioners. J Clin Psychiatry. 2021;82(3):20r13263.
To share: https://doi.org/10.4088/JCP.20r13263
© Copyright 2021 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Northwell Health System, New York, New York
bDepartment of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, New York
*Corresponding author: Joseph F. Goldberg, MD, 128 East Ave, Norwalk, CT 06851 ([email protected]).
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation. A $10 processing fee will apply.
CME Objective
After studying this article, you should be able to:
- Regularly weigh risks versus benefits of the components of a complex pharmacotherapy regimen for patients with bipolar disorder
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for
physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Nurses Credentialing Center (ANCC) and the American Academy of Physician Assistants (AAPA) accept certificates of participation for educational activities certified for AMA PRA Category 1 Credit™ from organizations accredited by the ACCME.
Release, Expiration, and Review Dates
This educational activity was published in June 2021 and is eligible for AMA PRA Category 1 Credit™ through June 30, 2023. The latest review of this material was May 2021.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Marlene P. Freeman, MD, Editor in Chief, has received research funding from JayMac and Sage; has been a member of the advisory boards for Otsuka, Alkermes, and Sunovion; and has been a member of the Independent Data Safety and Monitoring Committee for Janssen. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
Once shunned as a sign of suboptimal care, the use of complex pharmacotherapy has increasingly become the norm in the treatment of bipolar disorder.1–7 In part, elaborate medication combinations may reflect the potential shortcomings and incomplete efficacy of traditional monotherapies such as lithium, as well as the complexity of a disorder in which comorbidities are common.8,9 Domains of psychopathology in bipolar disorder span such diverse dimensions as mood, behavior, psychosis, cognition, speech-language, personality, and circadian dysrhythmias—making it unlikely that any single drug could reasonably provide comprehensive efficacy. The expansion of newer US Food and Drug Administration (FDA)–approved pharmacotherapy options across all phases of bipolar disorder during the past two decades also bears consideration as contributing to polypharmacy. Clinicians may sometimes implement new agents as augmentations without necessarily discarding existing medications, in the hopes of either additive or synergistic effects, or perhaps fear of clinical deterioration if existing medications were deprescribed. The absence of clear guidelines for when to discontinue certain medications—such as antidepressants or antipsychotics—after an acute episode may further add to overall pharmacotherapy burden. “Carryover” of medications prescribed by previous treaters may also sometimes become folded into current drug regimens without deliberation as to their necessity, relevance, tolerability, or ongoing efficacy.
To date, there has been little formal review in the literature regarding the nature and extent of complex combination pharmacotherapy, the theoretical if not empirical rationale for specific combinations, its use in subpopulations, and impact on clinical outcome. The aims of the current review were therefore as follows:
- To differentiate simple from complex pharmacotherapy and review prevalence of the latter across studies in bipolar disorder patients;
- To identify clinical characteristics associated with the use of complex pharmacotherapy in clinical practice; and
- To provide preliminary recommendations to clinicians about when complex pharmacotherapy for bipolar disorder is most often rationale-based and likely to be clinically advantageous.
METHODS
The systematic review was prepared in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA; http://www.prisma-statement.org) following a registered publicly available protocol.
Preliminary Screening
A general, preliminary search was carried out on MEDLINE. The initial search terms used were bipolar disorder and polypharmacy. Results were screened to determine specific inclusion and exclusion criteria. The initial search yielded 175 results from 1981 to 2019. Following review of abstracts, full articles, and references, the following criteria were imposed: for inclusion, studies needed to (1) specify a diagnosis of bipolar disorder; (2) focus on polypharmacy of psychotropic medications; (3) include multiple, diverse individuals (ie, single case reports or small case series were excluded); (4) be related at the clinical (rather than preclinical) level; and (5) be English-language publications. Exclusion criteria involved studies that (1) did not include or specify a bipolar disorder diagnosis (and if included bipolar disorder, was a not separate section from other diagnoses); (2) did not focus on adjunctive treatments other than pharmacologic (eg, psychosocial, chronobiological); (3) failed to identify the number of medications taken by study subjects; (4) focused primarily on medication side effects rather than the demographics of medication prescribing; (5) were case reports, book formats, basic science reports, review articles, practice guidelines, opinion articles, or consensus statements without empirical data presentations; (6) reported on patients under 18 years old; and (7) were non–English language publications.
A subsequent MEDLINE search using the terms bipolar disorder and combination treatment and pharmacotherapy through the year 2020 yielded 3,566 results.
Information Sources and Search Criteria
From this preliminary search, we determined a protocol to be used for a systematic literature review on polypharmacy in bipolar disorder with a focus on demographics and prescribing patterns. We limited our search to records published in peer-reviewed journals and without publication date restrictions. Sources of information were accessed through MEDLINE, PsycINFO, and Embase and uploaded into COVIDENCE (www.covidence.org), a technology platform to assist with systematic reviews through data extraction, collection, and sorting format that in 2015 was adopted as the standard platform for Cochrane Reviews. Search criteria for MEDLINE, PsycINFO, and Embase included bipolar disorder, polypharmacy, limited to English language, and limited to adulthood (age 18 years and older). Duplicate records were automatically removed by COVIDENCE.
Study Selection
Our intent was to focus mainly on studies that report on prevalence rates and/or outcomes of bipolar patients receiving complex polypharmacy defined as ≥ 3 psychotropic medications. We therefore did not include studies of an adjunctive drug versus placebo added to treatment as usual (TAU) if TAU did not explicitly entail at least 2 drugs, or studies for which the total number of psychotropic drugs taken was not specified.
Following the aggregation of records from these 3 search engines, all of the authors independently examined and sorted manuscripts. Reviewers were blinded from each other. If there was not information in the abstract, full texts were accessed and reviewed according to inclusion and exclusion criteria.
Data Collection and Processing
Individual results were reviewed by the authors to resolve disagreements and reach a final consensus based on strict inclusion and exclusion criteria. The consensus constituted articles for final, full reading and review (see Results and Discussion section). Risk of biases in individual studies and across studies have been addressed in the limitations paragraph in the Discussion section.
RESULTS
Further Study Selection
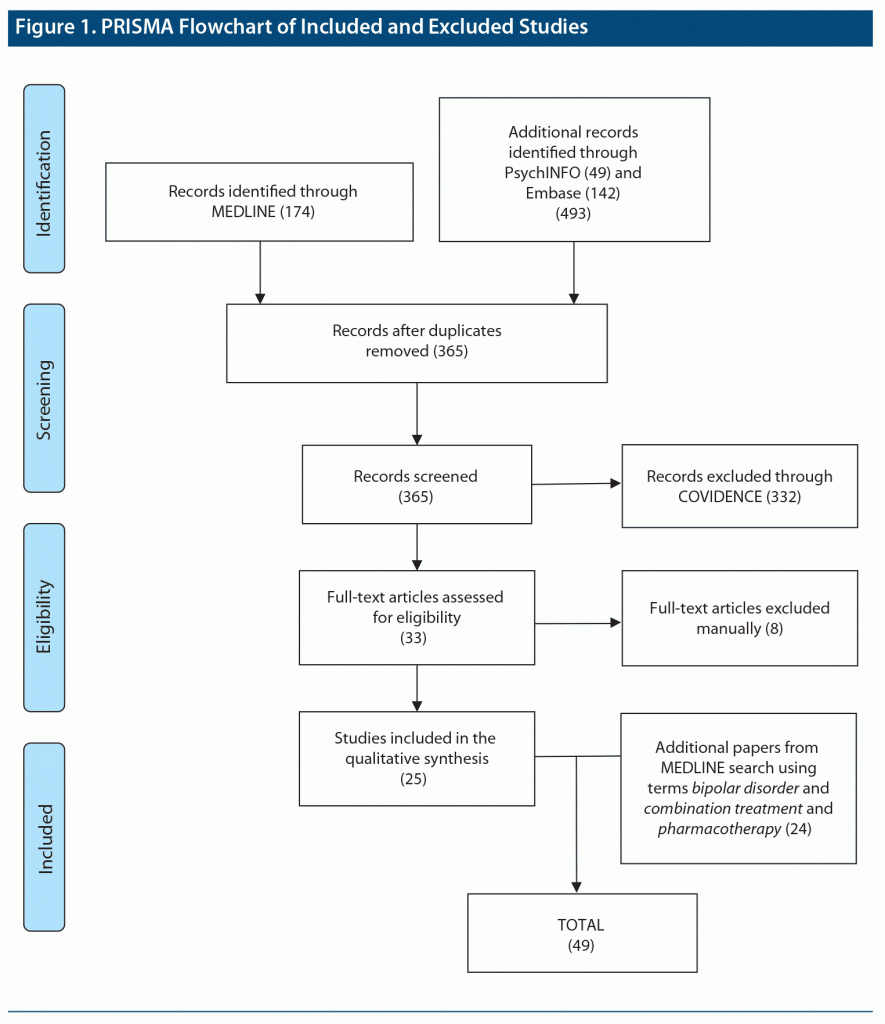
The 174 results from the initial MEDLINE search using the terms bipolar disorder and polypharmacy were uploaded into COVIDENCE. The following searches through PsycINFO yielded 49 additional results and through Embase, 142 additional results. These 3 search engines yielded a total of 493 results. Removal of duplicates resulted in 365 records, which were fully screened, blinded, following inclusion and exclusion criteria. Following resolution of disagreements by the authors, through the COVIDENCE platform, 33 records were selected for full review. These articles were then manually sorted, and 3 removed due to only abstract and poster being accessible, 2 were supplements, 1 was found to be Spanish-language only, and 2 included schizophrenia and bipolar disorder together. From this manual review and final reading, 25 articles from 1997 to 2019 were selected for initial qualitative analysis.
Hand-screening of publications generated from a second MEDLINE search, which yielded 3,566 results, resulted in 24 separate reports that met study criteria, increasing the final number of relevant publications to a total of 49.1–6,10–52 A PRISMA flowchart for study selection is presented in Figure 1.
Main Clinical Features
Definitions of polypharmacy and complex polypharmacy. Polypharmacy itself can be defined both conceptually and concretely. For the purposes of research, a more concrete, operative form is preferred as “the use of two or more psychiatric medications in the same patient.”2–5,7 We shall refer to the use of only 2 medications as simple polypharmacy. Complex polypharmacy has generally been defined in the literature as the use of either ≥ 3 medications (eg, Nierenberg et al10) or ≥ 4 medications (eg, Golden et al,6 Goldberg et al,15 Kim et al20). For the present study we define complex polypharmacy as “the use of 3 or more psychotropic medications.”
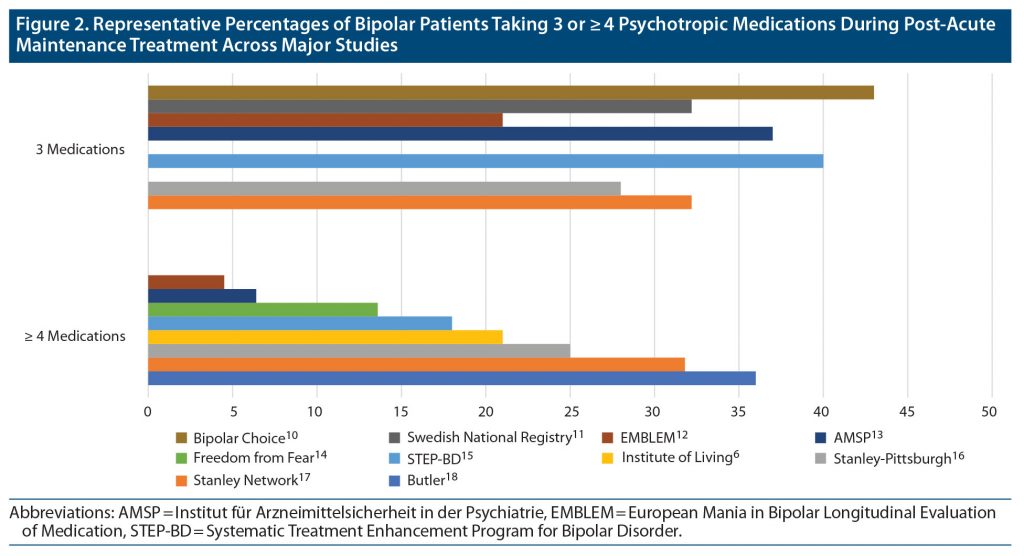
Prevalence of complex polypharmacy and trends over time. Figure 2 presents a summary of reported prevalence rates of bipolar patients taking either 3 or ≥ 4 medications based on studies from the literature search reporting those distinctions. From the studies identified in the figure, we calculated a weighted pooled mean percentage of 27.8% (2,614/9,414) taking (only) 3 medications and 22.3% (2,660/11,935) taking ≥ 4 medications. Discounting overlapping cases, a combined pooled total of 32.7% (4,535/13,863) took ≥ 3 medications.
In addition to the studies listed in Figure 2, several others met our definition of complex polypharmacy but reported prevalence rates of ≥ 3 medications without distinguishing more specific breakdowns. These included (a) a 2006 German cross-sectional study (published in 2010)21 in which antidepressants were the most often coprescribed drug among the 6.6% of patients who received ≥ 3 medications, (b) a study of 93 German private practitioners36 in which 5% of bipolar patients were prescribed ≥ 3 medications in both 2009 and 2018 (quetiapine was the most commonly coprescribed drug in 2018), (c) a 4-month survey of 16 Asian countries encompassing 348 patients (Research on Asian Psychotropic Prescription Pattern for Bipolar Disorder [REAP-BD])20 in which 34.3% took ≥ 3 medications, (d) a cohort of 80 bipolar outpatients from the Charité-University Medicine (Berlin)3 in which nearly all took a mean of 3.8 medications over 3 months, and (e) a cross-sectional study of 169 Brazilian bipolar outpatients37 in which 19% took ≥ 3 medications.
Trends over time suggest a rising frequency of combination pharmacotherapy over the past two decades. For example, observations from the National Institute of Mental Health (NIMH) intramural program1 indicated a sharp increase in polypharmacy regimens over a 22-year period among combined groups of refractory bipolar and unipolar mood disorder patients, with use of ≥ 3 medications increasing from 3.3% in the mid-to-late 1970s to 9.3% in the early 1980s to 34.9% in the mid-to-late 1980s to 43.8% in the early 1990s, irrespective of patient age. A Danish registry study38 found significant increases from 2000 to 2011 in rates of prescribing lithium plus an anticonvulsant or plus an antipsychotic, a doubling in the rate of coprescribing an antidepressant plus anticonvulsant or plus antipsychotic, and a 4-fold rise in the prescribing of an anticonvulsant plus antipsychotic. The European Institut für Arzneimittelsicherheit in der Psychiatrie (AMSP) Project found that the number of prescribed drugs (lithium, anticonvulsant, antipsychotic, or antidepressant) rose steadily from a mean of 2.1 during 1994–1997 to 2.9 during 2006–2009.13 By contrast, data from the Scottish registry22 found remarkably stable rates of complex polypharmacy from 2009 to 2016 (noted in Table 1).
In a number of studies, complex polypharmacy was more commonly encountered in bipolar disorder than in other disorders, although geographic variations were evident. For example, a retrospective study of German outpatients4 (n = 300) reported monotherapy as most common in schizophrenia patients (44.4%), followed by 16.1% in those with bipolar disorder and 11.9% in those with schizoaffective disorder. By contrast, in a cross-sectional Nigerian study (n = 278),2 simple or complex polypharmacy rates were lower in bipolar disorder (21.1%) than in schizophrenia (46.1%). In general, at least simple polypharmacy is often the initial treatment strategy in greater than 80% of bipolar disorder patients,5 usually as a combination of mood stabilizer and antipsychotic. A Taiwanese study40 points out that 71% of prescriptions in bipolar disorder involved between-class polypharmacy while 17% involved within-class polypharmacy.
Both simple and complex polypharmacy in special populations—notably, bipolar disorder in pregnancy and old age—have received limited study. One study of 533 pregnant women with bipolar disorder in Australia41 found that 30% were prescribed simple polypharmacy. A study of a Danish registry enrolling 336 pregnant women with bipolar disorder in 1997–200242 found that slightly less than 6% took a combination of an antidepressant plus an anticonvulsant mood stabilizer plus at least 1 other psychotropic medication, about 4% took an antidepressant plus an atypical antipsychotic plus at least 1 other psychotropic medication, 4% took an antidepressant plus anticonvulsant plus atypical antipsychotic, 4% took an antidepressant plus lithium plus at least 1 other psychotropic medication, about 2.5% took an antidepressant plus lithium plus an atypical antipsychotic, slightly more than 2% took an antidepressant plus lithium plus an anticonvulsant mood stabilizer, and less than 2% took lithium plus an anticonvulsant plus an atypical antipsychotic.
Among older adults with bipolar disorder, a Dutch cross-sectional study (n = 101) of mainly outpatients43 identified any polypharmacy in 31.7% of subjects; a median took 2 psychotropic compounds (most often involving a pairing of lithium, sedatives, antidepressants, antipsychotics, or anticonvulsant mood stabilizers), and nearly one-third took > 6 medications in total (including both psychotropic and nonpsychotropic medications). A cross-sectional study of 1,443 elderly Canadian bipolar disorder outpatients44 found at least simple polypharmacy in 81.5%. In the European AMSP database,45 triple drug therapy was significantly rarer in acutely manic patients older versus younger than age 70.
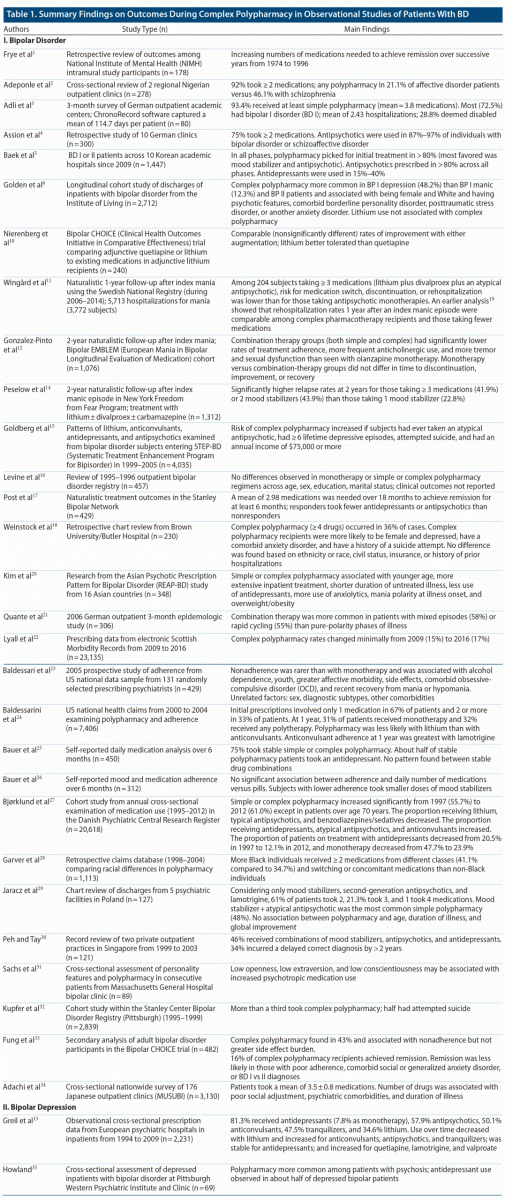
Clinical features associated with complex polypharmacy. Table 1 summarizes main findings from 31 of the 49 studies drawn from the final literature search that reported outcome data for bipolar disorder patients taking complex polypharmacy regimens.
Varied clinical features have been associated with complex polypharmacy. A German retrospective chart review of 80 bipolar disorder outpatients3 found a median age of 38 years old, 50% male and 50% female, 72.5% with bipolar I disorder (BD I) and 27.5% bipolar II disorder (BD II), 50% single and 50% married, and a history of 2.43 hospitalizations, with 28.8% on disability. A 2002 study characterizing a cohort of 2,839 outpatients32 identified through the Stanley Center Bipolar Disorder Registry found a median age of 40.1 years, 65.4% women, and 90% White, with 60% having completed some college, 30% having completed college, and 64% currently unemployed. Importantly 50.3% had attempted suicide, and the disparity between education level and employment was noted. A 2008 Singaporean retrospective chart review of 121 bipolar disorder outpatients notes that 58% were employed, 48% married, with 7% a diagnosis of BD I, 76% BD II, and 7% bipolar disorder not otherwise specified (BD NOS).30 No psychotic symptoms were observed in 75% of the population; 65% had never been hospitalized, 9% had past suicide attempts, and 16% had had electroconvulsive therapy (ECT).30 This study took particular care to comment on the diagnostic complexity of bipolar disorder, elaborating on the delay in diagnosis as contributing to polypharmacy as well as the challenges in achieving full remission.
In the 2009 report based on Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) data,15 likelihood of either simple or complex polypharmacy was found to be higher if individuals were ever on treatment with an antipsychotic, if there was a history of ≥ 6 lifetime episodes of depression, history of suicide attempts, or annual income of greater than $75,000. A history of psychosis, age at onset, BD I versus II, rapid cycling, prior hospitalizations, and alcohol and substance use were not found to alter risk of polypharmacy, although rapid cycling was still ascertained a predictor for extensive depression, antidepressant use, and suicidal behavior.15 Another STEP-BD report focusing on the naturalistic use of ≥ 2 antipsychotics in bipolar subjects46 (evident in 10% of the study group) found an increased overall burden of adverse effects and greater service utilization but no differences in global functioning or symptom status as compared to those taking ≤ 1 antipsychotic.
A 2014 chart review of 231 inpatients with BD I diagnoses31 found that any form of polypharmacy was more likely in depressed females with comorbid anxiety disorders and a history of suicide attempts, and no difference was found based on ethnicity or race, civil status, insurance, or history of prior hospitalizations. However, the data from this study were based on a population of 94% White and 91% non-Hispanic individuals,11 and contrastingly a study focusing on racial differences in prescribing in bipolar disorder found that Black individuals tended to have more Medicaid and Medicare complementary services as well as Axis I diagnoses than non-Black individuals.28 Additionally, more Black individuals received ≥ 2 medications from different classes (41.1% compared to 34.7%), and switching or use of concomitant medications was higher in Black individuals.28
Aside from race, other variables studied in relation to polypharmacy in bipolar disorder have included personality characteristics. Sachs et al31 examined personality styles and found low openness, low extraversion, and low conscientiousness to be associated with increased psychotropic medication use. A 2017 prospective cohort study of 2,712 inpatients6 concluded that variables associated with complex polypharmacy included White race, female sex, psychotic features, and comorbid diagnosis of borderline personality disorder, posttraumatic stress disorder (PTSD), or anxiety disorders. Interestingly, substance use was not associated with polypharmacy.6
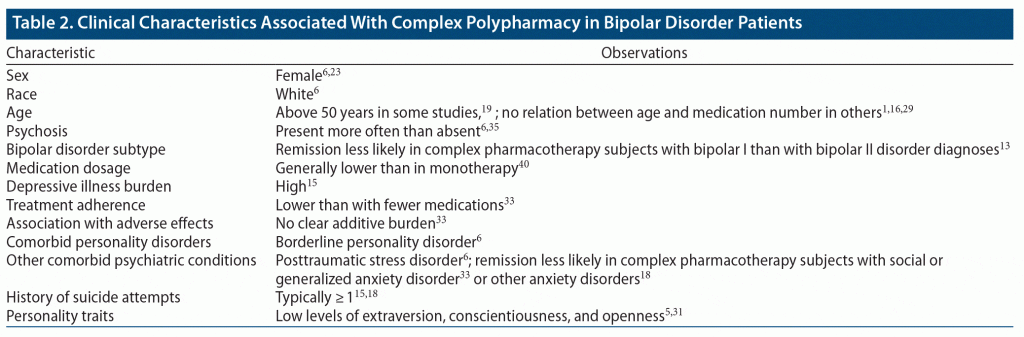
Table 2 summarizes demographic and clinical features associated with complex polypharmacy based on the present literature review.
Medication types within polypharmacy. Trends in medication types within simple or complex polypharmacy regimens were generally consistent throughout the studies. A 2016 nationwide register-based cohort study from 1995 to 201227 found that the proportion of lithium, benzodiazepines, and typical antipsychotics decreased over time, while the number of antidepressants, anticonvulsants, and atypical antipsychotics has increased.
In the AMSP Project,39 among patients taking at least 3 medications, the most commonly observed combinations (each 1%–2%) were a selective serotonin reuptake inhibitor (SSRI) plus lithium and quetiapine, an SSRI plus lithium and lamotrigine, an SSRI plus mirtazapine with lithium or divalproex or venlafaxine, or lithium plus mirtazapine plus venlafaxine; incident rates of 1.1% each were observed with an SSRI plus olanzapine plus lithium, lithium plus quetiapine plus venlafaxine, or lithium plus quetiapine plus lamotrigine. Clinical correlates or statistical predictors of complex polypharmacy in that cohort were not reported. In another contemporary multinational observational study,25 75% of those who were taking polypharmacy remained on a stable regimen for ≥ 50% of days. Individual polypharmacy patterns in that study varied, with no particular combination appearing especially prominent.
Antidepressant use and complex polypharmacy. Although expert consensus guidelines generally eschew antidepressant monotherapy in bipolar disorder,53 formal studies reporting on complex polypharmacy involving antidepressant use were surprisingly scarce. As noted in Table 1, the European AMSP study13 found decreased use of lithium; increased use of antipsychotics, anticonvulsants, and tranquilizers; and no change regarding antidepressant use from 2009 to 2018. A total of 81.3% of that study group received antidepressants (7.8% as monotherapy), and 57.9% took antipsychotics. Least associated with polypharmacy were lithium, divalproex, and carbamazepine, while most associated included antidepressants and atypical antipsychotics. In a Danish registry study,27 antidepressant monotherapy declined by about half from 1997 to 2012. Another 1997 study of 69 bipolar depressed inpatients found that 90% received one “anti-manic” drug, most often lithium, and 50% were maintained on antidepressants.35 In the Asian REAP-BD study,20 the combination of mood stabilizer and antipsychotic was most commonly used as the initial treatment strategy during maintenance phase therapy (80%), with antidepressant use ranging from 15% to 40%.5
Adherence and complex pharmacotherapy in bipolar disorder. A number of studies addressed polypharmacy in relation to medication adherence. As noted in Table 1, a health claims review by Baldessarini et al24 found that when examining adherence with anticonvulsants, patients were more likely to be adherent with lamotrigine as compared to valproate, carbamazepine, oxcarbazepine, and lithium, while nonadherent patients were more often prescribed divalproex, carbamazepine, and oxcarbazepine. Adherent patients also were less likely to use drugs or alcohol, more likely to have a comorbid anxiety disorder, and more likely to be treated by a psychiatrist rather than a primary care physician. Although adherent patients utilized 55% more ambulatory visits and 44% more emergency services compared to nonadherent individuals, overall, there was a 27% decrease in hospitalizations for all reasons. A prospective study by this team23 found that nonadherence during polypharmacy was associated with comorbid alcohol dependence, youth, greater affective morbidity, side effects, comorbid obsessive-compulsive disorder (OCD), and recent recovery from mania or hypomania. Factors unrelated to polypharmacy nonadherence included sex, diagnostic subtypes, and other comorbidities. The authors concluded that OCD and hypomania could reflect ambivalence and denial of symptoms related to bipolar disorder and that some of the consequences of the disease on the patient could go unrecognized, thus leading to more complex and aggressive regimens with “vicious” cycles of diminishing adherence. Others have suggested, rather, that a complex medication regimen itself could contribute to nonadherence; however, this has been contradicted by suggestions that concurrent psychotropic medications are not associated with nonadherence. Bauer et al26 found no association between medication adherence and the number of prescribed medications or actual number of pills consumed per day.
Outcomes. Functional and symptomatic outcomes for patients receiving extensive polypharmacy are difficult to judge from naturalistic studies because of the very high likelihood of confounding by indication and protopathic bias—that is, a higher number of medications is likely the result, rather than the cause, of greater illness severity and a presumably poorer previous response to simpler treatment regimens. This is certainly the impression generated by most observational studies linking lower remission rates with the use of more extensive medication regimens for bipolar disorder (eg, Chae et al48), although it contrasts with findings from the Swedish registry study11 showing higher rates of treatment failure (eg, relapse, rehospitalization) among monotherapy than polypharmacy recipients.
When searching the literature for controlled pharmacotherapy trials involving use of ≥ 3 psychotropic medications for any phase of bipolar disorder, we identified only a handful of studies. One randomized trial reported across 2 articles49,50 involved the use of 3 mood stabilizers (lithium plus divalproex plus lamotrigine) versus 2 (lithium plus divalproex plus placebo) in rapid-cycling outpatients for whom lithium plus divalproex failed to achieve stabilization. That trial failed to demonstrate an advantage for triple versus double mood stabilizer therapy, although high study dropout, poor tolerability and statistical underpowering were collectively perceived to contribute to an uninformative end result with regard to undemonstrated efficacy. Another trial in 45 acute manic patients from Iran52 found a significantly greater reduction in mania symptoms when the 5-HT2 antagonist ritanserin rather than placebo was added to the combination of lithium plus haloperidol. An additional small (n = 32) randomized study focusing on comorbid OCD in bipolar disorder patients51 found an advantage for reducing obsessive-compulsive symptoms with the combination of lithium plus olanzapine plus clonazepam plus topiramate versus lithium plus olanzapine plus clonazepam without topiramate.
DISCUSSION
Definitions of polypharmacy and complex polypharmacy were generally consistent throughout the literature. Polypharmacy, whether simple or complex, appears to have become the norm during acute and maintenance phases of bipolar disorder, occurring at least to some degree in more than half of adult patients. Populations with complex polypharmacy were of particular interest and concern, occurring in 21% to 43% of study populations, with bipolar depression at highest risk. Combination therapy was found to be implemented more often in women, White individuals, and those older than age 50 years. A later age may reflect lifelong attempts at varied management strategies for symptoms. Interestingly, female sex is often comorbid with anxiety disorders (social, general), PTSD, and borderline personality disorder, which were found to be clinical diagnoses associated with complex polypharmacy in our systematic literature review. Increased combination pharmacotherapy was noted in patients with a history of psychosis, greater burden of depressive illness, and a history of suicide attempt. Greater overall illness severity is reflected in extensive or complex pharmacotherapy in bipolar disorder.
Aside from patient characteristics, clinician prescribing practices provide insight as well. Prescriptions of lithium and typical antipsychotics have decreased over time, perhaps due to need for monitoring and unwanted side effect profile. Contrastingly, prescriptions of antidepressants have remained the same or elevated, and increases have been noted in the number of patients taking antipsychotics or other mood stabilizers. Manifold explanations for this trend exist and may include prescriber reluctance to remove medications that are perceived to be possibly beneficial, the wish to try new medications and/or combinations in the hopes of improved efficacy, lack of clear standardized guidelines (resulting in subtherapeutic dosing or multiple agents), consideration of patient personality traits and subjective report, and overall rapport. Perhaps prescribing practices respond to patient characteristics of low extraversion, conscientiousness, and openness.
The observational nature of the vast majority of the literature on complex polypharmacy precludes causal relationships and invites speculation about confounding by indication (that is, a presumption that more severely ill patients “require” more medications when simpler regimens prove ineffective). Hence, it is possible that complex polypharmacy might sometimes arise from the fact that available treatments are frequently not always efficacious as monotherapy, underscoring the need for development of novel therapeutics. Only in rare instances among naturalistic studies did we observe better outcomes with polypharmacy than with monotherapy (eg, Wingård et al11). Another perspective, articulated based on findings from the Stanley Bipolar Network, suggests that the phenomenology of modern-day bipolar disorder may be more inherently complex than in previous eras, such that greater numbers of medications are now needed to achieve stabilization, as suggested by Frye et al1 and Post et al.17
At the same time, given that clinical practice may often favor complex polypharmacy as an assumed necessity for more severely ill patients, it must also be noted that other factors that drive complex polypharmacy may be poorly accounted for in naturalistic studies—such as clinicians’ failure to deprescribe ineffective medications, use of inappropriate medications (eg, antidepressants during mania or episodes involving mixed features, use of drugs with duplicative mechanisms, or the intended thymoleptic use of anticonvulsants that lack mood-stabilizing properties), or failure to optimize dosing of appropriate medications, among other considerations.
Perhaps the most fundamental unanswerable question from the present review involves insight into when complex polypharmacy could yield better clinical outcomes than simpler regimens. The veritable absence of large randomized trials to address this issue seems to pose one of the greatest unmet needs in modern psychopharmacology, and the practical limitations of undertaking such efforts makes it unlikely that empirical findings to guide this question are forthcoming. It remains uncertain whether inclusion of certain specific agents within a broader regimen—such as lithium or olanzapine – may either lessen the need for complex polypharmacy or improve its likelihood of success; thus, Bipolar CHOICE (Clinical Health Outcomes Initiative in Comparative Effectiveness) subjects taking lithium had a somewhat lesser “burden” of antipsychotic cotherapy, but not better overall outcomes,10 while in the Swedish registry study19 complex polypharmacy recipients whose regimen included olanzapine had lower rehospitalization rates.
Because complex polypharmacy more often tends to be a marker for greater illness complexity and severity, it is likely also a proxy for treatment resistance to simpler regimens. On the other hand, such inferences are difficult to draw without knowing details about the adequacy, appropriateness, and adherence surrounding possible simpler pharmacotherapy efforts that may predate complex polypharmacy for some patients. Sachs et al31 coined the term ineffective complex chronic care (ICCC) to describe patients who “remain ill despite receiving 5 or more medications for 6 or more months.”31(p1065) Given our observation that one-third or more of bipolar patients receive complex polypharmacy, clinicians who encounter ICCC-type situations must differentiate pharmacologic futility despite strategic or heroic efforts to overcome true lack of efficacy from simpler regimens versus artifacts of past treatments that may have been inadequate, inappropriate, or undermined by nonadherence.
Because of the limited insight provided by the literature on complex polypharmacy, alongside the reality that many bipolar disorder patients receive extensive drug therapy regimens despite the lack of an empirical evidence base, we sought to identify basic concepts and principles that may aid clinicians in their efforts to determine the rationale and logical basis for considering complex polypharmacy. These provisional recommendations, pending the future emergence of a more rigorous clinical trials database, are summarized in Table 3.
A further recommendation relevant to the reporting of future clinical trials involving complex polypharmacy pertains to studies that randomize subjects to a particular adjunctive drug versus placebo when added to TAU. Surprisingly few existing published trials using that design specify precisely or systematically what drugs comprise TAU, in either number or intensity of therapy, limiting the extent to which inferences can be made about the relative impact of a unique agent added to an existing complex regimen without knowledge of the appropriateness or adequacy of baseline TAU.
Limitations of the study include the use of single covariate statistical analyses to examine individual predictors rather than risk profiles; recall and confounding bias within each study; varied diagnostic bias across studies, such as the overlap between schizoaffective disorder and bipolar disorder; inpatient versus outpatient settings; differences in sample sizes and selection bias (homogenous vs non-homogenous); and regional and cultural differences in addition to pharmacoeconomic issues that have an influence on treatment decision. Importantly, psychosocial factors such as quality of patient-clinician communication and family/environmental support are factors that could not be accounted for. Children were not included in attempts to simplify the analysis. Additionally, we were unable to assess health care–related factors that might influence polypharmacy, such as prescriber discipline (eg, MD versus non-MD midlevel provider) and expertise, treatment setting (clinic versus community mental health or private practice), and extent and use of nonpharmacologic interventions (eg, individual psychotherapy, groups, day treatment programs).
Strengths of the present study include the systematic and rigorous review of articles from multiple search engines, a holistic and integrated understanding of multiple factors contributing to polypharmacy, inclusion of recent articles, and applicability to identify populations at risk for polypharmacy as well as patterns in prescribers.
Both simple and complex polypharmacy are highly prevalent and, when not strategically devised, can become unduly costly, complex, and a driver of poor adherence and excessive adverse drug effects. Naturalistic studies do not suggest better outcomes for patients receiving more complex drug regimens. Formal clinical trials are needed to identify optimal drug combinations and durations when using ≥ 3 psychotropic medications to treat patients with bipolar disorder. Strategies such as simplifying regimens, optimizing doses prior to adding another agent, deprescribing ineffective medication, and carefully considering risks and benefit versus lack of benefit of each medication can be employed for improved clinical care, all the while incorporating known factors that place the patient at risk.
Submitted: February 7, 2021; accepted February 23, 2021.
Published online: June 1, 2021.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, venlafaxine, mirtazapine, ritanserin, haloperidol, topiramate, and clonazepam are not approved by the US Food and Drug Administration for the treatment of bipolar disorder.
Financial disclosure: Dr Goldberg is a consultant to BioXcel, Lundbeck, SAGE Therapeutics, MedScape, Intracellular Therapies, Otsuka, Psychiatry and Behavioral Health Learning Network, Sunovion, and WebMD; he serves on the speakers bureaus for Allergan, Intracellular Therapies, Otsuka, and Sunovion; and he receives royalties from American Psychiatric Publishing, Inc, and Cambridge University Press. Drs Kim and Salstein have no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
Funding/support: None.
Clinical Points
- Although extensive combination pharmacotherapy is common in many patients with bipolar disorder, naturalistic studies do not point to better outcomes for patients receiving more complex drug regimens.
- Simplifying regimens, optimizing doses prior to adding another agent, deprescribing ineffective medication, and carefully considering risks and benefit versus lack of benefit of each medication may improve clinical care while incorporating known factors that place the patient at risk.
References (53)

- Frye MA, Ketter TA, Leverich GS, et al. The increasing use of polypharmacotherapy for refractory mood disorders: 22 years of study. J Clin Psychiatry. 2000;61(1):9–15. PubMed CrossRef
- Adeponle AB, Obembe AO, Adeyemi SO, et al. Polypharmacy in psychiatric out-patient practice in northern Nigeria. Afr J Psychiatry (Johannesbg). 2007;10(4):215–218. PubMed
- Adli M, Whybrow PC, Grof P, et al. Use of polypharmacy and self-reported mood in outpatients with bipolar disorder. Int J Psychiatry Clin Pract. 2005;9(4):251–256. PubMed CrossRef
- Assion HJ, Schweppe A, Reinbold H, et al. Pharmacological treatment for schizoaffective disorder: a comparison with schizophrenia and bipolar disorder. Nervenarzt. 2019;90(suppl 1):1–8. PubMed CrossRef
- Baek JH, Ha K, Yatham LN, et al. Pattern of pharmacotherapy by episode types for patients with bipolar disorders and its concordance with treatment guidelines. J Clin Psychopharmacol. 2014;34(5):577–587. PubMed CrossRef
- Golden JC, Goethe JW, Woolley SB. Complex psychotropic polypharmacy in bipolar disorder across varying mood polarities: a prospective cohort study of 2712 inpatients. J Affect Disord. 2017;221:6–10. PubMed CrossRef
- Fornaro M, De Berardis D, Koshy AS, et al. Prevalence and clinical features associated with bipolar disorder polypharmacy: a systematic review. Neuropsychiatr Dis Treat. 2016;12:719–735. PubMed CrossRef
- McElroy SL, Altshuler LL, Suppes T, et al. Axis I psychiatric comorbidity and its relationship to historical illness variables in 288 patients with bipolar disorder. Am J Psychiatry. 2001;158(3):420–426. PubMed CrossRef
- Krishnan KR. Psychiatric and medical comorbidities of bipolar disorder. Psychosom Med. 2005;67(1):1–8. PubMed CrossRef
- Nierenberg AA, McElroy SL, Friedman ES, et al. Bipolar CHOICE (Clinical Health Outcomes Initiative in Comparative Effectiveness): a pragmatic 6-month trial of lithium versus quetiapine for bipolar disorder. J Clin Psychiatry. 2016;77(1):90–99. PubMed CrossRef
- Wingård L, Brandt L, Bodén R, et al. Monotherapy vs combination therapy for post mania maintenance treatment: a population based cohort study. Eur Neuropsychopharmacol. 2019;29(6):691–700. PubMed CrossRef
- Gonzalez-Pinto A, Vieta E, Reed C, et al. Effectiveness of olanzapine monotherapy and olanzapine combination treatment in the long term following acute mania–results of a two year observational study in bipolar disorder (EMBLEM). J Affect Disord. 2011;131(1–3):320–329. PubMed CrossRef
- Greil W, Häberle A, Haueis P, et al. Pharmacotherapeutic trends in 2231 psychiatric inpatients with bipolar depression from the International AMSP Project between 1994 and 2009. J Affect Disord. 2012;136(3):534–542. PubMed CrossRef
- Peselow ED, Naghdechi L, Pizano D, et al. Polypharmacy in maintenance of bipolar disorder. Clin Neuropharmacol. 2016;39(3):132–134. PubMed CrossRef
- Goldberg JF, Brooks JO 3rd, Kurita K, et al. Depressive illness burden associated with complex polypharmacy in patients with bipolar disorder: findings from the STEP-BD. J Clin Psychiatry. 2009;70(2):155–162. PubMed CrossRef
- Levine J, Chengappa KNR, Brar JS, et al. Psychotropic drug prescription patterns among patients with bipolar I disorder. Bipolar Disord. 2000;2(2):120–130. PubMed CrossRef
- Post RM, Altshuler LL, Frye MA, et al. Complexity of pharmacologic treatment required for sustained improvement in outpatients with bipolar disorder. J Clin Psychiatry. 2010;71(9):1176–1186, quiz 1252–1253. PubMed CrossRef
- Weinstock LM, Gaudiano BA, Epstein-Lubow G, et al. Medication burden in bipolar disorder: a chart review of patients at psychiatric hospital admission. Psychiatry Res. 2014;216(1):24–30. PubMed CrossRef
- Wingård L, Bodén R, Brandt L, et al. Reducing the rehospitalization risk after a manic episode: a population based cohort study of lithium, valproate, olanzapine, quetiapine and aripiprazole in monotherapy and combinations. J Affect Disord. 2017;217:16–23. PubMed CrossRef
- Kim K, Yang H, Na E, et al. Examining patterns of polypharmacy in bipolar disorder: findings from the REAP-BD Korea. Psychiatry Investig. 2019;16(5):397–402. PubMed CrossRef
- Quante A, Zeugmann S, Regen F, et al. Psychopharmacological treatment status in outpatients with bipolar disorder: a clinical survey in Germany. Psychiatry Investig. 2010;7(3):155–162. PubMed CrossRef
- Lyall LM, Penades N, Smith DJ. Changes in prescribing for bipolar disorder between 2009 and 2016: national-level data linkage study in Scotland. Br J Psychiatry. 2019;215(1):415–421. PubMed CrossRef
- Baldessarini RJ, Perry R, Pike J. Factors associated with treatment nonadherence among US bipolar disorder patients. Hum Psychopharmacol. 2008;23(2):95–105. PubMed CrossRef
- Baldessarini R, Henk H, Sklar A, et al. Psychotropic medications for patients with bipolar disorder in the United States: polytherapy and adherence. Psychiatr Serv. 2008;59(10):1175–1183. PubMed CrossRef
- Bauer M, Glenn T, Alda M, et al. Drug treatment patterns in bipolar disorder: analysis of long-term self-reported data. Int J Bipolar Disord. 2013;1(1):5. PubMed CrossRef
- Bauer M, Glenn T, Grof P, et al. The association between concurrent psychotropic medications and self-reported adherence with taking a mood stabilizer in bipolar disorder. Hum Psychopharmacol. 2010;25(1):47–54. PubMed CrossRef
- Bjørklund L, Horsdal HT, Mors O, et al. Trends in the psychopharmacological treatment of bipolar disorder: a nationwide register-based study. Acta Neuropsychiatr. 2016;28(2):75–84. PubMed CrossRef
- Garver D, Lazarus A, Rajagopalan K, et al. Racial differences in medication switching and concomitant prescriptions in the treatment of bipolar disorder. Psychiatr Serv. 2006;57(5):666–672. PubMed CrossRef
- Jaracz J, Rudnicka ET, Bierejszyk M, et al. The pattern of pharmacological treatment of bipolar patients discharged from psychiatric units in Poland. Pharmacol Rep. 2018;70(4):694–698. PubMed CrossRef
- Peh AL, Tay LK. Demographical profile and clinical features of patients with bipolar disorder in an outpatient setting in Singapore. Singapore Med J. 2008;49(5):380–383. PubMed
- Sachs GS, Peters AT, Sylvia L, et al. Polypharmacy and bipolar disorder: what’s personality got to do with it? Int J Neuropsychopharmacol. 2014;17(7):1053–1061. PubMed CrossRef
- Kupfer DJ, Frank E, Grochocinski VJ, et al. Demographic and clinical characteristics of individuals in a bipolar disorder case registry. J Clin Psychiatry. 2002;63(2):120–125. PubMed CrossRef
- Fung VC, Overhage LN, Sylvia LG, et al. Complex polypharmacy in bipolar disorder: side effect burden, adherence, and response predictors. J Affect Disord. 2019;257:17–22. PubMed CrossRef
- Adachi N, Azekawa T, Edagawa K, et al. Estimated model of psychotropic polypharmacy for bipolar disorder: analysis using patients’ and practitioners’ parameters in the MUSUBI study. Hum Psychopharmacol. 2020;e2764. PubMed
- Howland RH. Pharmacotherapy of inpatients with bipolar depression. Ann Clin Psychiatry. 1997;9(4):199–202. PubMed CrossRef
- Bohlken J, Bauer M, Kostev K. Drug treatment for patients with bipolar disorders in psychiatric practices in Germany in 2009 and 2018. Psychiatry Res. 2020;289:112965. PubMed CrossRef
- Gazalle FK, Hallal PC, Tramontina J, et al. Polypharmacy and suicide attempts in bipolar disorder. Br J Psychiatry. 2007;29:35–38.
- Kessing LV, Vradi E, Andersen PK. Nationwide and population-based prescription patterns in bipolar disorder. Bipolar Disord. 2016;18(2):174–182. PubMed CrossRef
- Haeberle A, Greil W, Russmann S, et al. Mono- and combination drug therapies in hospitalized patients with bipolar depression: data from the European drug surveillance program AMSP. BMC Psychiatry. 2012;12(1):153. PubMed CrossRef
- Hung GC, Yang SY, Chen Y, et al. Psychotropic polypharmacy for the treatment of bipolar disorder in Taiwan. Psychiatr Serv. 2014;65(1):125–128. PubMed CrossRef
- Galbally M, Frayne J, Watson SJ, et al. Psychopharmacological prescribing practices in pregnancy for women with severe mental illness: a multicentre study. Eur Neuropsychopharmacol. 2019;29(1):57–65. PubMed CrossRef
- Broeks SC, Thisted Horsdal H, Glejsted Ingstrup K, et al. Psychopharmacological drug utilization patterns in pregnant women with bipolar disorder: a nationwide register-based study. J Affect Disord. 2017;210:158–165. PubMed CrossRef
- Dols A, Rhebergen D, Beekman A, et al. Psychiatric and medical comorbidities: results from a bipolar elderly cohort study. Am J Geriatr Psychiatry. 2014;22(11):1066–1074. PubMed CrossRef
- Rej S, Herrmann N, Shulman K, et al. Current psychotropic medication prescribing patterns in late-life bipolar disorder. Int J Geriatr Psychiatry. 2017;32(12):1459–1465. PubMed CrossRef
- Kleimann A, Schrader V, Stübner S, et al. Psychopharmacological treatment of 1650 in-patients with acute mania-data from the AMSP study. J Affect Disord. 2016;191:164–171. PubMed CrossRef
- Brooks JO 3rd, Goldberg JF, Ketter TA, et al. Safety and tolerability associated with second-generation antipsychotic polytherapy in bipolar disorder: findings from the Systematic Treatment Enhancement Program for Bipolar Disorder. J Clin Psychiatry. 2011;72(2):240–247. PubMed CrossRef
- Hayes J, Prah P, Nazareth I, et al. Prescribing trends in bipolar disorder: cohort study in the United Kingdom THIN primary care database 1995-2009. PLoS One. 2011;6(12):e28725. PubMed CrossRef
- Chae WR, Nagel JM, Kuehl LK, et al. Predictors of response and remission in a naturalistic inpatient sample undergoing multimodal treatment for depression. J Affect Disord. 2019;252:99–106. PubMed CrossRef
- Kemp DE, Gao K, Fein EB, et al. Lamotrigine as add-on treatment to lithium and divalproex: lessons learned from a double-blind, placebo-controlled trial in rapid-cycling bipolar disorder. Bipolar Disord. 2012;14(7):780–789. PubMed CrossRef
- Wang Z, Gao K, Kemp DE, et al. Lamotrigine adjunctive therapy to lithium and divalproex in depressed patients with rapid cycling bipolar disorder and a recent substance use disorder: a 12-week, double-blind, placebo-controlled pilot study. Psychopharmacol Bull. 2010;43(4):5–21. PubMed
- Sahraian A, Bigdeli M, Ghanizadeh A, et al. Topiramate as an adjuvant treatment for obsessive compulsive symptoms in patients with bipolar disorder: a randomized double blind placebo controlled clinical trial. J Affect Disord. 2014;166:201–205. PubMed CrossRef
- Akhondzadeh S, Mohajari H, Reza Mohammadi M, et al. Ritanserin as an adjunct to lithium and haloperidol for the treatment of medication-naive patients with acute mania: a double blind and placebo controlled trial. BMC Psychiatry. 2003;3(1):7. PubMed CrossRef
- Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249–1262. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top