Background: Developing safe and effective long-term treatments for bipolar disorder remains a major challenge. Given available treatments, patients with bipolar disorder remain unwell in half of long-term follow-up, mostly in depression. As memantine, an N-methyl-d-aspartate (NMDA)-glutamate receptor antagonist used to treat dementia, has been proposed for testing in bipolar disorder, we carried out a 3 + 3-year, mirror-image, chart-review study of the effects of adding memantine to stably continued, but insufficiently effective, ongoing mood-stabilizing treatments.
Method: Outpatients diagnosed with DSM-IV-TR bipolar disorder (I or II), followed intensively at the Lucio Bini Mood Disorder Center, Rome, Italy, had responded consistently unsatisfactorily to standard treatments (lithium, anticonvulsants, antipsychotics, antidepressants, and electroconvulsive therapy) for ≥ 3 years (2005-2013). Memantine (20-30 mg/d) was added clinically to otherwise stable regimens for another 3 years. On the basis of chart review, we compared morbidity measures and Clinical Global Impressions scale for Bipolar Disorder (CGI-BP) score before versus during memantine treatment.
Results: The 30 bipolar I (n = 17) and II (n = 13) subjects showed consistent morbidity for 3 years before memantine, but improved progressively (r = 0.28, P < .01) over 3 years with memantine (23 ± 4.8 mg/d). Markedly decreased (all P values ≤ .01) were (1) percentage of time ill (total, mania, or depression; averaging -75.0%), (2) CGI-BP severity scores (-67.8%), (3) duration of new episodes (-58.6%), and (4) episodes/year (-55.7%). Subjects with previous rapid or continuous cycling were particularly improved (t = 2.61, P = .016). Adverse effects were mild and rare.
Conclusions: Memantine added substantial long-term benefits by preventing or ameliorating depressive as well as mania-like morbidity in previously consistently poorly responsive patients with bipolar disorder. Further testing in randomized, controlled trials is required.
Three-Year, Naturalistic, Mirror-Image Assessment of Adding Memantine to the Treatment of 30 Treatment-Resistant Patients With Bipolar Disorder

ABSTRACT
Background: Developing safe and effective long-term treatments for bipolar disorder remains a major challenge. Given available treatments, patients with bipolar disorder remain unwell in half of long-term follow-up, mostly in depression. As memantine, an N-methyl-d-aspartate (NMDA)-glutamate receptor antagonist used to treat dementia, has been proposed for testing in bipolar disorder, we carried out a 3 + 3-year, mirror-image, chart-review study of the effects of adding memantine to stably continued, but insufficiently effective, ongoing mood-stabilizing treatments.
Method: Outpatients diagnosed with DSM-IV-TR bipolar disorder (I or II), followed intensively at the Lucio Bini Mood Disorder Center, Rome, Italy, had responded consistently unsatisfactorily to standard treatments (lithium, anticonvulsants, antipsychotics, antidepressants, and electroconvulsive therapy) for ≥ 3 years (2005-2013). Memantine (20-30 mg/d) was added clinically to otherwise stable regimens for another 3 years. On the basis of chart review, we compared morbidity measures and Clinical Global Impressions scale for Bipolar Disorder (CGI-BP) score before versus during memantine treatment.
Results: The 30 bipolar I (n = 17) and II (n = 13) subjects showed consistent morbidity for 3 years before memantine, but improved progressively (r = 0.28, P < .01) over 3 years with memantine (23 ± 4.8 mg/d). Markedly decreased (all P values ≤ .01) were (1) percentage of time ill (total, mania, or depression; averaging -75.0%), (2) CGI-BP severity scores (-67.8%), (3) duration of new episodes (-58.6%), and (4) episodes/year (-55.7%). Subjects with previous rapid or continuous cycling were particularly improved (t = 2.61, P = .016). Adverse effects were mild and rare.
Conclusions: Memantine added substantial long-term benefits by preventing or ameliorating depressive as well as mania-like morbidity in previously consistently poorly responsive patients with bipolar disorder. Further testing in randomized, controlled trials is required.
J Clin Psychiatry 2015;76(1):e91-e97
© Copyright 2015 Physicians Postgraduate Press, Inc.
Submitted: December 19, 2013; accepted April 23, 2014 (doi:10.4088/JCP.13m08956).
‘ Deceased.
Corresponding author: Ross J. Baldessarini, MD, Mailman Research Center, McLean Hospital, 115 Mill St, Belmont, MA 02478-9106 ([email protected]).
Long-term treatment of bipolar disorder (BD) aimed at preventing recurrences of the various phases of this complex illness is a leading clinical and research challenge for contemporary psychiatry. With the possible exception of lithium carbonate, it has been difficult to develop unambiguous evidence for effective, long-term mood stabilization in BD uncomplicated by artifacts associated with trials involving treatment-discontinuation designs, particularly following incomplete recovery from acute episodes of illness.1 Treatments with evidence of long-term beneficial effects and with current regulatory approval for prophylactic applications include lithium carbonate, lamotrigine, aripiprazole, and olanzapine, as well as quetiapine as an adjunctive agent.1-3 Most antipsychotic drugs and the anticonvulsants carbamazepine and valproate are useful in acute manic or mixed-states but lack regulatory approval for long-term applications in BD and have variable effects in acute bipolar depression.4 Antidepressants remain controversial for use in acute bipolar depression and lack evidence of substantial long-term preventive effects.1,5,6
All of these treatments, as well as others sometimes used on an empirical or “off-label” basis, appear to be incompletely effective, alone or in various combinations. It is remarkable that, even with currently available treatments used by community standards of care, patients with BD remain unwell in approximately half of weeks of long-term follow-up, both during the midcourse of the illness and from onset.7 Moreover, fully three-quarters of this unresolved morbidity is depressive, dysthymic, or dysphoric.7 These considerations underscore the urgent need for more effective treatments that can provide long-term protective effects in BD patients, particularly for depressive components of the disorder that are closely associated with disability, substance abuse, and premature mortality.8-10
One potential candidate is memantine (1-amino-3,5-dimethyladamantane hydrochloride; Namenda and others), currently indicated for the treatment of some forms of dementia. This pharmacologically complex adamantane derivative was first synthesized in the 1960s.11 It is a low-affinity, rapidly dissociating, voltage-dependent, uncompetitive antagonist at glutamate N-methyl-d-aspartate (NMDA) receptors, which inhibits neuronal influx of Ca2+ ions, but does not prevent functioning of glutamate excitatory neurotransmission.11,12 Memantine also has noncompetitive activity at some cerebral nicotinic acetylcholine receptors (including α7) that is followed by their rapid up-regulation, perhaps contributing to antidementia effects. In addition, memantine has noncompetitive antagonistic activity at serotonin 5-HT3 receptors, of unknown significance, with additional agonistic actions at dopamine D2 receptors.11 A proposed laboratory model of BD involves up-regulation of dopamine D2 receptors by repeated treatment with antidepressants, associated with increased, possibly mania-related, behavioral responsiveness of laboratory animals to dopamine agonists, followed by a “depressive” state after discontinuing antidepressants.13 Such effects of antidepressant treatment are prevented by cotreatment with NMDA antagonists including memantine, but not by standard mood-stabilizing drugs.14,15
In daily doses of 10-30 mg, memantine is used in the treatment of moderate-to-severe,16 but not mild,17 Alzheimer’s disease with a favorable efficacy/risk ratio; it also may be effective in other types of nonvascular dementia.18 Memantine undergoes little hepatic metabolism and has a relatively long elimination half-life (60-100 hours). It appears to be less likely than other NMDA antagonists, such as phencyclidine and ketamine, to induce psychotic reactions, although such effects may occur infrequently with memantine.19 Memantine has been considered for use in the treatment of various psychiatric disorders, usually with inconsistent or inconclusive findings, particularly in schizophrenia.20,21 In patients with dementia, clinical benefits may include reduction of irritability, agitation, aggression, and some psychotic symptoms, as well as a reduced need for other psychotropic drugs.22,23
Studies of effects of memantine in BD, specifically, remain limited and tentative, though suggestive. Memantine monotherapy was reported to show evidence of antimanic effects at well-tolerated daily doses (20-50 mg) in a 3-week open-label trial in 33 acutely manic patients.24 Our group found suggestive evidence of mood-stabilizing actions in 40 patients with BD in an unblinded, 12-month trial when memantine was added to stable, ongoing, but inadequately effective, treatments.25 Memantine as a monotherapy also has been reported to show beneficial effects in mania and possibly for long-term mood stabilization in a few individual BD patients, including after discontinuation of lithium treatment.26,27 Another short-term study found memantine (20 mg/d; n = 14) to be more effective than placebo (n = 15) when added to lamotrigine for 4 weeks to treat acute bipolar depression in a randomized, controlled trial, but this effect was no longer significant at 8 weeks.28 A recent 12-week trial found little overall difference in effects of small doses of memantine (5 mg/d; n = 62) versus placebo (n = 73) added to valproate in patients with bipolar II disorder for 12 weeks.29
The few studies that have evaluated effects of memantine in BD, therefore, are inconsistent regarding short-term effects but vary in the current morbid status of subjects tested, doses of memantine, and duration of treatment. More prolonged treatments might have been more helpful. Given this suggestive but inconclusive background of possible beneficial effects on mania and bipolar depression, we evaluated the effects of adding memantine clinically for 3 years to stable, ongoing, mood-stabilizing treatments that had proved to be consistently unsatisfactory over several preceding years in types I and II BD patients in varied states of initial morbidity. We hypothesized that adding memantine in such circumstances might yield long-term reductions of morbidity involving manic and possibly also depressive recurrences based on selected outcome measures.

- Memantine may have substantial, long-term, mood-stabilizing effects in otherwise treatment-resistant bipolar disorder (BD) patients and had excellent tolerability at daily doses of 20-30 mg.
- Clinical improvements with memantine occurred in both type I and II BD patients, including reduction of the number, duration, and severity of episodes in both mania-like and depressive morbidity, and were independent of initial clinical status at the start of memantine treatment.
- Subjects with previous rapid or continuous cycling were particularly improved with memantine treatment.
METHOD
Subjects were evaluated and treated as outpatients at the Lucio Bini Mood Disorder Center in Rome, Italy (2005-2013). In an effort to improve their clinical status, patients were given memantine augmentation clinically and individually after failing to respond satisfactorily to prolonged trials of standard mood-stabilizing treatments over several years. Patients provided informed consent for the off-label administration of a drug with regulatory approval for other indications and for possible future anonymous analysis of their clinical data and its reporting in aggregate form. These procedures are consistent with current Italian law pertaining to individually, clinically decided, off-label use of marketed drugs. The study was a retrospective chart review of clinically acquired data that was collected prospectively by the same expert psychiatrist (A.K.).
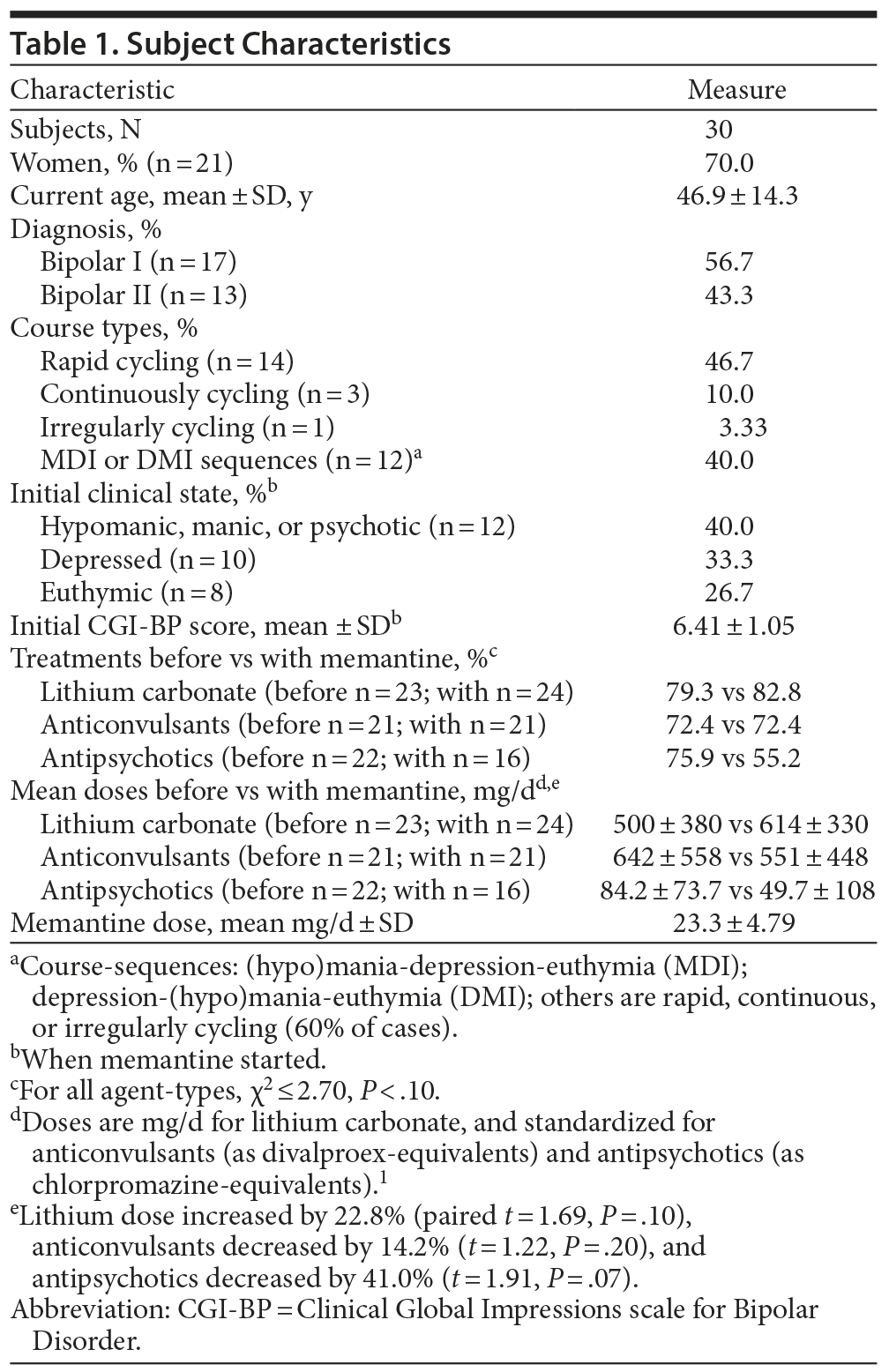
Memantine was given to patients meeting DSM-IV-TR diagnostic criteria for type I or II BD, aged 18-70 years, lacking major, unstable medical illnesses, and with adequate contraception for women of childbearing age. The same clinician (A.K.) had followed all study patients personally for several years and documented their clinically unsatisfactory responses to standard mood-stabilizing treatments for several years before considering them for addition of memantine. He then added memantine on an individually decided, clinical basis to clinically unsatisfactory treatment regimens, not according to a formal protocol. Treatments were held constant throughout the 3 years of addition of memantine in this open-label, naturalistic, clinical study. Assessments were made regularly and prospectively to compare clinical status in the 3 years during versus the 3 years before memantine. We evaluated morbidity on a per-time-at-risk basis, evaluated consistency of observed changes before, and tested for possible temporal changes during memantine treatment. Ongoing treatments included standard mood-stabilizing agents, including lithium at daily trough serum concentrations of 0.6-1.0 mEq/L, selected anticonvulsants (carbamazepine, lamotrigine, and valproate), or antipsychotic agents (in various combinations, usually adjunctively in low doses), as well as antidepressants when required. A third of the subjects (10/30) had also received trials of electroconvulsive shock treatment in the past, consistent with relatively severe illness. All study subjects were considered to respond consistently unsatisfactorily for at least 3 years prior to trial entry based on clinical assessments and repeatedly elevated ratings of overall morbidity with the Clinical Global Impressions scale for Bipolar Disorder (CGI-BP-total30), with scores of > 5 at the point of starting memantine. A majority of participants (20/30) had been ill more than half of the time during the 3 years prior to starting memantine.
Data collected for each subject included the number, type (polarity), and estimated duration of episodes of BD illness based on regular assessments every 2-4 weeks for the 3 years before memantine treatment was given and every 2 weeks during the 3 years of prospective memantine treatment based on semistructured clinical assessments and recording of clinical status using life charts.31 In addition, all subjects were rated with the CGI-BP scale at least once yearly to assess the average interval severity of overall illness, as well as that of manic and depressive episodes. Clinical assessments were recorded systematically and prospectively before and during memantine and summarized in life charts, all in accordance with the standard clinical practices of the study center; these records provided data for the present analyses.
Memantine hydrochloride at daily oral doses of 20-30 mg was added to ongoing, mood-stabilizing treatment, which was held constant across the 3 years of the trial but for adjusting doses to current clinical requirements. For new acute episodes of BD illness, temporary addition only of treatments that had been given previously to the same patient for similar acute illnesses was allowed, including an antipsychotic agent for mania and an antidepressant for acute major depression based on the discretion of the senior treating psychiatrist.
We compared the number of illness episodes and the estimated duration of episodes considered as mania-related (mania, hypomania, psychosis) or depression-related (depression with or without psychosis, dysthymia, dysphoric-agitated mixed-states), as well as their severity based on CGI-BP ratings. Systematically collected and recorded clinical assessment data allowed quarterly estimates of episode counts and durations and computation of the percentage of months of mania-related illness, depression, and all affective illness. These measures were averaged for 12-month intervals and compared yearly for the 3 years before versus 3 years during treatment with memantine added.
Averages for individual subjects were compared between times before versus during memantine treatment using paired t tests. We also employed linear regression to test for changes in percentage of time ill and of mean CGI-BP scores versus year of observation during periods before and during memantine treatment. Finally, we considered selected factors (age, sex, diagnosis, total years of illness, polarity of episodes closest to the start of memantine, and predominant cycling pattern) in preliminary bivariate comparisons for before versus during memantine treatment, followed by multivariate, linear regression modeling of factors tentatively associated with change in total percentage of time ill. Data are means (SDs) or medians with interquartile ranges (IQR), unless stated otherwise. Statistical analyses were made with commercial software (Stata.12, StataCorp, College Station, Texas; including spreadsheets based on Statview.5, SAS Institute, Cary, North Carolina).
RESULTS
Subject Characteristics
We reviewed and analyzed the medical records of 30 patients with DSM-IV-TR bipolar I (n = 17) or II (n = 13) disorder to compare morbidity during 36 months of illness course before to 36 months with memantine added to otherwise stable, but clinically unsatisfactory, treatment regimens. A total of 44 BD patients had been started on memantine augmentation at the study center through 2013. Ten patients discontinued or were lost at follow-up before 3 years of treatment, at 6-18 months after starting memantine. Of these, 4 patients were rated as unchanged (CGI-BP improvement score of 4) at their last assessment; 1 patient was minimally improved (CGI-BP improvement score of 3), and 5 patients (50.0%) were rated as very much or much improved (CGI-BP improvement score of 1 or 2). Reasons for dropout included complaints of drowsiness (1 patient), lack of immediate efficacy (2 patients), and loss of hypomania (1 patient). Another 4 subjects with major changes in other medications also were omitted but appear elsewhere in case reports.26,27
Participant age averaged 46.9 years, with a mean of 23.7 years from illness onset, and assessment and treatment at the study site averaged 7.53 ± 7.19 years; 70.0% of participants were women (Table 1). The subjects had been consistently symptomatic in the 3 years before memantine treatment was added: median percentage of time ill was 66.7% (IQR: 23.3%-100%); 66.7% had been ill > 50% of the time and 33.3% for > 90%. A high proportion of subjects (56.7%) had shown rapid (≥ 4 discrete episodes/year) or continuous cycling at some time during the 6 years of study; when a course pattern could be identified, it was characterized as being dominated by episodes of mania preceding depression episodes or the opposite.31 Overall, CGI-BP scores averaged 6.41 ± 1.05 at the start of memantine treatment. These several measures indicate relatively high levels of previous and initial morbidity. Initial clinical states at the start of memantine treatment were hypomanic or manic in 40.0% of patients, depressive in 33.3% of patients, and more or less euthymic in 26.7% of patients.
The proportions and average doses of other treatments were typical of patients with BD and very similar before and during addition of memantine, thus supporting the conclusion that treatments were substantially held constant (Table 1).
Morbidity Before Versus During Memantine Treatment
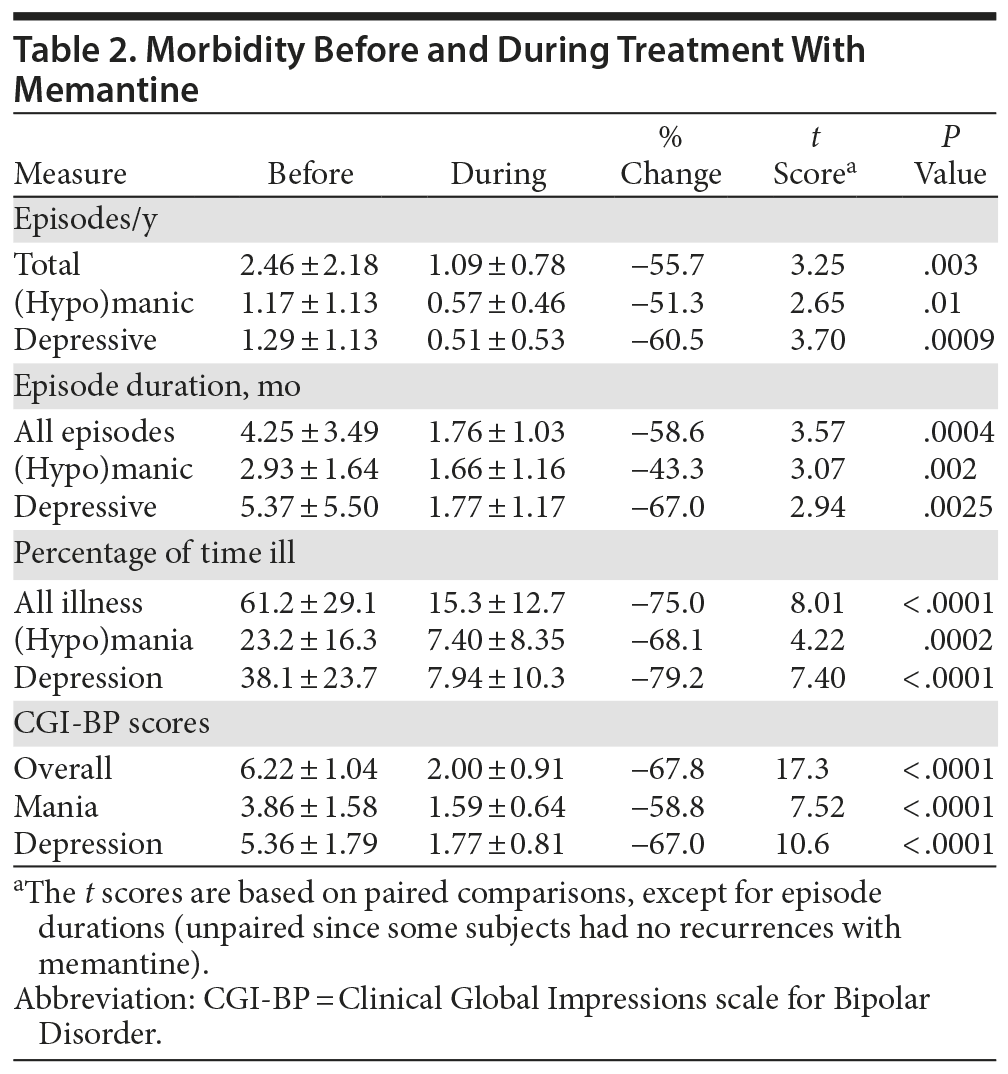
Several measures indicated marked differences in morbidity between the years during versus the years before starting adjunctive memantine treatment, consistently in the direction of less severe illness with memantine (Table 2). These outcome measures included episodes/year (total, depressive, and manic or hypomanic), estimated average duration of episodes (total and specific types), proportion of time ill (and time in depressive or manic illness), and mean annual CGI-BP scores for overall morbidity, as well as for mania-like and depression-related illness states. Improvements ranged from a low of 43.3% shorter mania-like recurrences to a high of 79.2% less time in depression; all of the changes were highly significant, based on paired comparisons (Table 2). Several of the previously treatment-resistant study participants (4/30; 13.3%) had no new illness recurrences in the 3 years of treatment with memantine; a substantial proportion (12/30; 40.0%) were ill ≤ 10% of the entire 3 years during such treatment, and 33.3% (10/30) were illness-free in at least 1 of the years during memantine treatment (all in years 2 and 3).
As expected,7,9 time in depressive states was much greater than time in mania-like states before adding memantine, but, surprisingly, this difference was much less during memantine treatment (Table 2). This change reflects the particularly noteworthy finding that depressive morbidity tended to be reduced by at least as much, and possibly more than mania-like illness, in terms of episodes/year, months/episode, and in percentage of time ill (percentage change in time depressed decreased by 79.2%, and in time manic by 68.1%; t = 1.29, P = .21; Table 2). These findings suggest substantial long-term improvements in all aspects of BD morbidity, with somewhat greater effects on depressive than on mania-like illness.
That episode recurrences decreased by an average of 55.7%, and their duration decreased by 58.6% during versus before memantine treatment, with similar decreases in both mania-like and depression-like episodes, suggests that the duration and perhaps severity, as well as risk of new episodes, were reduced during treatment with memantine (Table 2). The impression that the intensity of morbidity may have been reduced is further suggested by indications that in type I BD patients, the rate of recurrence of manic episodes decreased markedly with memantine (by 89.3%, from 0.58 ± 0.48 to 0.06 ± 0.13 episodes/year; paired t = 4.04, P = .001), whereas recurrences of hypomania tended to increase (by 66.7%, from 0.38 ± 0.42 to 0.63 ± 0.48 episodes/year; paired t = 2.02, P = .06; not shown). There were no instances of increased hypomania among BD type II patients (percentage time in hypomania was reduced by 75.7%, t = 2.56, P = .03, and the number of hypomanic recurrences was reduced by 69.6%, t = 2.05, P = .05). That is, there was an evident shift from mania to hypomania, adding to the impression that the severity of mania was reduced.
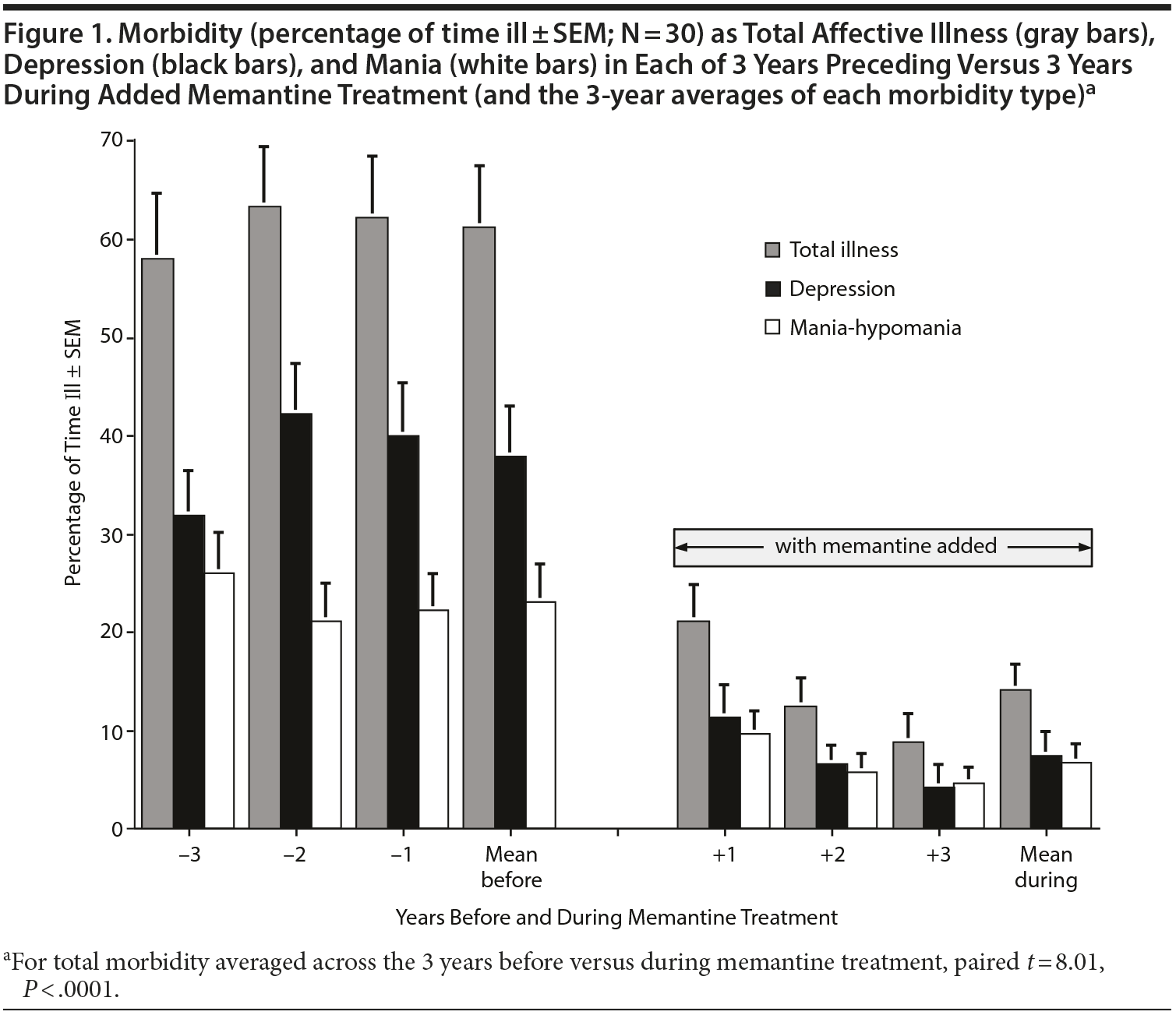
Overall morbidity as well as the proportion of time in mania-like and depression-like states before and during addition of memantine for each of the 3 years before and 3 years during addition of memantine is illustrated (Figure 1). These findings indicate that morbidity was quite stable over the 3 years of observation before memantine, but has declined over the years of memantine treatment. This hypothesis was tested by regressing the proportion of time ill versus years, separately, for before and during memantine treatment. Before memantine, there was no significant change over time (r = 0.050, P = .638), whereas during the 3 years of memantine treatment, morbidity declined significantly over time (r = -0.288, P = .006). Similar relationships were found for changes of yearly-averaged CGI-BP ratings of overall morbidity: before (r = 0.059, P = .603) versus during memantine treatment (r = -0.244, P = .03).
Factors Associated With Improvement
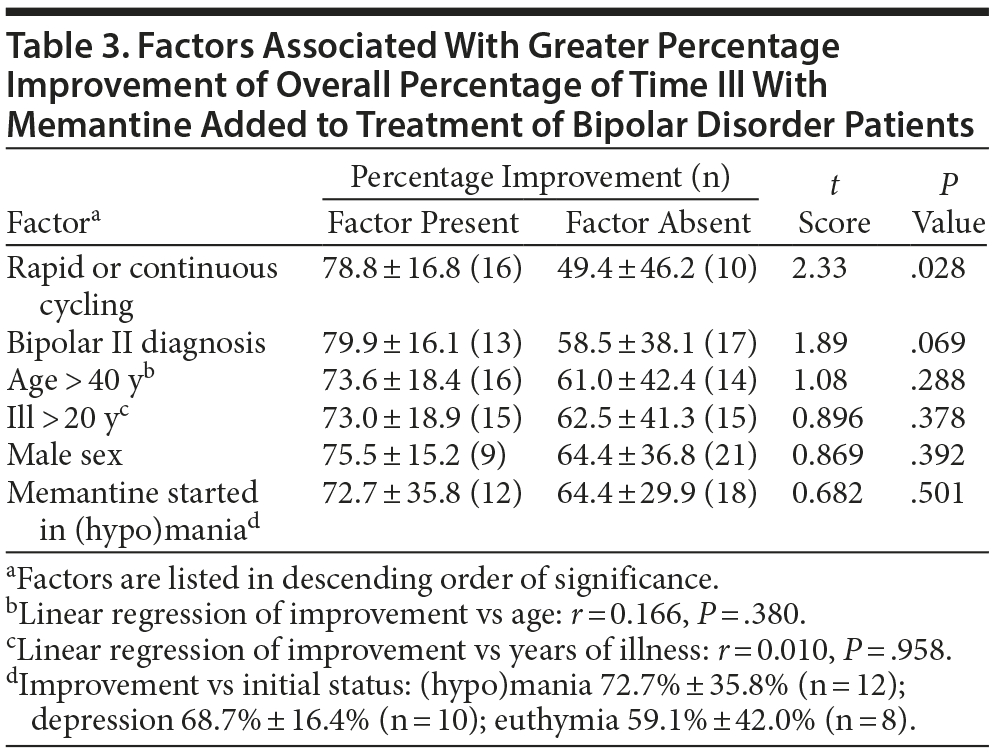
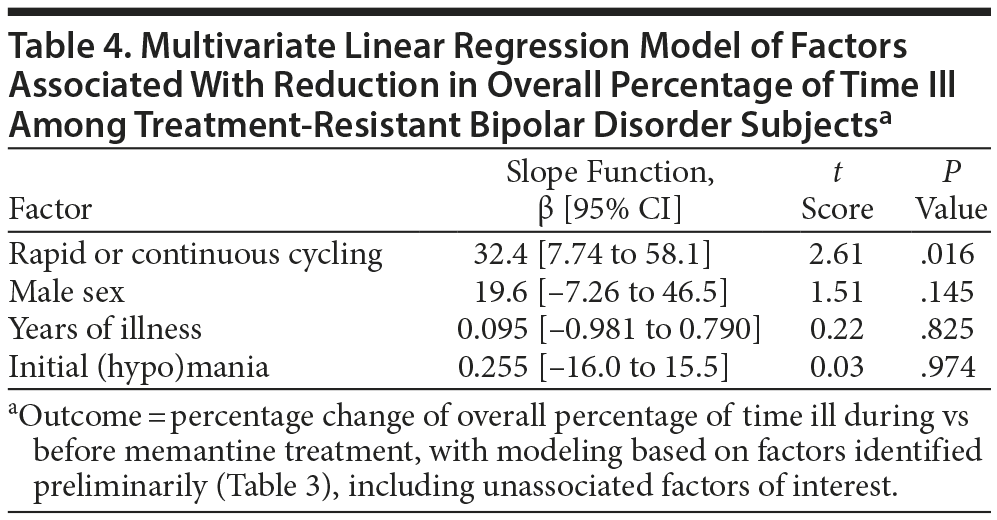
We compared the mean 3-year percentage change in overall proportion of time ill with versus before memantine-treatment as an outcome measure (Table 3). In bivariate comparisons, only rapid or continuous cycling (vs slower or erratic cycling) was significantly associated with greater benefit with memantine (possibly an artifact of greater initial deviance). There also was a nonsignificant tendency for somewhat better responses among subjects diagnosed with type II versus type I BD. Other factors not significantly associated with percentage change in overall proportion of time ill included sex, current age, years of illness, and clinical status (in mania-like or depressive episodes, or euthymic) at the start of memantine treatment (Table 3). In multivariate linear regression modeling of factors even suggestively associated with the outcome measure in the preliminary bivariate analyses, only a rapid or continuously cycling course type remained significantly associated with superior outcome, regardless of other covariates included (Table 4).
DISCUSSION
The present findings support a growing body of evidence suggesting that memantine may have substantial long-term benefits for a variety of measures of morbidity among BD patients (Table 2). In this long-term, open, naturalistic study, standard treatments had been persistently unsatisfactory in reducing BD morbidity for at least 3 years before memantine was added to otherwise stable treatment regimens (Figure 1). Addition of memantine, even to relatively complex treatment regimens, was remarkably well tolerated for 3 years.
In the present multiyear study, improvements were seen among both type I and II BD patients in mania-like and depressive morbidity and were independent of initial clinical status at the start of memantine treatment. In addition, there were indications that illness severity may have diminished with memantine in that recurrences were briefer, and there appeared to be a substantial shift from manic to hypomanic recurrences. Given the difficulty of long-term control of the depressive components of BD and their major clinical importance in terms of disability and excess mortality,8-10,32,33 it may be particularly significant that improvements in depressive morbidity, on average, were somewhat greater than in mania-like morbidity (Table 2). On the other hand, this observation may reflect a statistical consequence of the generally higher starting levels of depressive morbidity.
Previous clinical observations had suggested possible antimanic and mood-stabilizing effects of memantine in BD patients,24-27 as well as reduction of possibly mania-like manifestations associated with other neuropsychiatric disorders.22,23,34,35 The antimanic effects of memantine may exceed those in acute depression in short-term studies,28,36,37 in contrast to the rapid antidepressant actions of the more potent NMDA-antagonist ketamine.38 However, in the present observation, long-term benefits appeared to be at least as great against bipolar depression as for mania-like morbidity. The present long-term benefits for depressive aspects of BD associated with adding memantine to other standard treatments for BD might also reflect reductions of mixed, agitated-dysphoric features, or indirect amelioration of depression secondary to prevention or amelioration of manic aspects of the disorder. Broader effects of preventing mania, including on bipolar depression, have been hypothesized previously.39,40
As the reported mechanisms of action of memantine are complex and implicate neurotransmission through glutamate, serotonin, dopamine, and nicotinic acetylcholine receptors, it is not clear why this agent should have mood-stabilizing effects. It is also not clear whether its beneficial effects in dementia are based on pharmacodynamic actions similar to its apparent effects in BD. Mechanisms that may plausibly be considered include modulation of dopaminergic neurotransmission,15,41,42 perhaps neurotrophic or neuroprotective effects,43,44 or reduction of proposed pathophysiologic excitotoxic effects.45-47
Clinical questions arising from this study that remain to be examined are whether memantine will prove useful in the long-term treatment of BD as a monotherapy or in specific and limited combinations with other agents, as well as its optimal dosing. Also remaining to be determined is the extent to which memantine may exert short-term antimanic compared to antidepressive effects in BD. These questions require additional, adequately designed and controlled trials.
Limitations of the present study are clear and substantial. They include a lack of adequate controls or blinding and a small number of patients who may not be representative of broader samples of BD patients. On the other hand, striking benefits were found in a particularly challenging sample of patients who had shown clinically inadequate responses to standard treatments for several years. Lack of a randomly assigned placebo control group led to use of a within-subject analytic design based on paired comparisons. Moreover, unresolved morbidity had been stable for at least 3 years before showing not only major, but also progressive improvements with memantine (Figure 1). These aspects of the findings tend to support their plausibility.
In conclusion, this preliminary, naturalistic study evaluated effects of adding memantine in doses used to treat dementias in type I and II BD patients who had consistently shown clinically unsatisfactory responses to standard mood-stabilizing treatments for at least 3 years. It found major improvements in recurrence rates, duration, and possibly the severity of both mania-like and depressive morbidity that were sustained and may have increased over the 3 years of memantine treatment. Particularly important may be suggestions of superior benefits in the especially hard-to-treat depressive components of BD illness.
Drug names: aripiprazole (Abilify), carbamazepine (Carbatrol, Equetro, and others), divalproex (Depakote and others), ketamine (Ketalar and others), lamotrigine (Lamictal and others), lithium (Lithobid and others), memantine (Namenda), olanzapine (Zyprexa), quetiapine (Seroquel).
Author affiliations: NESMOS Department, Sant’ Andrea Hospital, Sapienza University, Rome, Italy (Drs Giulia Serra, De Chiara, A. E. Koukopoulos, and Girardi); Centro Lucio Bini Mood Disorder Center, Rome, Italy (Drs Giulia Serra, De Chiara, A. Koukopoulos, A. E. Koukopoulos, Tondo, and Girardi); Department of Psychiatry, Harvard Medical School, Boston, Massachusetts and International Consortium for Bipolar Disorder Research, McLean Hospital, Belmont, Massachusetts (Drs Giulia Serra, Tondo, and Baldessarini); Centro Lucio Bini Mood Disorder Center, Cagliari, Sardinia (Dr Tondo); and Department of Biomedical Sciences, University of Sassari, Sardinia (Dr Gino Serra).
Potential conflicts of interest: Dr Gino Serra has applied for a patent for use of memantine to treat bipolar disorder. Dr Girardi has received grant/research support from Eli Lilly and Janssen; has received honoraria from Eli Lilly and Organon; and has served on the speakers or advisory boards for Eli Lilly, Organon, Pfizer, and Schering. Drs Giulia Serra, A. E. Koukopoulos, De Chiara, Tondo, Baldessarini, and Gino Serra and their immediate family members have no current financial relationships with commercial entities that might appear to represent potential conflicts of interest with the material presented. Dr A. Koukopoulos had no conflicts of interest.
Funding/support: Supported, in part, by a Research Fellowship from the Sapienza Foundation, Rome, Italy (Dr Giulia Serra), an award from the Aretצus Foundation of Rome and by the Lucio Bini/Cagliari Private Donors Research Fund (Dr Tondo), and by a grant from the Bruce J. Anderson Foundation and by the McLean Private Donors Research Fund (Dr Baldessarini).
Role of the sponsor: The sponsors had no role in the conduct, analysis, or reporting of this study.
REFERENCES
1. Baldessarini RJ. Chemotherapy in Psychiatry. 3rd ed. New York, NY: Springer Press; 2013. doi:10.1007/978-1-4614-3710-9
2. Popovic D, Reinares M, Amann B, et al. Number needed to treat analyses of drugs used for maintenance treatment of bipolar disorder. Psychopharmacology (Berl). 2011;213(4):657-667. PubMed doi:10.1007/s00213-010-2056-8
3. Vieta E, Günther O, Locklear J, et al. Effectiveness of psychotropic medications in the maintenance phase of bipolar disorder: a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2011;14(8):1029-1049. PubMed doi:10.1017/S1461145711000885
4. Selle V, Schalkwijk S, Vázquez GH, et al. Treatments for acute bipolar depression: meta-analyses of placebo-controlled, monotherapy trials of anticonvulsants, lithium and antipsychotics. Pharmacopsychiatry. 2014;47(2):43-52. PubMed doi:10.1055/s-0033-1363258
5. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249-1262. PubMed doi:10.1176/appi.ajp.2013.13020185
6. Vázquez GH, Tondo L, Undurraga J, et al. Overview of antidepressant treatment of bipolar depression. Int J Neuropsychopharmacol. 2013;16(7):1673-1685. PubMed doi:10.1017/S1461145713000023
7. Baldessarini RJ, Salvatore P, Khalsa HM, et al. Morbidity in 303 first-episode bipolar I disorder patients. Bipolar Disord. 2010;12(3):264-270. PubMed doi:10.1111/j.1399-5618.2010.00812.x
8. ×–sby U, Brandt L, Correia N, et al. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58(9):844-850. PubMed doi:10.1001/archpsyc.58.9.844
9. Baldessarini RJ, Vieta E, Calabrese JR, et al. Bipolar depression: overview and commentary. Harv Rev Psychiatry. 2010;18(3):143-157. PubMed doi:10.3109/10673221003747955
10. Ferrari AJ, Saha S, McGrath JJ, et al. Health states for schizophrenia and bipolar disorder within the Global Burden of Disease 2010 Study. Popul Health Metr. 2012;10(1):16-23. PubMed doi:10.1186/1478-7954-10-16
11. Noetzli M, Eap CB. Pharmacodynamic, pharmacokinetic and pharmacogenetic aspects of drugs used in the treatment of Alzheimer’s disease. Clin Pharmacokinet. 2013;52(4):225-241. PubMed doi:10.1007/s40262-013-0038-9
12. Olivares D, Deshpande VK, Shi Y, et al. N-methyl-d-aspartate (NMDA) receptor antagonists and memantine treatment for Alzheimer’s disease, vascular dementia and Parkinson’s disease. Curr Alzheimer Res. 2012;9(6):746-758. PubMed doi:10.2174/156720512801322564
13. D’ Aquila PS, Peana AT, Panin F, et al. Reversal of antidepressant-induced dopaminergic behavioural supersensitivity after long-term chronic imipramine withdrawal. Eur J Pharmacol. 2003;458(1-2):129-134. PubMed doi:10.1016/S0014-2999(02)02731-0
14. D’ Aquila PS, Panin F, Serra G. Chronic valproate fails to prevent imipramine-induced behavioural sensitization to the dopamine D2-like receptor agonist quinpirole. Eur J Pharmacol. 2006;535(1-3):208-211. PubMed doi:10.1016/j.ejphar.2006.02.016
15. Demontis F, Cubeddu A, Serra G. Memantine prevents the “bipolar-like behavior” induced by chronic imipramine in rats [abstract]. Poster presented at the 12th International Review of Bipolar Disorders; May 21-23, 2012; Nice, France.
16. Reisberg B, Doody R, Stöffler A, et al; Memantine Study Group. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med. 2003;348(14):1333-1341. PubMed doi:10.1056/NEJMoa013128
17. Schneider LS, Dagerman KS, Higgins JP, et al. Lack of evidence for the efficacy of memantine in mild Alzheimer disease. Arch Neurol. 2011;68(8):991-998. PubMed doi:10.1001/archneurol.2011.69
18. Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8(7):613-618. PubMed doi:10.1016/S1474-4422(09)70146-2
19. Canan F, Ataoglu A. Memantine-related psychotic symptoms in a patient with bipolar disorder. J Clin Psychiatry. 2010;71(7):957. PubMed doi:10.4088/JCP.09l05802gry
20. Zdanys K, Tampi RR. A systematic review of off-label uses of memantine for psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1362-1374. PubMed doi:10.1016/j.pnpbp.2008.01.008
21. Sani G, Serra G, Kotzalidis GD, et al. The role of memantine in the treatment of psychiatric disorders other than the dementias: a review of current preclinical and clinical evidence. CNS Drugs. 2012;26(8):663-690. PubMed doi:10.2165/11634390-000000000-00000
22. Vidal JS, Lacombe JM, Dartigues JF, et al. Evaluation of the impact of memantine treatment initiation on psychotropics use: a study from the French national health care database. Neuroepidemiology. 2008;31(3):193-200. PubMed doi:10.1159/000158226
23. Wilcock GK, Ballard CG, Cooper JA, et al. Memantine for agitation/aggression and psychosis in moderately severe to severe Alzheimer’s disease: a pooled analysis of 3 studies. J Clin Psychiatry. 2008;69(3):341-348. PubMed doi:10.4088/JCP.v69n0302
24. Keck PE Jr, Hsu HA, Papadakis K, et al. Memantine efficacy and safety in patients with acute mania associated with bipolar I disorder: a pilot evaluation. Clin Neuropharmacol. 2009;32(4):199-204. PubMed doi:10.1097/WNF.0b013e318184fae2
25. Koukopoulos A, Serra G, Koukopoulos AE, et al. The sustained mood-stabilizing effect of memantine in the management of treatment resistant bipolar disorders: findings from a 12-month naturalistic trial. J Affect Disord. 2012;136(1-2):163-166. PubMed doi:10.1016/j.jad.2011.09.040
26. Serra G, De Chiara L, Koukopoulos A, et al. Antimanic and long-lasting mood stabilizing effect of memantine in bipolar I mood disorder: two case reports. J Clin Psychopharmacol. 2013;33(5):715-717. PubMed doi:10.1097/JCP.0b013e31829b62ba
27. Serra G, De Chiara L, Manfredi G, et al. Memantine in the management of affective recurrences of bipolar disorders after the discontinuation of long-term lithium treatment: three case histories. Ther Adv Psychopharmacol. 2014;4(1):53-55. PubMed doi:10.1177/2045125313507737
28. Anand A, Gunn AD, Barkay G, et al. Early antidepressant effect of memantine during augmentation of lamotrigine inadequate response in bipolar depression: a double-blind, randomized, placebo-controlled trial. Bipolar Disord. 2012;14(1):64-70. PubMed doi:10.1111/j.1399-5618.2011.00971.x
29. Lee SY, Chen SL, Chang YH, et al. Add-on memantine to valproate treatment increased HDL-C in bipolar II disorder. J Psychiatr Res. 2013;47(10):1343-1348. PubMed doi:10.1016/j.jpsychires.2013.06.017
30. Spearing MK, Post RM, Leverich GS, et al. Modification of the Clinical Global Impressions (CGI) scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Res. 1997;73(3):159-171. PubMed doi:10.1016/S0165-1781(97)00123-6
31. Koukopoulos A, Reginaldi D, Tondo L, et al. Course sequences in bipolar disorder: depressions preceding or following manias or hypomanias. J Affect Disord. 2013;151(1):105-110. PubMed doi:10.1016/j.jad.2013.05.059
32. Tondo L, Isacsson G, Baldessarini RJ. Suicidal behaviour in bipolar disorder: risk and prevention. CNS Drugs. 2003;17(7):491-511. PubMed doi:10.2165/00023210-200317070-00003
33. Tondo L, Baldessarini RJ, Vázquez GH, et al. Clinical responses to antidepressants among 1036 acutely depressed patients with bipolar or unipolar major affective disorders. Acta Psychiatr Scand. 2013;127(5):355-364. PubMed doi:10.1111/acps.12023
34. Grant JE, Odlaug BL, Mooney M, et al. Open-label pilot study of memantine in the treatment of compulsive buying. Ann Clin Psychiatry. 2012;24(2):119-126. PubMed
35. Ghaleiha A, Asadabadi M, Mohammadi MR, et al. Memantine as adjunctive treatment to risperidone in children with autistic disorder: a randomized, double-blind, placebo-controlled trial. Int J Neuropsychopharmacol. 2013;16(4):783-789. PubMed doi:10.1017/S1461145712000880
36. Zarate CA Jr, Singh JB, Quiroz JA, et al. A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am J Psychiatry. 2006;163(1):153-155. PubMed doi:10.1176/appi.ajp.163.1.153
37. Smith EG, Deligiannidis KM, Ulbricht CM, et al. Antidepressant augmentation using the N-methyl-d-aspartate antagonist memantine: randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2013;74(10):966-973. PubMed doi:10.4088/JCP.12m08252
38. Nugent AC, Diazgranados N, Carlson PJ, et al. Neural correlates of rapid antidepressant response to ketamine in bipolar disorder. Bipolar Disord. 2014;16(2):119-128. PubMed doi:10.1111/bdi.12118
39. Kukopulos A, Reginaldi D. Does lithium prevent depressions by suppressing manias? Int Pharmacopsychiatry. 1973;8(3):152-158. PubMed
40. Koukopoulos A, Ghaemi SN. The primacy of mania: a reconsideration of mood disorders. Eur Psychiatry. 2009;24(2):125-134. PubMed
41. Berk M, Dodd S. Kauer-Sant’ Anna M, et al. Dopamine dysregulation syndrome: implications for a dopamine hypothesis of bipolar disorder. Acta Psychiatr Scand. 2007;116(suppl 434):41-49. doi:10.1111/j.1600-0447.2007.01058.x
42. Cousins DA, Butts K, Young AH. The role of dopamine in bipolar disorder. Bipolar Disord. 2009;11(8):787-806. PubMed doi:10.1111/j.1399-5618.2009.00760.x
43. Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med. 2004;5(1):11-25. PubMed doi:10.1385/NMM:5:1:011
44. La Spada AR. Memantine strikes the perfect balance. Nat Med. 2009;15(12):1355-1356. PubMed doi:10.1038/nm1209-1355
45. Ongür D, Jensen JE, Prescot AP, et al. Abnormal glutamatergic neurotransmission and neuronal-glial interactions in acute mania. Biol Psychiatry. 2008;64(8):718-726. PubMed doi:10.1016/j.biopsych.2008.05.014
46. Gao Y, Payne RS, Schurr A, et al. Memantine reduces mania-like symptoms in animal models. Psychiatry Res. 2011;188(3):366-371. PubMed doi:10.1016/j.psychres.2010.12.030
47. Atmaca M, Yildirim H. Altered neurochemical ingredient of hippocampus in patients with bipolar depression. Depress Res Treat. 2012;2012:485249. PubMed doi:10.1155/2012/485249
Please sign in or purchase this PDF for $40.00.
Save
Cite