Termination of Clozapine Treatment Due to Medical Reasons:
When Is It Warranted and
How Can It Be Avoided?
ABSTRACT
Objective: To identify the outcome of potentially serious adverse effects of clozapine, particularly those frequently cited as reasons for clozapine discontinuation, and to characterize management strategies for adverse effects that do not warrant discontinuation.
Data Sources: A structured search was performed of PubMed and EMBASE from database inception until September 10, 2012, without any language restrictions, using clozapine as the search term. Reference lists of retrieved articles were cross-checked for additional relevant studies.
Study Selection: Included in this review were studies that reported on the frequency of the specified clozapine-related adverse effects and that either reported on the grounds for or against clozapine discontinuation or reported on management techniques to maintain patients on clozapine or enable successful clozapine rechallenge. The following side effects were considered important for this review as potential grounds for clozapine discontinuation: neutropenia or agranulocytosis, leukocytosis, thrombocytopenia, thrombocytosis, eosinophilia, leukocytosis, QTc prolongation, electrocardiogram changes, atrial flutter, tachycardia, myocarditis, cardiomyopathy, fever, syncope, diabetes mellitus, diabetic ketoacidosis, diabetic hyperosmolar coma, neuroleptic malignant
syndrome, ileus, liver enzyme elevation, or seizure.
Data Extraction: Study results that supported continuation or discontinuation of clozapine or that provided information
on management techniques were abstracted.
Results: Of a total of 13,385 search results, data from 81 studies were included in this review. Results suggest that prompt discontinuation of clozapine without rechallenge is indicated for agranulocytosis, myocarditis, cardiomyopathy, and a QTc interval > 500 milliseconds that is confirmed and derived using the appropriate correction method. Clozapine discontinuation with potential rechallenge (provided there is appropriate surveillance and management or prophylactic therapy) is indicated for ileus or subileus, neuroleptic malignant syndrome, venous thromboembolism, and diabetic ketoacidosis or hyperosmolar coma. Neutropenia, leukocytosis, seizures, orthostatic hypotension, severe constipation, and weight gain and metabolic abnormalities, including metabolic syndrome and its components, as well as moderately prolonged myocardial repolarization, need to be managed but do not generally warrant clozapine discontinuation. Eosinophilia, leukocytosis, drug-induced fever, and tachycardia (provided that myocarditis and neuroleptic malignant syndrome are ruled out) can be managed and should rarely lead to clozapine discontinuation.
Conclusions: A number of side effects commonly cited as medical reasons for clozapine discontinuation do not necessarily warrant such action. Management techniques are available that allow continuation or rechallenge in relation to a number of clozapine-related side effects.
J Clin Psychiatry 2013;74(6):603–613
© Copyright 2013 Physicians Postgraduate Press, Inc.
Submitted: July 31, 2012; accepted January 28, 2013 (doi:10.4088/JCP.12r08064).
Corresponding author: Jimmi Nielsen, MD, Centre for Schizophrenia, Aalborg Psychiatric Hospital, Brandevej 5, 9210 Aalborg, N/A 9000, Denmark ([email protected]).
Clozapine remains the gold standard for treatment-resistant schizophrenia, and its impressive efficacy has been demonstrated in several clinical trials1–4 and a meta-analysis.5 In addition to its unique efficacy in patients with treatment-resistant schizophrenia, clozapine possesses antisuicidal6 and antiaggressive7 properties, and its efficacy for treatment-resistant bipolar disorder has also been documented.8a,8b Treatment with clozapine is highly cost-effective due to significant reductions in hospitalization.9 Many clozapine-treated patients and their relatives report a substantial positive effect on their lives and well-being.10 However, clozapine is also associated with a wide range of side effects, some of which are potentially fatal. The most salient among them, agranulocytosis, led to the suspension of clozapine treatment after 8 fatal cases in Finland in 1974 and demonstrated the need for the current mandatory hematologic monitoring program. Other side effects, such as myocarditis,11 aspiration pneumonia,12 ileus,13 and weight gain,14 have in fact caused more deaths than agranulocytosis. Weight gain alone has been estimated to cause 412 deaths per 100,000 patients over 10 years.14 Although the estimate of Fontaine et al14 remains untested in prospective samples, overall, weight gain–induced mortality is balanced by
clozapine’s antisuicidal properties, illustrated by 416 additional weight gain–induced deaths counteracted by saving 492 per every 100,000 patients from suicide.14
Because of the paucity of alternative treatments, the discontinuation of clozapine remains an issue of utmost importance. Almost half of the patients who abruptly discontinue clozapine treatment experience rapid psychotic deterioration,15 exposing some of them to an increased risk of suicide.16 With rates of up to 65%,17 discontinuation
is highest during the first year of treatment, but there is
evidence of a 2- to 3-fold variation from study to study. There is growing evidence that psychiatrists’ lack of experience with clozapine contributes to the high discontinuation rate.16–20 A recent study17 found that medical reasons accounted for 20% of discontinuation decisions and cited as the most common reasons seizure (45.5%), severe constipation (36.4%), somnolence (27.3%), and neutropenia (18.2%). A cause for concern is that the literature provides consistent evidence that the overwhelming majority of decisions to discontinue clozapine treatment are made on the basis of side effects, such as leukocytosis, seizures, and fever,16–20 which, under normal circumstances, can be managed and should not lead to such a decision.
As strategies for counteracting many of the troublesome side effects are available,21 it is highly likely that the termination of treatment is unwarranted in many cases. A lack of experience with clozapine seems to often explain why many psychiatrists are unaware of these strategies22 and unnecessarily discontinue clozapine treatment, thus depriving patients of what could be the only effective intervention for them. Limited evidence combined with inexperience in using known remedies for side effects may thus jeopardize patients’ overall well-being.
While the complexities of clozapine treatment, including potentially life-threatening adverse events, may cause doctors to refrain from reintroducing clozapine after witnessing serious side effects, several case reports23 document that a rechallenge may offer patients another chance to benefit from clozapine treatment. However, the rarity of life-threatening side effects, on the one hand, and the prospect of increased risk of death following rechallenge, on the other hand, explain why this option has received only scant attention. The severe clinical consequences of discontinuation of clozapine treatment and the difficult questions facing psychiatrists have prompted this clinical overview, which aims to help psychiatrists handle the most common pitfalls in treatment with clozapine, particularly with regard to decisions on the discontinuation or continuation of clozapine treatment.
METHOD
We conducted a structured search of PubMed and EMBASE from database inception until September 10, 2012, without any language restrictions. References were imported to EndNote X4 (Thomson Reuters, New York, New York), and duplicates were removed. On the basis of review of discontinuation studies12,20,24 and the authors’ clinical experience, the following side effects were considered important for this review as potential grounds for discontinuation of clozapine treatment: neutropenia or agranulocytosis, leukocytosis, thrombocytopenia, thrombocytosis, eosinophilia, leukocytosis, QTc prolongation, electrocardiogram changes, atrial flutter, tachycardia, myocarditis, cardiomyopathy, fever, syncope, diabetes mellitus, diabetic ketoacidosis, diabetic hyperosmolar coma, neuroleptic malignant syndrome, ileus, liver enzyme elevation, or seizure. Although merely bothersome side effects may also have led to discontinuation, these are not considered here. The search term we used was clozapine because searches using the above-mentioned side effects in conjunction with clozapine did not retrieve enough articles.
Included in this review are studies that reported on the frequency of the specified clozapine-related adverse effects and that either reported on the grounds for or against clozapine discontinuation or reported on management techniques to maintain patients on clozapine or enable successful clozapine rechallenge. Review articles and meta-analyses were given precedence over case series or individual studies. Case reports were excluded unless they provided important new evidence that was not contained in prior studies or reviews. Manuscripts were reviewed manually for relevance. Reference lists of the retrieved journal articles were cross-checked for additional relevant studies.
RESULTS
Of a total of 13,385 search results, data from 81 studies were included in this review. The remaining studies were excluded upon title or abstract review or full text review because they did not provide specific information for the topic of this review. A summary of potential reasons for discontinuation of clozapine and of justifications and management techniques detailed in the text below is provided in Tables 1–4.
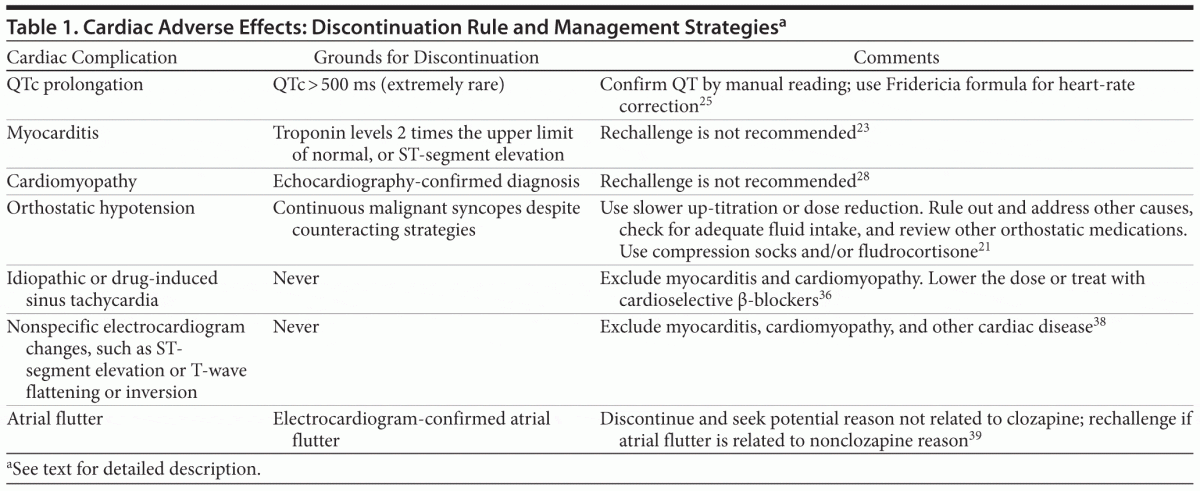
Cardiac Adverse Effects (Table 1)
QTc prolongation. Prolongation of the QTc interval to levels > 500 milliseconds has been associated with an increased risk of developing life-threatening polymorphic ventricular arrhythmia, or torsade de pointes. The QTc interval is a measure of the time from the beginning of ventricular depolarization to the end of repolarization obtained from surface electrocardiogram.25 However, since a heart rate elevation has the effect of narrowing the QT interval, a correction formula is used.
While a blockage of the fast rectifier potassium channel causes many antipsychotics to prolong the QTc interval25,26 (an effect most pronounced for ziprasidone and sertindole, with mean prolongation up to 20 milliseconds), QTc intervals above 500 milliseconds that should lead to discontinuation are rarely seen during clozapine treatment. In fact, the extent to which clozapine is capable of prolonging the QTc interval remains largely unknown, as no studies have taken account of 2 important pitfalls: (1) tachycardia is common during treatment with clozapine and (2) the Bazett formula is valid only for heart rates below 80 bpm; since most machines use this formula for correcting the QT interval, overcorrections are likely to occur.25
For heart rates exceeding 80 bpm, the Fridericia formula offers a more reliable correction.25 Two sets of scores illustrate the widening gap between the results for the 2 correction formulas: for a QT interval of 420 milliseconds, corrected figures of 454 milliseconds and 442 milliseconds are elicited at a heart rate of 70 bpm using the Bazett and Fridericia formulas, respectively. At the elevated heart rate of 90 bpm, the corresponding figures are 514 milliseconds and 481 milliseconds. The conclusion is that, very likely, a high number of cases with a QTc > 500 milliseconds during treatment with clozapine represents an artifact of the Bazett formula rather than the Fridericia formula being employed.
Another pitfall in clozapine treatment is presented by occasional changes in nonspecific T-wave morphology, in particular, flattening.27 This occurrence complicates both automatic and manual reading of the QT interval and is best controlled for by using manual readings based on a determination of the QT interval in the lead where the T-wave exhibits the greatest amplitude.25 Termination of clozapine treatment on the grounds of QTc prolongation is rarely
warranted; other causes of prolongation, such as concomitant medications or unrecognized cardiac disease, should therefore be sought.
Myocarditis. The absolute risk of clozapine-induced myocarditis is 0.015%–0.188%.28 Myocarditis occurs within the first months of treatment and is diagnosed by ST-segment elevation and tachycardia using an electrocardiogram or by increased troponin levels (troponin levels twice the upper limit of normal warrant discontinuation).29 Flu-like symptoms, fever, fatigue, and dyspnea are the most common symptoms.28 A recent study found that, in most cases, patients had no symptoms of myocarditis before death, which raises the question of whether weekly monitoring of troponin levels is warranted.29,30 Clozapine should be discontinued immediately in case of myocarditis, and rechallenge is not recommended.23 Although, in a report31 of 4 cases, clozapine rechallenge was successful in 3 patients, a publication bias is likely. Moreover, with the addition of 8 additional cases published recently,32 4 of which were successful, clozapine rechallenge would still not be recommended according to the metrics published by Manu et al23 for recommending rechallenge.
Cardiomyopathy. Clozapine-induced cardiomyopathy is a serious medical condition. Typically, it is of dilated type, and sinus tachycardia may be accompanied by fatigue, dyspnea, and tachypnea.28 Electrocardiogram signs are typically nonspecific but frequently include T-wave and P-wave abnormalities and signs of left ventricular hypertrophy. A cardiomyopathy diagnosis confirmed by echocardiography should lead to prompt discontinuation of clozapine.
Orthostatic hypotension. Especially during up-titration of clozapine, orthostatic hypotension may cause problems. The mechanism is extensive antagonism at the noradrenergic α1 receptor and is probably exacerbated by the sedating properties of clozapine.21 Slower up-titration may solve the problem, but if orthostatic hypotension is present in long-term treatment, patients are advised to drink plenty of fluid and eat a salt-containing diet. If this recommendation does not solve the problem, compression socks and/or fludrocortisone may help.33,34
Sinus tachycardia. Sinus tachycardia is a frequent side effect of clozapine. It is most pronounced at the start of treatment and during the up-titration phase. Clozapine-induced sinus tachycardia is usually harmless but could be the first sign of life-threatening conditions, such as myocarditis,
cardiomyopathy, or neuroleptic malignant syndrome. Newly occurring sinus tachycardia in a patient who has been in stable and unchanged treatment for at least a month should raise suspicion of cardiomyopathy.
Dose reduction should be attempted in case of idiopathic sinus tachycardia; if this attempt proves ineffective or if psychotic symptoms preclude dose reduction, a cardioselective β-blocker may be tried after cardiomyopathy and myocarditis, as well as neuroleptic malignant syndrome, have been ruled out.35 Treatment with a β-blocker should not be initiated within the first months of clozapine treatment because tolerance to the heart-rate inducing effect of clozapine is likely to develop after 4 to 6 weeks of treatment.36 Moreover, the use of β-blockers may complicate the diagnosis of myocarditis. Ivabradine may also be used in case of lack of response to or intolerability of β-blockers.37 In conclusion, idiopathic or drug-induced sinus tachycardia should not lead to clozapine discontinuation.
Other cardiac complications. Nonspecific electrocardiogram changes, such as ST-segment elevation, T-wave flattening, or T-wave inversion, have been reported during treatment with clozapine in which no connection to cardiac disease was found. Provided that cardiac disease can be excluded, such symptoms appear to be of no clinical importance.27,38 Atrial flutter of unknown origin has also been reported and should lead to the discontinuation of clozapine or relevant medical treatment.39
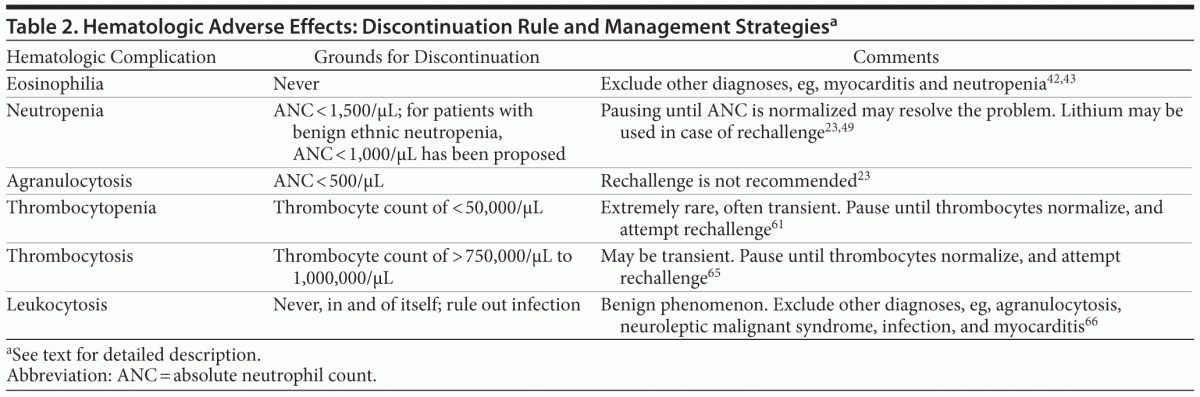
Hematologic Adverse Effects (Table 2)
Eosinophilia. An increase in eosinophil production may indicate allergic reactions and is therefore considered to be a predictor of other clozapine-induced side effects such as myocarditis,11 agranulocytosis,40 and toxic hepatitis.41 Clozapine-induced eosinophilia is not predictive of other side effects, but, since many of these potentially life-threatening side effects are accompanied by concomitant eosinophilia, a thorough examination should be performed.42 Eosinophilia (eosinophil level > 3,000/μL) is usually transient and occurs mainly during the first year of treatment. The manufacturer of clozapine recommends discontinuation and eventually restarting when the level of eosinophils is below 1,000/μL.43 Continuous eosinophilia (> 3,000/μL) is extremely rare, and the consequences remain unclear. It is our opinion that the potential consequences of discontinuation of clozapine outweigh this risk if all other medical conditions are excluded.43
Neutropenia or agranulocytosis. Agranulocytosis indicates a condition in which the absolute neutrophil count is below 500/μL, whereas neutropenia is characterized by absolute neutrophil counts between 500/μL and 1,500/μL.44 The risk of agranulocytosis is 0.7%; the risk of neutropenia is approximately 3%. The current mandatory monitoring system has made fatal agranulocytosis extremely rare, with incidences as low as 0%–0.03%.19 Occurrence of neutropenia typically provokes concern that the absolute neutrophil count will continue to drop and reach the agranulocytosis level, a development that is unpredictable. As clozapine treatment is generally terminated for safety reasons, the elucidation of this question is difficult. As a practical but unreliable rule of thumb, it can be assumed that neutropenia can occur at any time during treatment with clozapine, whereas agranulocytosis tends to develop predominantly during the first 6 months of treatment.17,45–48 The problem is particularly pronounced with the so-called leukocyte and granulocyte “dips,” which can occur at any time during treatment with clozapine. Although the absolute neutrophil count may have been stable for months to years, it may suddenly drop and reach neutropenia levels, in which case clozapine should be interrupted. The treatment may be resumed under increased surveillance when the absolute neutrophil count returns to normal levels.49
Moreover, clinicians should be aware of the potential for morning pseudoneutropenia, a physiologic state that can be attributed to increased neutrophil demargination as plasma cortisol levels reach their morning peak. However, morning pseudoneutropenia is no cause for concern; blood sampling should merely be transferred to midmorning50 or the afternoon.51 In patients of African and Middle Eastern descent, benign ethnic neutropenia may be present with a low baseline count, a biological variation in which increased infection rates have not been recorded.52 In general, a low baseline absolute neutrophil count is a risk factor for neutropenia but not for agranulocytosis.19 In Great Britain, adjusted thresholds have been proposed and used for neutropenia in patients with benign ethnic neutropenia that are 500/μL lower than in the general guidelines, recommending clozapine discontinuation only if the absolute neutrophil count is < 1,000/μL.52 However, severe cases of ethnic neutropenia may require concomitant treatment with granulocyte colony–stimulating factor, and a long-acting preparation of pegfilgrastim administered subcutaneously every 14 days has been shown to be effective.53
Lithium may be used to raise the neutrophil count in patients with a history of neutropenia as it stimulates the bone marrow and is a frequent cause of leukocytosis.54 The dosage should be therapeutic, ie, > 0.4 mmol/L (12-hour value); after administration, a period of at least 1 to 2 weeks should follow before the reinitiation of clozapine, if it was deemed necessary to discontinue clozapine. Despite the widespread use of this regimen, no investigation of its effect on infection rate has been undertaken. Lithium inhibits myeloperioxidase, which may reduce the aggressiveness of neutrophils, thereby masking the problem.55 Despite this fact, several studies report the successful use of lithium to boost neutrophils, and it is a recommended strategy in case of leukopenia.23,56,57 Other bone marrow–stimulating drugs, such as filgrastim, have also been tried successfully.58
Agranulocytosis should always lead to prompt discontinuation of clozapine. Rechallenge should not be attempted in patients with a history of agranulocytosis.23,54
Some patients have their clozapine treatment discontinued because they refuse to follow the hematologic monitoring. After 6 months of treatment, the risk of agranulocytosis with clozapine is similar to other drugs used in psychiatry, such as mianserin. For this reason, and if the patient accepts the potential risk, an option may be to accept longer intervals between blood sampling or to draw blood only whenever an infection is suspected.59 Another option may be to introduce hematologic monitoring with a point-of-care device, which may facilitate continuous monitoring on-site using capillary blood.60
Thrombocytopenia. Low thrombocyte counts may occur in isolation or accompanied by other cell line changes.61,62 The sequelae of thrombocytopenia are petechiae and increased risk of bleeding. Clozapine-induced thrombocytopenia
is usually transient and rarely merits a discontinuation of
clozapine. Persistent thrombocytopenia is rare.63 Thrombocyte counts below 50,000/μL should lead to discontinuation. The problem may be resolved by pausing until values return to a normal level. Lithium may also increase the thrombocyte count.64 It is currently being debated whether mandatory thrombocyte monitoring should be introduced.61
Thrombocytosis. While extremely rare, elevated thrombocyte counts have been reported during clozapine treatment.65 Although thrombocytosis predisposes to thrombosis, mild, transient thrombocytosis should not lead to discontinuation, but thrombocyte levels > 750,000/μL to 1,000,000/μL
warrant the discontinuation of clozapine.
Leukocytosis. Paradoxically, clozapine may cause not only leukopenia, but also leukocytosis. The sparse work done in relation to leukocytosis has failed to uncover the underlying mechanism, but the condition is viewed as benign in relation to clozapine treatment and should not lead to discontinuation.66–68 An unreplicated study found an increased risk of acute myeloid leukemia associated with clozapine, but the incidence was extremely low.69 Obviously, clinicians should rule out any non–clozapine-related reason for the elevated leukocyte count, including neuroleptic malignant syndrome (see later section on neuroleptic malignant syndrome), but, if there is none, then leukocytosis should not lead to clozapine discontinuation. Leukocytosis occurs very often transiently in the first weeks of treatment, and, in this period, additional medical examination is usually not warranted in comparison to the development of leukocytosis after years of treatment.
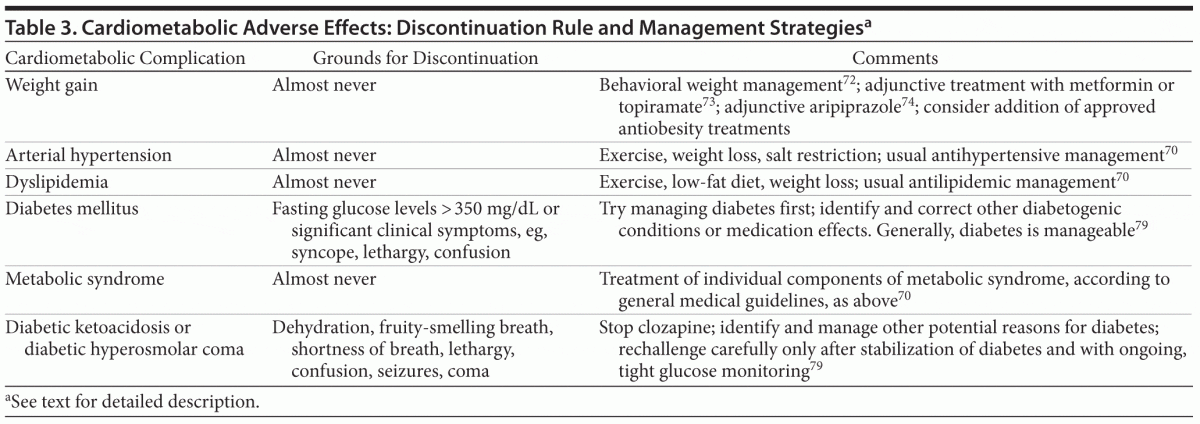
Cardiometabolic Adverse Effects (Table 3)
Weight gain, arterial hypertension, dyslipidemia, and metabolic syndrome. Clozapine is among the antipsychotics with the strongest liability for weight gain and lipid and glucose abnormalities. Although most antipsychotics can lower blood pressure via blockade of α1 adrenergic receptors and although clozapine can be associated with orthostatic hypotension (as described in the section on cardiac adverse effects), the often-severe weight gain can offset this effect, raising the risk for elevated blood pressure over time. Clozapine is among the antipsychotics with the greatest risk for the development of metabolic syndrome, a constellation of at least 3 of the following 5 cardiovascular risk factors: abdominal obesity, arterial hypertension, hypertriglyceridemia, low high-density lipoprotein cholesterol, and elevated blood glucose.70
Metabolic syndrome or its components should generally not lead to clozapine discontinuation. Although these cardiometabolic risk factors are of clinical concern, they should each and all be managed appropriately during ongoing clozapine treatment by following general medical guidelines. Healthy lifestyle guidance should be offered as early as possible when initiating clozapine treatment, but clearly when cardiometabolic side effects emerge.71 Since weight gain and metabolic abnormalities may be at least to some degree dose-related,70,71 the lowest effective clozapine dose should be used. Behavioral weight-management treatment72 should be initiated when cardiometabolic adverse effects worsen. When behavioral interventions fail or patients cannot adequately comply with them, adjunctive treatment with metformin or topiramate73 or with aripiprazole74 should be considered. These options have the best evidence base for weight loss in patients treated with clozapine. Novel approved drugs for obesity, such as lorcaserin, the combination of phentermine plus topiramate, or naltrexone plus bupropion, could also be attempted, although no data currently exist for patients with antipsychotic-induced weight gain and metabolic abnormalities.
If arterial hypertension, dyslipidemia, or hyperglycemia emerge, common medical guidelines should be followed for the management of these conditions, which should generally be managed by a medical specialist in conjunction with
the psychiatric health care team.70
Diabetes mellitus, diabetic ketoacidosis, and diabetic hyperosmolar coma. Diabetes mellitus is a potential adverse effect of antipsychotic agents, but clozapine has been most frequently associated with this potential complication.70,71,75,76 In addition to long-term diabetogenic effects of increased body weight and adiposity, clozapine seems to have unique molecular effects that increase the risk for diabetes mellitus, possibly mediated via muscarinic M3 receptor blockade decrease of cholinergic-dependent and glucose-dependent insulin secretion from pancreatic
β cells.70 However, it is sometimes difficult to determine the magnitude of the mediation effect, as most patients who develop diabetes while taking clozapine have one or more risk factors for diabetes, including a diagnosis of schizophrenia, family history of diabetes mellitus, advanced age, body mass index of ≥ 25 (calculated as kg/m2), dyslipidemia, central obesity, gestational diabetes, or nonwhite ethnicity.70,76–78 Since diabetes can be managed medically, diabetes mellitus development should not automatically lead to clozapine discontinuation. Rather, clinicians should identify and address other diabetogenic conditions (eg, infection, obesity, hyperthyroidism) or medication effects (eg, β-blockers, steroids) and coordinate diabetes care in collaboration with medical specialists. If fasting glucose levels are too high (ie, > 350 mg/dL) or if significant clinical symptoms develop (eg, dizziness, syncope, lethargy, confusion) that cannot be managed acutely with rehydration and lowering of blood sugar, clozapine may need to be held or discontinued until adequate glucose control has been achieved. However, clozapine should generally be reintroduced with appropriate antidiabetic management and close glucose monitoring.79
Diabetic ketoacidosis and diabetic hyperosmolar coma are very rare adverse effects of antipsychotics, but clozapine has been the agent most likely to be associated with this potentially life-threatening effect.78 This side effect can either occur suddenly early in treatment or be a late sign of longer-standing diabetes that was unrecognized and untreated.77,80 Patients present with dehydration, fruity-smelling breath, shortness of breath, lethargy, and confusion. In more severe forms, patients can develop seizures, coma, and death. Clozapine should be discontinued immediately, and the diabetic condition should be managed appropriately in an acute-care setting. Careful reintroduction of clozapine can be attempted after sustained control of diabetes and removal of potentially diabetogenic medications or treatment of diabetogenic comorbidities as listed above—and with careful glucose monitoring in place.
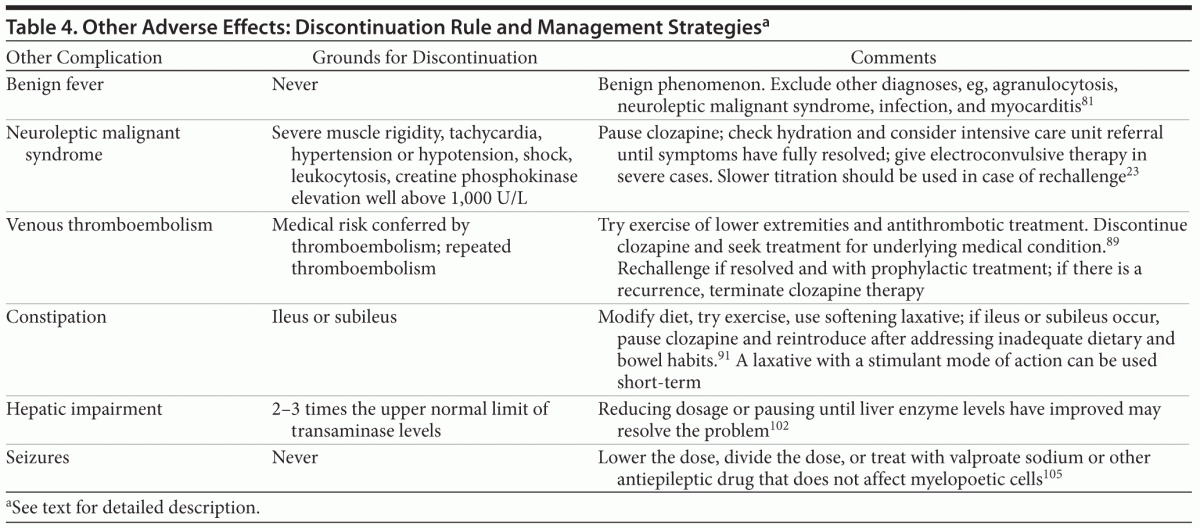
Other Adverse Effects (Table 4)
Benign hyperthermia. The benign fever and flu-like symptoms, or hyperthermia, encountered by up to 55% of patients during the first month of clozapine treatment81 are explained by an increase in pyrogens and bear no relation to the long-term tolerability of clozapine.82 While the fever may be caused by several other conditions, psychiatrists tend to focus on excluding agranulocytosis through white blood cell counts and absolute neutrophil counts, leading to a risk of missing other potentially life-threatening side effects. There are a variety of immunologic conditions, such as pancreatitis,83 polyserositis,84 colitis,85 and myocarditis,11 of which the latter in particular may produce symptoms resembling benign hyperthermia. Symptoms of any of these conditions should always lead to abrupt discontinuation of clozapine. Therefore, all patients with fever who are in their first month of clozapine treatment should be thoroughly examined; examinations should include white blood cell and absolute neutrophil counts, electrocardiogram, and troponin enzyme level measurements. Differential diagnoses for benign hyperthermia should also include neuroleptic malignant syndrome. The possibility of infection should also be considered, as patients treated with clozapine may be more prone to infections.86
Neuroleptic malignant syndrome. Neuroleptic malignant syndrome is a rare but potentially life-threatening condition associated with clozapine treatment and presents with autonomic instability (ie, tachycardia, hypertension or hypotension, shock), altered consciousness, fever, leukocytosis, and elevated creatine phosphokinase levels well above 1,000 U/L, as well as often-severe parkinsonism. However, parkinsonism is not always present during neuroleptic malignant syndrome that is induced by atypical antipsychotics.87,88 Pausing clozapine and hydrating until symptoms have fully resolved is the treatment of choice; electroconvulsive therapy can also be helpful in severe and refractory cases. However, rechallenge can be attempted, with slower titration of clozapine recommended.21
Venous thromboembolism. Treatment with antipsychotics seems to increase the risk of venous thromboembolism, with clozapine causing the highest risk.89 The mechanism remains largely unknown, and, because most of the studies have not been able to control for additional risk factors, such as obesity, lack of exercise, and smoking, it remains unclear whether the increased risk is a direct effect of the drug or is due to the effect of additional risk factors that are present in patients receiving antipsychotic drugs. Patients who are experiencing thromboembolism should receive traditional medical treatment and prophylactic measures, such as antithrombotic treatment. Whether clozapine should be discontinued needs to be decided by balancing the expected consequences of discontinuing or continuing clozapine treatment. Patients should be involved in this discussion. A recurrence of thromboembolism despite prophylactic treatment should always lead to clozapine discontinuation.
Constipation. Clozapine possesses extensive anticholinergic properties in combination with sedative properties, constituting a serious risk factor for constipation and, subsequently, ileus.90 Clozapine-induced ileus may be fatal, and, in fact, more deaths are caused by clozapine-induced ileus than by agranulocytosis.13 Patients should be asked weekly during up-titration and every month during maintenance treatment about their defecation frequency, and a frequency of less than 4 to 5 times per week should lead to intervention. A high-fiber diet, plenty of fluid, and exercise are often ineffective in clozapine-induced constipation, and often a softening laxative is warranted.91,92 It remains controversial whether a laxative with a stimulant mode of action can be used safely during long-term treatment because of risk of cathartic colon.93
Hepatic impairment. Clozapine has been associated with several types of hepatic impairment ranging from asymptomatic increases in liver function tests to fulminant liver failure with hepatic encephalopathy.94–96 Up to 40% of clozapine patients experience alanine transaminase levels above 2 times the upper limit of normal97; icteric hepatitis is observed in only 0.06% of clozapine patients.94 Abnormal liver function tends to occur during the first months of treatment but has proven to be transient in 60% of cases.97 Hepatitis tends to occur during the same period and is reversible if clozapine is terminated in a timely manner.41,96 Drug-induced hepatic impairment is usually a diagnosis of exclusion, the causality of which is difficult to determine.98 Clozapine-induced hepatic impairment has received scant attention in the literature, and no clear recommendations can be given. The routine measurement of liver function enzymes has occasionally been recommended94,99,100 but involves a risk of discontinuation based on a false indication. Clinical symptoms, such as rash, jaundice, or malaise, should always prompt the measurement of liver function. Although 2-fold and 3-fold increases in transaminase levels should not lead to discontinuation, careful monitoring is recommended. Increases in transaminase levels beyond 3 times the normal upper limit usually warrant discontinuation, but the temporary disruption of treatment while the liver function is subnormal may overcome the problem. Dose reduction may likewise be helpful, as there seems to be a dose dependency of the hepatic effects.36,97 Cases of successful rechallenge in patients with a marked elevation of liver enzyme levels have been described.101,102
Seizures. Clozapine lowers the seizure threshold, and dose-dependent fluctuation in electroencephalographic activity and risk of seizure are seen.103,104 Clozapine doses above 600 mg/d cause seizures in 4.4% of patients as compared to only 2.7% and 1.0% of patients receiving 300–600 mg/d and < 300 mg/d, respectively.103 A careful analysis105 of 101 clozapine-induced seizures indicated paroxysmal tonic-clonic activity in 67 patients, myoclonic seizures in 27 patients, atonic seizure in 1 patient, and partial simple or partial complex seizures in 6 patients. The mean daily dosage of clozapine at the time of the seizure ranged from 275 mg in patients with partial complex seizure to 535 mg in patients with myoclonic activity.105 A 50% dose reduction and assessment of other causes for the decreased seizure threshold (eg, drug toxicity or withdrawal of benzodiazepines or antiepileptics, electrolyte abnormalities, diabetic ketoacidosis, organic brain disorders, or sleep deprivation) must follow the first seizure. Seizures or electroencephalographic activity indicating an epileptiform focus should not lead to discontinuation; either a reduction in dosage or a division of dosage (if administered once daily), or treatment with antiepileptic medication such as valproate sodium,92 is recommended. Lamotrigine,106 gabapentin,107 and other antiepileptics have also been tried with success. Refractory epilepsy is also not an absolute contraindication to initiating clozapine.108
DISCUSSION
The evidence for handling the discussed side effects is sparse and is based largely on published case reports and rational deduction. Psychiatrists should be aware that discontinuing clozapine may have severe consequences, such as psychotic relapse and suicidal behavior, which underscores the importance of psychiatrists’ knowledge of appropriate strategies for managing clozapine side effects and of their ability to determine when it is safe to continue clozapine. It is noteworthy that the discontinuation cases described in many of the studies have since been shown to be prompted by unwarranted alarm over relatively harmless side effects.
As the mechanisms behind many of these side effects are largely unknown, it is difficult to determine the best time to discontinue clozapine treatment. Many of the side effects, eg, abnormal liver function and neutropenia, are often transient and benign in nature, whereas, in other cases, the effects can progress to life-threatening conditions, such as liver failure or agranulocytosis. Unfortunately, the course of these side effects is unpredictable, leaving the psychiatrist with no other option than to discontinue clozapine treatment and then reinitiate clozapine when parameters have stabilized. Other side effects are more clearly handled because discontinuation is the only option (eg, agranulocytosis, myocarditis, cardiomyopathy) or because the effects are harmless and should not lead to discontinuation (eg, benign hyperthermia, benign leukocytosis or neutrophilia, eosinophilia, idiopathic sinus tachycardia, seizures).
Many of the side effects are idiosyncratic, with no dose dependency, such as agranulocytosis and myocarditis, whereas some dose dependency exists for others, such as tachycardia and seizure. For the latter, it is important to remember that clozapine is susceptible to several pharmacokinetic interactions that may change plasma levels and contribute to toxic clozapine levels. Smoking cessation is the most important one because of the high prevalence of smoking among patients with schizophrenia. Plasma clozapine levels may in extreme cases increase by 80% and have in some cases caused seizures.109 Infection and inflammation can also cause extreme plasma levels.110,111 In these conditions, dose reduction will solve the problem. Furthermore, the addition of medications that are strong cytochrome P450 inhibitors, such as fluvoxamine or ketoconazole, can also significantly increase clozapine levels.
When discontinuation of clozapine has been decided, the dosage should be tapered off over a period of weeks to months, depending on variables such as size of the dosage, psychotic symptoms, and the duration of treatment. Only in cases of agranulocytosis, myocarditis, or other severe medical conditions should treatment be terminated abruptly, as abrupt discontinuation increases the risk of rebound psychosis and anticholinergic withdrawal symptoms. The latter can be avoided by adding an anticholinergic drug as a temporary substitution, followed by a gradual tapering of the clozapine dose.15 Guidance on the best antipsychotic drug replacement is more difficult, as clozapine patients have typically failed to respond to several other antipsychotic drugs. Some case reports112 of agranulocytosis and neutropenia have raised concern that replacement with olanzapine could prolong the neutropenia period due to olanzapine’s chemical similarity with clozapine, while other case reports113 have demonstrated that olanzapine could be used safely during clozapine-induced agranulocytosis. In conclusion,
olanzapine currently seems to have the best evidence for preventing rebound psychosis or withdrawal psychosis.
This review has several limitations. Foremost, high-quality data on specific side effects, risk factors, and the most effective management strategies are scarce. Most of the cited evidence is based on small samples, uncontrolled observations, and clinical guidelines rather than randomized controlled trials or population-based register studies. Furthermore, this overview is selective, as it was intended to cover only the most common pitfalls of clozapine therapy. Therefore, it should be stressed that additional conditions not covered in this review may occur, in which case psychiatrists are recommended to seek assistance from colleagues who have greater experience with clozapine, from the published literature, or from physicians in the corresponding medical specialties.
Clearly, since clozapine is such a valuable resource for a substantial subgroup of treatment-refractory patients with psychotic disorders, further study of the development,
trajectory, and best management of serious clozapine-related side effects and of appropriate grounds for clozapine discontinuation is warranted.
Drug names: aripiprazole (Abilify), bupropion (Wellbutrin, Aplenzin, and others), clozapine (Clozaril, FazaClo, and others), fluvoxamine (Luvox and others), gabapentin (Neurontin, Gralise, and others), ketoconazole (Ketozole, Nizoral, and others), lamotrigine (Lamictal and others), lithium (Lithobid and others), lorcaserin (Belviq), metformin (Glucophage, Fortamet, and others), naltrexone (Vivitrol, ReVia, and others), olanzapine (Zyprexa and others), phentermine (Adipex-P, Suprenza, and others), topiramate (Topamax and others), valproate sodium (Depacon and others), ziprasidone (Geodon and others).
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, aripiprazole, bupropion, and topiramate are not approved by the US Food and Drug Administration for minimizing clozapine-induced weight gain, and lithium is not approved for increasing absolute neutrophil count.
Author affiliations: Centre for Schizophrenia, Aalborg Psychiatric Hospital, Aalborg University Hospital, Aalborg, Denmark (Dr Nielsen); Division of Psychiatry Research (Dr Correll), Department of Medical Services
(Dr Manu), and Department of Psychiatry (Dr Kane), The Zucker Hillside Hospital, Glen Oaks, New York; Departments of Psychiatry and Molecular Medicine (Drs Correll and Kane) and Department of Medicine (Dr Manu), Hofstra North Shore–Long Island Jewish School of Medicine, Hempstead, New York; and Universitatea Transilvania, Brasov, Romania (Dr Manu).
Financial disclosure: Dr Nielsen has received research support from Pfizer and has received speakers or advisory board honoraria from AstraZeneca, Bristol-Myers Squibb, HemoCue, and Lundbeck. Dr Correll has received grant/research support from Bristol-Myers Squibb, Ortho-McNeil/Janssen/Johnson & Johnson, and Otsuka; has been a consultant to Alexza, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Genentech, Gerson Lehrman Group, Intra-Cellular Therapies, Lundbeck, MedAvante, Pfizer, Otsuka, Roche, Takeda, Teva, and Vanda; has been a member of the speakers or advisory boards for Actelion, Alexza, AstraZeneca, Bristol-Myers Squibb, Intra-Cellular Therapies, MedAvante, Merck, Novartis, Otsuka, Pfizer, and Sunovion; and has received honoraria from Bristol-Myers Squibb, Cephalon, Ortho-McNeil/Janssen/Johnson & Johnson, Medscape, Otsuka, ProPhase, Takeda, Teva, and Vanda. Dr Kane has received grant support from the National Institute of Mental Health; has been a consultant to Alkermes, Amgen, Bristol-Myers Squibb, Eli Lilly, Intra-Cellular Therapies, Janssen, Johnson & Johnson, Lundbeck, Merck, Novartis, Otsuka, Pfizer, Pierre Fabre, Proteus, Roche, and Sunovion; and is a stock shareholder of and has received honoraria from MedAvante. Dr Manu has no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
Funding/support: None reported.
REFERENCES
1. Nielsen J, Nielsen RE, Correll CU. Predictors of clozapine response in patients with treatment-refractory schizophrenia: results from a Danish Register Study. J Clin Psychopharmacol. 2012;32(5):678–683. doi:10.1097/JCP.0b013e318267b3cd PubMed
2. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. a double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789–796. doi:10.1001/archpsyc.1988.01800330013001 PubMed
3. Azorin JM, Spiegel R, Remington G, et al. A double-blind comparative study of clozapine and risperidone in the management of severe chronic schizophrenia. Am J Psychiatry. 2001;158(8):1305–1313. doi:10.1176/appi.ajp.158.8.1305 PubMed
4. Conley RR, Kelly DL, Richardson CM, et al. The efficacy of high-dose olanzapine versus clozapine in treatment-resistant schizophrenia: a double-blind crossover study. J Clin Psychopharmacol. 2003;23(6):668–671. doi:10.1097/01.jcp.0000096246.29231.73 PubMed
5. Leucht S, Corves C, Arbter D, et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;
373(9657):31–41. doi:10.1016/S0140-6736(08)61764-X PubMed
6. Meltzer HY, Alphs L, Green AI, et al; International Suicide Prevention Trial Study Group. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry. 2003;60(1):82–91. doi:10.1001/archpsyc.60.1.82 PubMed
7. Chengappa KN, Vasile J, Levine J, et al. Clozapine: its impact on aggressive behavior among patients in a state psychiatric hospital. Schizophr Res. 2002;
53(1–2):1–6. doi:10.1016/S0920-9964(00)00175-4 PubMed
8a. Ciapparelli A, Dell’Osso L, Bandettini di Poggio A, et al. Clozapine in treatment-resistant patients with schizophrenia, schizoaffective disorder, or psychotic bipolar disorder: a naturalistic 48-month follow-up study. J Clin Psychiatry. 2003;64(4):451–458. doi:10.4088/JCP.v64n0416 PubMed
8b. Nielsen J, Kane JM, Correll CU. Real-world effectiveness of clozapine in patients with bipolar disorder: results from a 2-year mirror-image study. Bipolar Disord. 2012;14(8):863–869. doi:10.1111/bdi.12018 PubMed
9. Pollack S, Woerner MG, Howard A, et al. Clozapine reduces rehospitalization among schizophrenia patients. Psychopharmacol Bull. 1998;34(1):89–92. PubMed
10. Angermeyer MC, Löffler W, Müller P, et al. Patients’ and relatives’ assessment of clozapine treatment. Psychol Med. 2001;31(3):509–517. doi:10.1017/S0033291701003749 PubMed
11. Merrill DB, Ahmari SE, Bradford JM, et al. Myocarditis during clozapine treatment. Am J Psychiatry. 2006;163(2):204–208. doi:10.1176/appi.ajp.163.2.204 PubMed
12. Taylor DM, Douglas-Hall P, Olofinjana B, et al. Reasons for discontinuing clozapine: matched, case-control comparison with risperidone long-acting injection. Br J Psychiatry. 2009;194(2):165–167. doi:10.1192/bjp.bp.108.051979 PubMed
13. Nielsen J, Meyer JM. Risk factors for ileus in patients with schizophrenia. Schizophr Bull. 2012;38(3):592–598. doi:10.1093/schbul/sbq137 PubMed
14. Fontaine KR, Heo M, Harrigan EP, et al. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res. 2001;101(3):277–288. doi:10.1016/S0165-1781(01)00234-7 PubMed
15. Seppälä N, Kovio C, Leinonen E. Effect of anticholinergics in preventing acute deterioration in patients undergoing abrupt clozapine withdrawal.
CNS Drugs. 2005;19(12):1049–1055. doi:10.2165/00023210-200519120-00006 PubMed
16. Krivoy A, Malka L, Fischel T, et al. Predictors of clozapine discontinuation in patients with schizophrenia. Int Clin Psychopharmacol. 2011;26(6):311–315. doi:10.1097/YIC.0b013e32834ab34c PubMed
17. Pai NB, Vella SC. Reason for clozapine cessation. Acta Psychiatr Scand. 2012;
125(1):39–44. doi:10.1111/j.1600-0447.2011.01776.x PubMed
18. Whiskey E, Wykes T, Duncan-McConnell D, et al. Continuation of clozapine treatment: practice makes perfect. Psychiatr Bull. 2003;27(6):211–213. doi:10.1192/pb.27.6.211
19. Munro J, O’Sullivan D, Andrews C, et al. Active monitoring of 12,760 clozapine recipients in the UK and Ireland: beyond pharmacovigilance.
Br J Psychiatry. 1999;175(6):576–580. doi:10.1192/bjp.175.6.576 PubMed
20. Moeller FG, Chen YW, Steinberg JL, et al. Risk factors for clozapine discontinuation among 805 patients in the VA hospital system. Ann Clin Psychiatry. 1995;7(4):167–173. doi:10.3109/10401239509149622 PubMed
21. Nielsen J, Damkier P, Lublin H, et al. Optimizing clozapine treatment.
Acta Psychiatr Scand. 2011;123(6):411–422. doi:10.1111/j.1600-0447.2011.01710.x PubMed
22. Nielsen J, Dahm M, Lublin H, et al. Psychiatrists’ attitude towards and knowledge of clozapine treatment. J Psychopharmacol. 2010;24(7):965–971. doi:10.1177/0269881108100320 PubMed
23. Manu P, Sarpal D, Muir O, et al. When can patients with potentially life-threatening adverse effects be rechallenged with clozapine? a systematic review of the published literature. Schizophr Res. 2012;134(2–3):180–186. doi:10.1016/j.schres.2011.10.014 PubMed
24. Lindström LH. The effect of long-term treatment with clozapine in schizophrenia: a retrospective study in 96 patients treated with clozapine for up to 13 years. Acta Psychiatr Scand. 1988;77(5):524–529. doi:10.1111/j.1600-0447.1988.tb05164.x PubMed
25. Nielsen J, Graff C, Kanters JK, et al. Assessing QT interval prolongation and its associated risks with antipsychotics. CNS Drugs. 2011;25(6):473–490. doi:10.2165/11587800-000000000-00000 PubMed
26. Nielsen J, Andersen MP, Graff C, et al. The effect of sertindole on QTD and TPTE. Acta Psychiatr Scand. 2010;121(5):385–388. doi:10.1111/j.1600-0447.2009.01534.x PubMed
27. Kang UG, Kwon JS, Ahn YM, et al. Electrocardiographic abnormalities in patients treated with clozapine. J Clin Psychiatry. 2000;61(6):441–446. doi:10.4088/JCP.v61n0609 PubMed
28. Merrill DB, Dec GW, Goff DC. Adverse cardiac effects associated with clozapine. J Clin Psychopharmacol. 2005;25(1):32–41. doi:10.1097/01.jcp.0000150217.51433.9f PubMed
29. Ronaldson KJ, Fitzgerald PB, Taylor AJ, et al. A new monitoring protocol for clozapine-induced myocarditis based on an analysis of 75 cases and 94 controls. Aust N Z J Psychiatry. 2011;45(6):458–465. doi:10.3109/00048674.2011.572852 PubMed
30. Ronaldson KJ, Fitzgerald PB, Taylor AJ, et al. Clinical course and analysis of ten fatal cases of clozapine-induced myocarditis and comparison with 66 surviving cases. Schizophr Res. 2011;128(1–3):161–165. doi:10.1016/j.schres.2011.01.017 PubMed
31. Bray A, Reid R. Successful clozapine rechallenge after acute myocarditis. Aust N Z J Psychiatry. 2011;45(1):90. doi:10.3109/00048674.2010.533365 PubMed
32. Ronaldson KJ, Fitzgerald PB, Taylor AJ, et al. Observations from 8 cases of clozapine rechallenge after development of myocarditis. J Clin Psychiatry. 2012;73(2):252–254. doi:10.4088/JCP.11l07467 PubMed
33. Testani M Jr. Clozapine-induced orthostatic hypotension treated with fludrocortisone. J Clin Psychiatry. 1994;55(11):497–498. PubMed
34. Low PA, Singer W. Management of neurogenic orthostatic hypotension:
an update. Lancet Neurol. 2008;7(5):451–458. doi:10.1016/S1474-4422(08)70088-7 PubMed
35. Stryjer R, Timinsky I, Reznik I, et al. Beta-adrenergic antagonists for the treatment of clozapine-induced sinus tachycardia: a retrospective study.
Clin Neuropharmacol. 2009;32(5):290–292. doi:10.1097/WNF.0b013e3181a620b2 PubMed
36. Marinkovic D, Timotijevic I, Babinski T, et al. The side-effects of clozapine: a four year follow-up study. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18(3):537–544. doi:10.1016/0278-5846(94)90010-8 PubMed
37. Rakovec P. Treatment of inappropriate sinus tachycardia with ivabradine. Wien Klin Wochenschr. 2009;121(21–22):715–718. doi:10.1007/s00508-009-1265-9 PubMed
38. Ketch J, Herd A, Ludwig L. ST segment elevations without myocardial infarction in a patient on clozapine. Am J Emerg Med. 1996;14(1):111–112. doi:10.1016/S0735-6757(96)90036-4 PubMed
39. Low RA Jr, Fuller MA, Popli A. Clozapine induced atrial fibrillation. J Clin Psychopharmacol. 1998;18(2):170. doi:10.1097/00004714-199804000-00010 PubMed
40. Amital D, Gross R, Amital H, et al. Coexistence of eosinophilia and agranulocytosis in a clozapine-treated patient. Br J Psychiatry. 1997;170:194. PubMed
41. Fong SY, Au Yeung KL, Tosh JM, et al. Clozapine-induced toxic hepatitis with skin rash. J Psychopharmacol. 2005;19(1):107. doi:10.1177/0269881105047287 PubMed
42. Ames D, Wirshing WC, Baker RW, et al. Predictive value of eosinophilia for neutropenia during clozapine treatment. J Clin Psychiatry. 1996;57(12):579–581. doi:10.4088/JCP.v57n1205 PubMed
43. Lucht MJ, Rietschel M. Clozapine-induced eosinophilia: subsequent neutropenia and corresponding allergic mechanisms. J Clin Psychiatry. 1998;59(4):195–197. doi:10.4088/JCP.v59n0409c PubMed
44. Duggal HS, Singh I. Psychotropic drug–induced neutropenia. Drugs Today (Barc). 2005;41(8):517–526. doi:10.1358/dot.2005.41.8.893629 PubMed
45. Duggal HS, Singh I. Re: late-onset neutropenia with clozapine [letter]. Can J Psychiatry. 2006;51(2):125. Author reply: 125–126. PubMed
46. Small JG, Weber MC, Klapper MH, et al. Rechallenge of late-onset neutropenia with clozapine. J Clin Psychopharmacol. 2005;25(2):185–186. doi:10.1097/01.jcp.0000155831.72154.bc PubMed
47. Thompson A, Girishchandra B, Castle D, et al. Late onset neutropenia with clozapine. Can J Psychiatry. 2004;49(9):647–648. PubMed
48. Tamam L, Kulan E, Ozpoyraz N. Late onset neutropenia during clozapine treatment. Psychiatry Clin Neurosci. 2001;55(5):547–548. doi:10.1046/j.1440-1819.2001.904(1).x PubMed
49. Hummer M, Kurz M, Barnas C, et al. Transient neutropenia induced by clozapine. Psychopharmacol Bull. 1992;28(3):287–290. PubMed
50. McKee JR, Wall T, Owensby J. Impact of complete blood count sampling time change on white blood cell and absolute neutrophil count values in clozapine recipients. Clin Schizophr Relat Psychoses. 2011;5(1):26–32. doi:10.3371/CSRP.5.1.4 PubMed
51. Esposito D, Chouinard G, Hardy P, et al. Successful initiation of clozapine treatment despite morning pseudoneutropenia. Int J Neuropsychopharmacol. 2006;9(4):489–491. doi:10.1017/S146114570500605X PubMed
52. Rajagopal S. Clozapine, agranulocytosis, and benign ethnic neutropenia. Postgrad Med J. 2005;81(959):545–546. doi:10.1136/pgmj.2004.031161 PubMed
53. Spencer BW, Williams HR, Gee SH, et al. Granulocyte colony stimulating factor (G-CSF) can allow treatment with clozapine in a patient with severe benign ethnic neutropaenia (BEN): a case report. J Psychopharmacol. 2012;26(9):1280–1282. doi:10.1177/0269881112450782 PubMed
54. Whiskey E, Taylor D. Restarting clozapine after neutropenia: evaluating the possibilities and practicalities. CNS Drugs. 2007;21(1):25–35. doi:10.2165/00023210-200721010-00003 PubMed
55. Flanagan RJ, Dunk L. Haematological toxicity of drugs used in psychiatry. Hum Psychopharmacol. 2008;23(suppl 1):27–41. doi:10.1002/hup.917 PubMed
56. Brunoni AR, Kobuti Ferreira LR, Gallucci-Neto J, et al. Lithium as a treatment of clozapine-induced neutropenia: a case report. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):2006–2007. doi:10.1016/j.pnpbp.2008.09.005 PubMed
57. Kutscher EC, Robbins GP, Kennedy WK, et al. Clozapine-induced leukopenia successfully treated with lithium. Am J Health Syst Pharm. 2007;64(19):2027–2031. doi:10.2146/ajhp060319 PubMed
58. Hägg S, Rosenius S, Spigset O. Long-term combination treatment with clozapine and filgrastim in patients with clozapine-induced agranulocytosis. Int Clin Psychopharmacol. 2003;18(3):173–174. doi:10.1097/01.yic.0000062800.74434.6c PubMed
59. Schulte PF. Risk of clozapine-associated agranulocytosis and mandatory white blood cell monitoring. Ann Pharmacother. 2006;40(4):683–688. doi:10.1345/aph.1G396 PubMed
60. Nielsen J, Thode D, Stenager E, et al. Hematological clozapine monitoring with a point-of-care device: a randomized cross-over trial. Eur Neuropsychopharmacol. 2012;22(6):401–405. doi:10.1016/j.euroneuro.2011.10.001 PubMed
61. Tharyan P. Is monitoring of platelets necessary during clozapine therapy? Indian J Psychiatry. 1999;41(3):263–264. PubMed
62. Mahendran R. Leukopenia and thrombocytopenia induced by clozapine. Hong Kong J Psychiatry. 2002;12(3):19–20.
63. Lambertenghi Deliliers G. Blood dyscrasias in clozapine-treated patients in Italy. Haematologica. 2000;85(3):233–237. PubMed
64. Focosi D, Azzarà A, Kast RE, et al. Lithium and hematology: established and proposed uses. J Leukoc Biol. 2009;85(1):20–28. doi:10.1189/jlb.0608388 PubMed
65. Hampson ME. Clozapine-induced thrombocytosis. Br J Psychiatry. 2000;176(4):400. doi:10.1192/bjp.176.4.400-a PubMed
66. Sopko MA, Caley CF. Chronic leukocytosis associated with clozapine treatment. Clin Schizophr Relat Psychoses. 2010;4(2):141–144. doi:10.3371/CSRP.4.2.6 PubMed
67. Madhusoodanan S, Cuni L, Brenner R, et al. Chronic leukocytosis associated with clozapine: a case series. J Clin Psychiatry. 2007;68(3):484–488. doi:10.4088/JCP.v68n0320b PubMed
68. Popli A, Pies R. Clozapine and leukocytosis. J Clin Psychopharmacol. 1995;15(4):286–287. doi:10.1097/00004714-199508000-00010 PubMed
69. Nielsen J, Boysen A. Clozapine treatment associated with increased risk of acute myeloid leukemia (AML). Schizophr Res. 2010;123(2–3):270–272. doi:10.1016/j.schres.2010.08.035 PubMed
70. De Hert M, Detraux J, van Winkel R, et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2012;8(2):114–126. doi:10.1038/nrendo.2011.156 PubMed
71. Correll CU, Lencz T, Malhotra AK. Antipsychotic drugs and obesity. Trends Mol Med. 2011;17(2):97–107. doi:10.1016/j.molmed.2010.10.010 PubMed
72. Caemmerer J, Correll CU, Maayan L. Acute and maintenance effects of non-pharmacologic interventions for antipsychotic associated weight gain and metabolic abnormalities: a meta-analytic comparison of randomized controlled trials. Schizophr Res. 2012;140(1–3):159–168. doi:10.1016/j.schres.2012.03.017 PubMed
73. Maayan L, Vakhrusheva J, Correll CU. Effectiveness of medications used to attenuate antipsychotic-related weight gain and metabolic abnormalities: a systematic review and meta-analysis. Neuropsychopharmacology. 2010;35(7):1520–1530. doi:10.1038/npp.2010.21 PubMed
74. Gallego JA, Nielsen J, De Hert M, et al. Safety and tolerability of antipsychotic polypharmacy. Expert Opin Drug Saf. 2012;11(4):527–542. doi:10.1517/14740338.2012.683523 PubMed
75. Nielsen J, Skadhede S, Correll CU. Antipsychotics associated with the development of type 2 diabetes in antipsychotic-naïve schizophrenia patients. Neuropsychopharmacology. 2010;35(9):1997–2004. doi:10.1038/npp.2010.78 PubMed
76. Henderson DC, Nguyen DD, Copeland PM, et al. Clozapine, diabetes mellitus, hyperlipidemia, and cardiovascular risks and mortality: results of a 10-year naturalistic study. J Clin Psychiatry. 2005;66(9):1116–1121. doi:10.4088/JCP.v66n0905 PubMed
77. Cohen D. Atypical antipsychotics and new onset diabetes mellitus: an overview of the literature. Pharmacopsychiatry. 2004;37(1):1–11. doi:10.1055/s-2004-815468 PubMed
78. Cohen D, Correll CU. Second-generation antipsychotic-associated diabetes mellitus and diabetic ketoacidosis: mechanisms, predictors, and screening need. J Clin Psychiatry. 2009;70(5):765–766. doi:10.4088/JCP.09ac05255 PubMed
79. Raja M. Clozapine safety, 35 years later. Curr Drug Saf. 2011;6(3):164–184. doi:10.2174/157488611797579230 PubMed
80. Henderson DC, Cagliero E, Copeland PM, et al. Elevated hemoglobin A1c as a possible indicator of diabetes mellitus and diabetic ketoacidosis in schizophrenia patients receiving atypical antipsychotics. J Clin Psychiatry. 2007;68(4):533–541. doi:10.4088/JCP.v68n0407 PubMed
81. Lowe CM, Grube RR, Scates AC. Characterization and clinical management of clozapine-induced fever. Ann Pharmacother. 2007;41(10):1700–1704. doi:10.1345/aph.1K126 PubMed
82. Tham JC, Dickson RA. Clozapine-induced fevers and 1-year clozapine discontinuation rate. J Clin Psychiatry. 2002;63(10):880–884. doi:10.4088/JCP.v63n1005 PubMed
83. Martin A. Acute pancreatitis associated with clozapine use. Am J Psychiatry. 1992;149(5):714. PubMed
84. Catalano G, Catalano MC, Frankel Wetter RL. Clozapine induced polyserositis. Clin Neuropharmacol. 1997;20(4):352–356. doi:10.1097/00002826-199708000-00007 PubMed
85. Friedberg JW, Frankenburg FR, Burk J, et al. Clozapine-caused eosinophilic colitis. Ann Clin Psychiatry. 1995;7(2):97–98. doi:10.3109/10401239509149034 PubMed
86. Nielsen J, Foldager L, Meyer JM. Increased use of antibiotics in patients treated with clozapine. Eur Neuropsychopharmacol. 2009;19(7):483–486. doi:10.1016/j.euroneuro.2009.03.002 PubMed
87. Nielsen J, Bruhn AM. Atypical neuroleptic malignant syndrome caused by olanzapine. Acta Psychiatr Scand. 2005;112(3):238–240. Discussion: 240. doi:10.1111/j.1600-0447.2005.00578.x PubMed
88. Baciewicz AM, Chandra R, Whelan P. Clozapine-associated neuroleptic malignant syndrome. Ann Intern Med. 2002;137(5 pt 1):374. PubMed
89. Jönsson AK, Spigset O, Hägg S. Venous thromboembolism in recipients of antipsychotics: incidence, mechanisms and management. CNS Drugs. 2012;26(8):649–662. doi:10.2165/11633920-000000000-00000 PubMed
90. Rondla S, Crane S. A case of clozapine-induced paralytic ileus. Emerg Med J. 2007;24(2):e12. doi:10.1136/emj.2006.042630 PubMed
91. Palmer SE, McLean RM, Ellis PM, et al. Life-threatening clozapine-induced gastrointestinal hypomotility: an analysis of 102 cases. J Clin Psychiatry. 2008;69(5):759–768. doi:10.4088/JCP.v69n0509 PubMed
92. Fitzsimons J, Berk M, Lambert T, et al. A review of clozapine safety.
Expert Opin Drug Saf. 2005;4(4):731–744. doi:10.1517/14740338.4.4.731 PubMed
93. Flanagan RJ, Ball RY. Gastrointestinal hypomotility: an under-recognised life-threatening adverse effect of clozapine. Forensic Sci Int. 2011;206(1–3):e31–e36. doi:10.1016/j.forsciint.2010.07.021 PubMed
94. Macfarlane B, Davies S, Mannan K, et al. Fatal acute fulminant liver failure due to clozapine: a case report and review of clozapine-induced hepatotoxicity. Gastroenterology. 1997;112(5):1707–1709. doi:10.1016/S0016-5085(97)70054-4 PubMed
95. Thatcher GW, Cates M, Bair B. Clozapine-induced toxic hepatitis.
Am J Psychiatry. 1995;152(2):296–297. PubMed
96. Luo D, McColl P, Walmsley R. Acute onset of ascites with clozapine-induced hepatitis. Intern Med J. 2007;37(3):204–205. doi:10.1111/j.1445-5994.2006.01300.x PubMed
97. Hummer M, Kurz M, Kurzthaler I, et al. Hepatotoxicity of clozapine.
J Clin Psychopharmacol. 1997;17(4):314–317. doi:10.1097/00004714-199708000-00012 PubMed
98. Stine JG, Lewis JH. Drug-induced liver injury: a summary of recent advances. Expert Opin Drug Metab Toxicol. 2011;7(7):875–890. doi:10.1517/17425255.2011.577415 PubMed
99. Berk M, Fitzsimons J, Lambert T, et al. Monitoring the safe use of clozapine: a consensus view from Victoria, Australia. CNS Drugs. 2007;21(2):117–127. doi:10.2165/00023210-200721020-00003 PubMed
100. Markowitz JS, Grinberg R, Jackson C. Marked liver enzyme elevations with clozapine. J Clin Psychopharmacol. 1997;17(1):70–71. doi:10.1097/00004714-199702000-00025 PubMed
101. Eggert AE, Crismon ML, Dorson PG, et al. Clozapine rechallenge after marked liver enzyme elevation. J Clin Psychopharmacol. 1994;14(6):425–426. doi:10.1097/00004714-199412000-00010 PubMed
102. Erdogan A, Kocabasoglu N, Yalug I, et al. Management of marked liver enzyme increase during clozapine treatment: a case report and review of the literature. Int J Psychiatry Med. 2004;34(1):83–89. doi:10.2190/44WA-WXF7-3UHA-FDV1 PubMed
103. Devinsky O, Honigfeld G, Patin J. Clozapine-related seizures. Neurology. 1991;41(3):369–371. doi:10.1212/WNL.41.3.369 PubMed
104. Freudenreich O, Weiner RD, McEvoy JP. Clozapine-induced electroencephalogram changes as a function of clozapine serum levels.
Biol Psychiatry. 1997;42(2):132–137. doi:10.1016/S0006-3223(96)00298-3 PubMed
105. Wong J, Delva N. Clozapine-induced seizures: recognition and treatment. Can J Psychiatry. 2007;52(7):457–463. PubMed
106. Muzyk A, Gala G, Kahn DA. Use of lamotrigine in a patient with a
clozapine-related seizure. J Psychiatr Pract. 2010;16(2):125–128. doi:10.1097/01.pra.0000369974.18274.e2 PubMed
107. Usiskin SI, Nicolson R, Lenane M, et al. Gabapentin prophylaxis of clozapine-induced seizures. Am J Psychiatry. 2000;157(3):482–483. doi:10.1176/appi.ajp.157.3.482 PubMed
108. Jarbin H, Johansson BA, Lundgren J. Clozapine and therapy-refractory epileptic seizures. Eur Child Adolesc Psychiatry. 2001;10(3):209–210. doi:10.1007/s007870170029 PubMed
109. Meyer JM. Individual changes in clozapine levels after smoking cessation: results and a predictive model. J Clin Psychopharmacol. 2001;21(6):569–574. doi:10.1097/00004714-200112000-00005 PubMed
110. Haack MJ, Bak ML, Beurskens R, et al. Toxic rise of clozapine plasma concentrations in relation to inflammation. Eur Neuropsychopharmacol. 2003;13(5):381–385. doi:10.1016/S0924-977X(03)00042-7 PubMed
111. Jecel J, Michel TM, Gutknecht L, et al. Toxic clozapine serum levels during acute urinary tract infection: a case report. Eur J Clin Pharmacol. 2005;
60(12):909–910. doi:10.1007/s00228-004-0867-4 PubMed
112. Sayin A, Cosar B. Prolongation of clozapine-induced leukopenia with olanzapine treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2006;
30(5):958–959. doi:10.1016/j.pnpbp.2005.11.032 PubMed
113. Tollefson GD, Birkett MA, Kiesler GM, et al; The Lilly Resistant Schizophrenia Study Group. Double-blind comparison of olanzapine versus clozapine in schizophrenic patients clinically eligible for treatment with clozapine. Biol Psychiatry. 2001;49(1):52–63. doi:10.1016/S0006-3223(00)01026-X PubMed




