Objective: The present placebo-controlled study evaluated the efficacy and safety of 8 weeks of treatment with tianeptine 25-50 mg/d in elderly patients suffering from major depressive disorder (MDD) according to DSM-IV-TR. Escitalopram 5-10 mg/d was used as an active comparator.
Methods: Elderly outpatients aged at least 65 years with a primary diagnosis of moderate to severe episode of recurrent MDD were recruited by psychiatrists in 44 clinical centers in 10 countries from October 2013 to January 2016. Patients were randomly assigned to receive tianeptine (n = 105), placebo (n = 107), or escitalopram (n = 99) for 8 weeks. The primary outcome measure was the 17-item Hamilton Depression Rating Scale (HDRS17) total score.
Results: Tianeptine improved depressive symptoms, as evaluated by the HDRS17 total score in terms of absolute change from baseline (week 0) to week 8 (placebo-tianeptine difference [SE] of 3.84 [0.85] points, P < .001, using a last-observation-carried-forward approach) and response to treatment (tianeptine: 46.7%; placebo: 34.0%, estimate [SE] = 12.70% [6.70], P = .06). A sensitivity analysis using a mixed model for repeated measures confirmed the main results on HDRS total s’ ‹core. The placebo-tianeptine difference (SE) was 0.66 (0.15) for Clinical Global Impressions-Severity of Illness (95% CI, 0.37 to 0.96; P < .001) and 0.57 (0.14) for Clinical Global Impressions- Improvement (95% CI, 0.30 to 0.83; P < .001). Positive results were also obtained with the active control escitalopram (HDRS17 total score placebo-escitalopram difference of 4.09 ± 0.86 points, P < .001), therefore validating the sensitivity of the studied population. Tianeptine was well tolerated, with only minimal differences in tolerability from placebo.
Conclusions: The present study provides robust evidence that an 8-week treatment period with tianeptine 25-50 mg is efficacious and well tolerated in depressed patients aged 65 years or older.
Trial Registration: EudraCT identifier: 2012-005612-26‘ ‹
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.

Efficacy of Tianeptine 25-50 mg in Elderly Patients With Recurrent Major Depressive Disorder:
An 8-Week Placebo- and Escitalopram-Controlled Study

ABSTRACT
Objective: The present placebo-controlled study evaluated the efficacy and safety of 8 weeks of treatment with tianeptine 25-50 mg/d in elderly patients suffering from major depressive disorder (MDD) according to DSM-IV-TR. Escitalopram 5-10 mg/d was used as an active comparator.
Methods: Elderly outpatients aged at least 65 years with a primary diagnosis of moderate to severe episode of recurrent MDD were recruited by psychiatrists in 44 clinical centers in 10 countries from October 2013 to January 2016. Patients were randomly assigned to receive tianeptine (n = 105), placebo (n = 107), or escitalopram (n = 99) for 8 weeks. The primary outcome measure was the 17-item Hamilton Depression Rating Scale (HDRS17) total score.
Results: Tianeptine improved depressive symptoms, as evaluated by the HDRS17 total score in terms of absolute change from baseline (week 0) to week 8 (placebo-tianeptine difference [SE] of 3.84 [0.85] points, P < .001, using a last-observation-carried-forward approach) and response to treatment (tianeptine: 46.7%; placebo: 34.0%, estimate [SE] = 12.70% [6.70], P = .06). A sensitivity analysis using a mixed model for repeated measures confirmed the main results on HDRS total score. The placebo-tianeptine difference (SE) was 0.66 (0.15) for Clinical Global Impressions-Severity of Illness (95% CI, 0.37 to 0.96; P < .001) and 0.57 (0.14) for Clinical Global Impressions- Improvement (95% CI, 0.30 to 0.83; P < .001). Positive results were also obtained with the active control escitalopram (HDRS17 total score placebo-escitalopram difference of 4.09 ± 0.86 points, P < .001), therefore validating the sensitivity of the studied population. Tianeptine was well tolerated, with only minimal differences in tolerability from placebo.
Conclusions: The present study provides robust evidence that an 8-week treatment period with tianeptine 25-50 mg is efficacious and well tolerated in depressed patients aged 65 years or older.
Trial Registration: EudraCT identifier: 2012-005612-26
J Clin Psychiatry 2018;79(4):17m11741
To cite: Emsley R, Ahokas A, Suarez A, et al. Efficacy of tianeptine 25-50 mg in elderly patients with recurrent major depressive disorder: an 8-week placebo- and escitalopram-controlled study. J Clin Psychiatry. 2018;79(4):17m11741.
To share: https://doi.org/10.4088/JCP.17m11741
© Copyright 2018 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Faculty of Medicine and Health Sciences, University of Stellenbosch, Parow, Cape Town, South Africa
bMehilפinen Clinic, Helsinki, Finland
cEstimulación Magnética Transcraneal de México S.C., Mexico City, Mexico
dClinica de Psihiatrie 1, Spitalul Clinic de Neuropsihiatrie, Craiova, Romania
eII. Psychiatrická Klinika Lekárskej Fakulty UPJÅ a Univerzitnej Nemocnice L. Pasteura, KoÅ¡ice, Slovakia
fWest-Tallinn Central Hospital Centre of Psychiatry, Tallinn, Estonia
gClinic of Psychiatry, University Hospital “Alexandrovska,” Sofia, Bulgaria
hDepartment of Psychiatry, Korea University Anam Hospital, Seoul, Korea
iPsychiatrie Générale Secteur, CHS La Chartreuse, Dijon, France
jNZOZ Centrum Kultury, Higieny i Zdrowia Psychicznego, Bydgoszcz, Poland
kDepartment of Psychological Medicine, University Malaya Medical Centre, Kuala Lumpur, Malaysia
lInstitut de Recherches Internationales Servier, Suresnes, France
*Corresponding author: Robin Emsley, MD, Department of Psychiatry, Faculty of Medicine and Health Sciences, University of Stellenbosch, Clinical Bldg Fransie van Zyl Ave, Parow, Cape Town 8000 South Africa ([email protected]).
Depression is common among elderly people,1-4 although a majority either are undiagnosed5 or do not receive appropriate treatment. Reasons for underdiagnosis include the atypical presentation of the illness, concomitant cognitive decline, inadequate diagnostic tools, and a preconception that depression is a normal part of aging.6,7 Depression is frequently confused with the effects of multiple illnesses and the medicines used to treat them.8-10 The main reasons for undertreatment include multimorbidity, concerns about adverse events and drug interactions, and lack of confidence in the efficacy and safety of treatments.
Placebo-controlled trials in elderly depressed patients have examined the efficacy of different classes of antidepressants.11-24 The efficacy of second-generation antidepressants is generally modest25,26; no class has been shown to have superior efficacy over any other, and published guidelines from the United States, United Kingdom, and Canada for antidepressant prescribing in older age are influenced more by expert opinions than by evidence given the few positive placebo-controlled randomized clinical trials.27 The choice of treatment is usually made on a case-by-case basis, taking into account patient characteristics and the tolerability and safety profile of a particular drug. The latter is a major concern given the sensitivity of elderly patients, who frequently present with comorbidities and multiple comedications, as adverse events may lead to premature treatment cessation and consequent depressive relapse.
The antidepressant efficacy of tianeptine has been demonstrated in depressed adults and is associated with a good acceptability profile.28-36 Previous trials have also shown the efficacy and safety of tianeptine in elderly patients, including beneficial effects on cognitive performance.37-39 However, a double-blind, randomized, placebo-controlled trial in elderly patients has not been conducted. Given the widespread use of antidepressant medications in elderly people, and the data indicating that the efficacy of these treatments is limited, an antidepressant such as tianeptine with a possible distinctive mechanism of action may be of interest. Tianeptine modulates monoaminergic neurotransmission, counteracts stress-induced impairment in synaptic glutamatergic neurotransmission and neuroplasticity in limbic areas and decreases stress-related hypothalamic-pituitary-adrenal axis overactivity,40 is a weak agonist of mu- and delta-opioid receptors,41,42 and has anti-inflammatory properties.43
Furthermore, given that tianeptine is generally well tolerated, with no drug-drug interactions, it may be particularly useful in elderly people with major depressive disorder (MDD).
The primary objective of this double-blind study was to evaluate the efficacy of tianeptine 25-50 mg compared with placebo in the 8-week treatment of elderly patients suffering from recurrent MDD. Escitalopram 5-10 mg was used as an active comparator. The secondary objectives were to evaluate the potential clinical benefit of tianeptine on clinical measures including response rates, functional impairment, and overall acceptability in this population.
METHODS
The study was approved by local ethics committees and was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki, as revised in Fortaleza, 2013. Participants provided written informed consent prior to participating in the trial. The study started in October 2013 and ended in January 2016, and it is registered with EudraCT (identifier: 2012-005612-26).
Patients and Assessments
Elderly outpatients (n = 311), aged at least 65 years, with a primary diagnosis of moderate to severe episode of recurrent MDD according to DSM-IV-TR criteria, were recruited by psychiatrists from 44 sites in Bulgaria, Estonia, Finland, France, Republic of Korea, Malaysia, Mexico, Poland, Romania, and Slovakia. The Mini-International Neuropsychiatric Interview44 was used to confirm the diagnosis of MDD and check for potential comorbid psychiatric disorders. Symptom severity was assessed at 0, 2, 4, 6, and 8 weeks using the 17-item Hamilton Depression Rating Scale (HDRS17)45 and the Clinical Global Impressions (CGI) scale46 and at 0, 2, and 8 weeks by the Hospital Anxiety and Depression scale (HAD)47 and the Sheehan Disability Scale (SDS).48 Subjects had to have a HDRS17 total score ≥ 22, a score ≥ 4 on item 1 of the CGI scale,46 and a HAD47 depression subscore ≥ 11. Subjects with a greater than 20% reduction on the HDRS17 total score between the selection visit (7 to 3 days before inclusion) and the inclusion visit (week 0) were excluded. The current depressive episode must have lasted at least 4 weeks (but no more than 12 months), with or without melancholic features, without seasonal pattern, without psychotic features, and without catatonic features. Comorbid generalized anxiety disorder was allowed.

- While use of antidepressants in later-life major depressive disorder (MDD) is widespread, there is a paucity of placebo-controlled studies indicating the efficacy of these treatments.
- Tianeptine is a viable and attractive option for treating the medically complex population of elderly patients with MDD.
All patients must have had a stable medical history and underwent 12-lead electrocardiogram (ECG) and clinical laboratory tests.
The following exclusion criteria were applied. Patients with any of the following disorders were excluded: MDD single episode, bipolar I and II disorder, dysthymic disorder, depression superimposed on dysthymic disorder (double depression), schizoaffective depressive disorder or bipolar type, MDD associated with Alzheimer’s disease or other dementia or mild cognitive impairment, panic disorder, agoraphobia, specific phobia, social phobia, obsessive-compulsive disorder, posttraumatic stress disorder, acute stress disorder, and any psychotic disorder diagnosed according to DSM-IV-TR criteria. Patients with severe neurologic disorders or severe or unstable medical conditions were excluded, as were patients with alcohol or drug abuse or dependence within the past 12 months, those with marked suicidal intent and/or known suicidal tendencies during the current episode, and those with transaminase values > 2 times the upper limit of normal reference range (ULN), alkaline phosphatase > 3 ULN, and/or total bilirubin > 2 ULN. Finally, patients were excluded if they had not responded to an appropriate dose of 2 antidepressant drugs of different classes (used for at least 4 weeks) for the current episode or if they had received any of the following therapies: electroconvulsive therapy, transcranial magnetic stimulation, or insight-oriented and structured psychotherapy started within 3 months before inclusion; light therapy started within 2 weeks; or depot neuroleptics within 6 months. Washout times for medications were 1 week for antidepressants (2 weeks for nonselective monoamine oxidase inhibitors, 5 weeks for fluoxetine), 12 weeks for antipsychotics given at therapeutic doses, and 2 weeks for antipsychotics given at low doses. Benzodiazepines, zolpidem, and zopiclone were permitted if they had been initiated at least 4 weeks before inclusion and used at stable dosage up to week 8.
Trial Medication
Randomization was balanced (nonadaptive), and stratified by center using an Interactive Response System. Treatments were identically labeled.
Escitalopram was initiated at 5 mg/d, and at week 2, as specified in its Summary of Product Characteristics, the dose was increased to 10 mg/d. For tianeptine-treated patients, in the event of insufficient improvement of depressive symptoms, the initial dosage of 25 mg could be increased up to 50 mg/d, according to a predefined dose adjustment algorithm, comprising either a HDRS total score decrease from inclusion of less than 20% or CGI-Improvement (CGI-I) score ≥ 4 (ie, no improvement or worsening). Both investigators and subjects were blind to the up-titration. The criteria for the dose adjustment, as well as the identity of those participants who underwent dose adjustment, was not disclosed to the investigators or the patients in order to minimize any subjective effects that may be associated with dose adjustment. At the end of the 8-week mandatory period (or in cases of premature withdrawal if, in the investigator’s opinion, this was advisable) the dose of escitalopram was gradually reduced during a double-blind tapering period of 1 week to avoid possible withdrawal reactions after the 8-week treatment period. Given that tianeptine is not associated with withdrawal symptoms,32 there was no down-titration for tianeptine or placebo.
Outcome Measures
The primary outcome measure was the absolute change from the inclusion visit (week 0) to week 8 on the HDRS17 total score. The response to treatment was a secondary measure performed at each visit after week 0.
Secondary outcome measures included response rates (defined as HDRS17 total score decrease from week 0 ≥ 50%; response to treatment according to CGI-I was defined as a score of 1 or 2). The SDS and HAD self-rating questionnaires were rated at the selection visit and at weeks 2 and 8.
Safety measures included adverse events reported at each visit, vital signs (heart rate and blood pressure recorded at each visit; weight at the selection visit and at weeks 0, 4, and 8), 12-lead ECGs at the selection visit, and laboratory tests (biochemistry, hematology) at the selection visit and week 8.
Training
All investigators underwent training in the use of the assessment instruments at the start of the study and once during the recruitment period. Videos of clinical cases were used to improve interrater reliability, but formal interrater reliability testing was not conducted.
Statistical Analyses
The efficacy analyses were performed in the full analysis set (FAS), defined as randomized patients who had taken at least 1 dose of medication, with a value at week 0 and at least 1 subsequent value for the primary efficacy measure. The primary analysis examined tianeptine-placebo differences on absolute change from week 0 to week 8 of the HDRS17 total score using a 2-way analysis of covariance model with treatment (including the 3 treatment groups) as a factor, with center as a random effect and week 0 HDRS17 total score as fixed covariate. Missing data were imputed using the last-observation-carried-forward (LOCF) approach. Escitalopram was compared with placebo as an active comparator.
A sensitivity analysis to the method of handling missing values was performed in the FAS. Treatment groups were compared on the absolute change from week 0 to week 8, using a mixed model for repeated measures (MMRM), including terms for the effects of treatment, week 0 HDRS17 total score, center as random effect, visit, and an interaction term for treatment and visit.
Additional analyses using a χ2 test assessed tianeptine-placebo differences on HDRS response to treatment (defined as a decrease from week 0 ≥ 50%) at week 8.
The tianeptine- and escitalopram-placebo differences were studied in the FAS over the 8-week treatment period on (1) value at week 8 of CGI-Severity of Illness (CGI-S) score and CGI-I score using a 2-sided Student t test for independent samples, (2) the response to treatment by CGI-I at week 8, using a χ2 test and the LOCF approach, and (3) the absolute change from week 0 to week 8 of HAD Anxiety and Depression subscores and total score and SDS work/daily activities, social life, and family life scores using 2-sided Student t tests for independent samples (post hoc analyses). Missing data were imputed using the LOCF approach.
For every safety measurement, descriptive statistics were provided by treatment group in the safety set (all included patients having taken at least 1 dose of study medication). For the rates of emergent adverse events (EAEs) related to study drug over the 8-week period, tianeptine- and escitalopram-placebo differences were compared using a χ2 test (post hoc analysis).
Except when specified as post hoc, the analyses were prespecified before breaking the blind. Statistical analysis was performed on SAS software, version 9.2 (Cary, North Carolina). The type I error was set at 5% (2-sided tests).
RESULTS
Patients
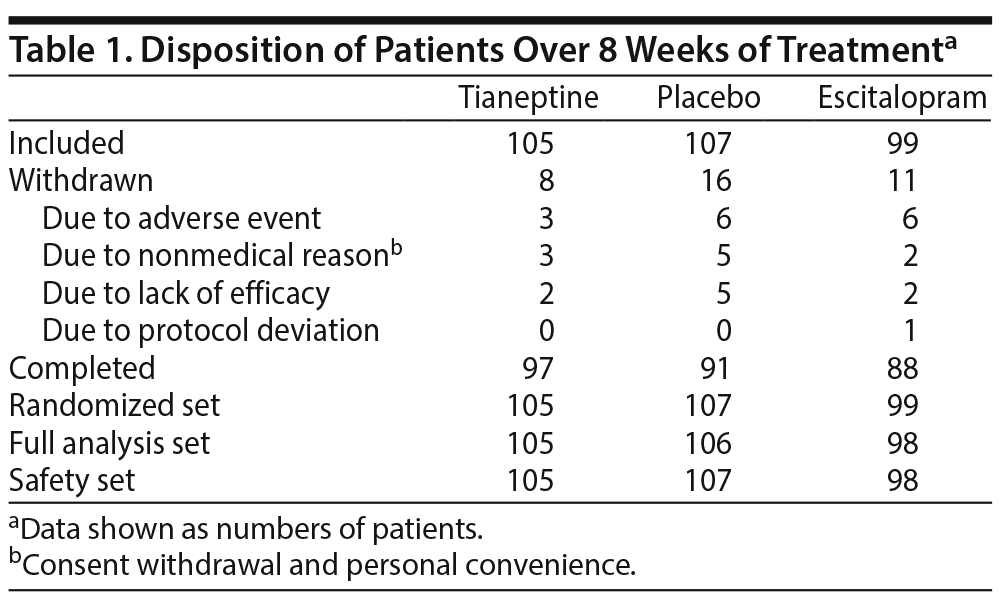
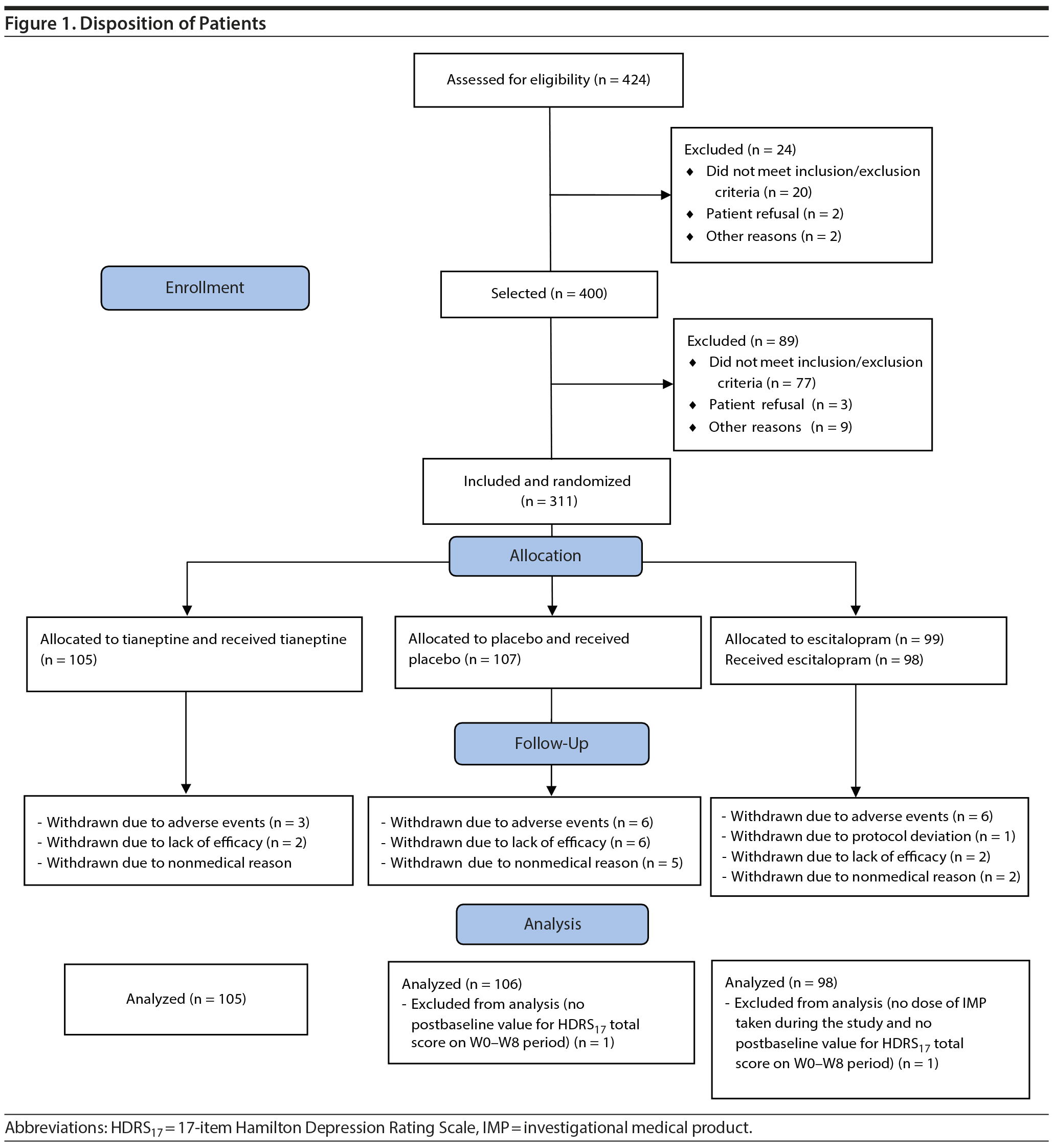
Patients were randomized to receive tianeptine (105 patients), escitalopram (99 patients), or placebo (107 patients), and 276 (88.7%) completed the 8-week treatment period. Reasons for withdrawal were mainly adverse events, lack of efficacy, and nonmedical reason (consent withdrawal and personal convenience) (Table 1, Figure 1).
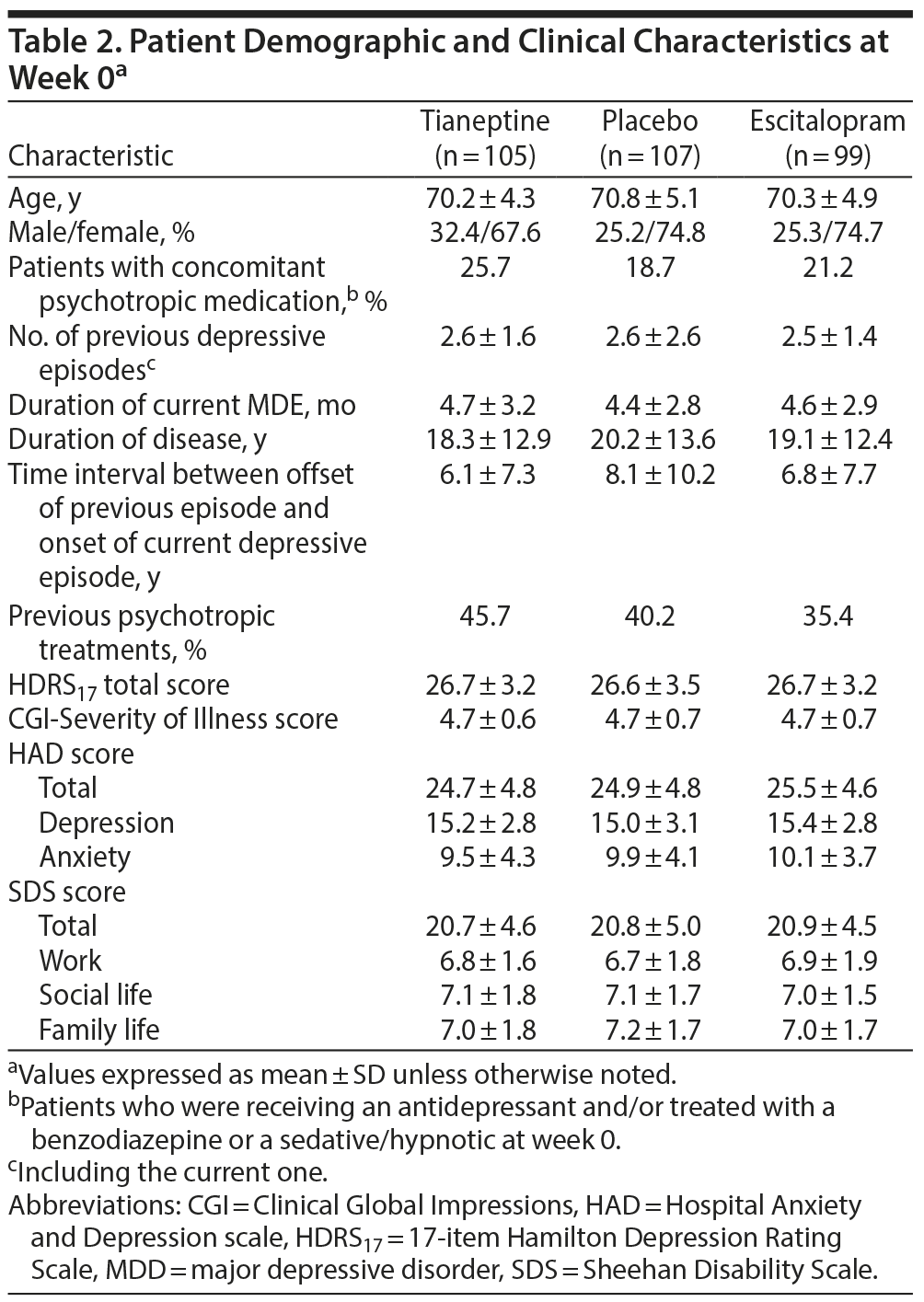
The patients’ mean ± SD age was 70.4 ± 4.8 years; 19.6% were aged ≥ 75 years, and 72.4% were women. At week 0, 62.7% of patients had moderate MDD and 37.3% had severe MDD without psychotic features, while 74.6% had melancholic features. There were no clinically relevant differences between groups for demographic criteria and clinical characteristics (Table 2). In all, 77.1% of patients taking tianeptine and 95.9% of patients taking escitalopram had a dose increase.
Efficacy
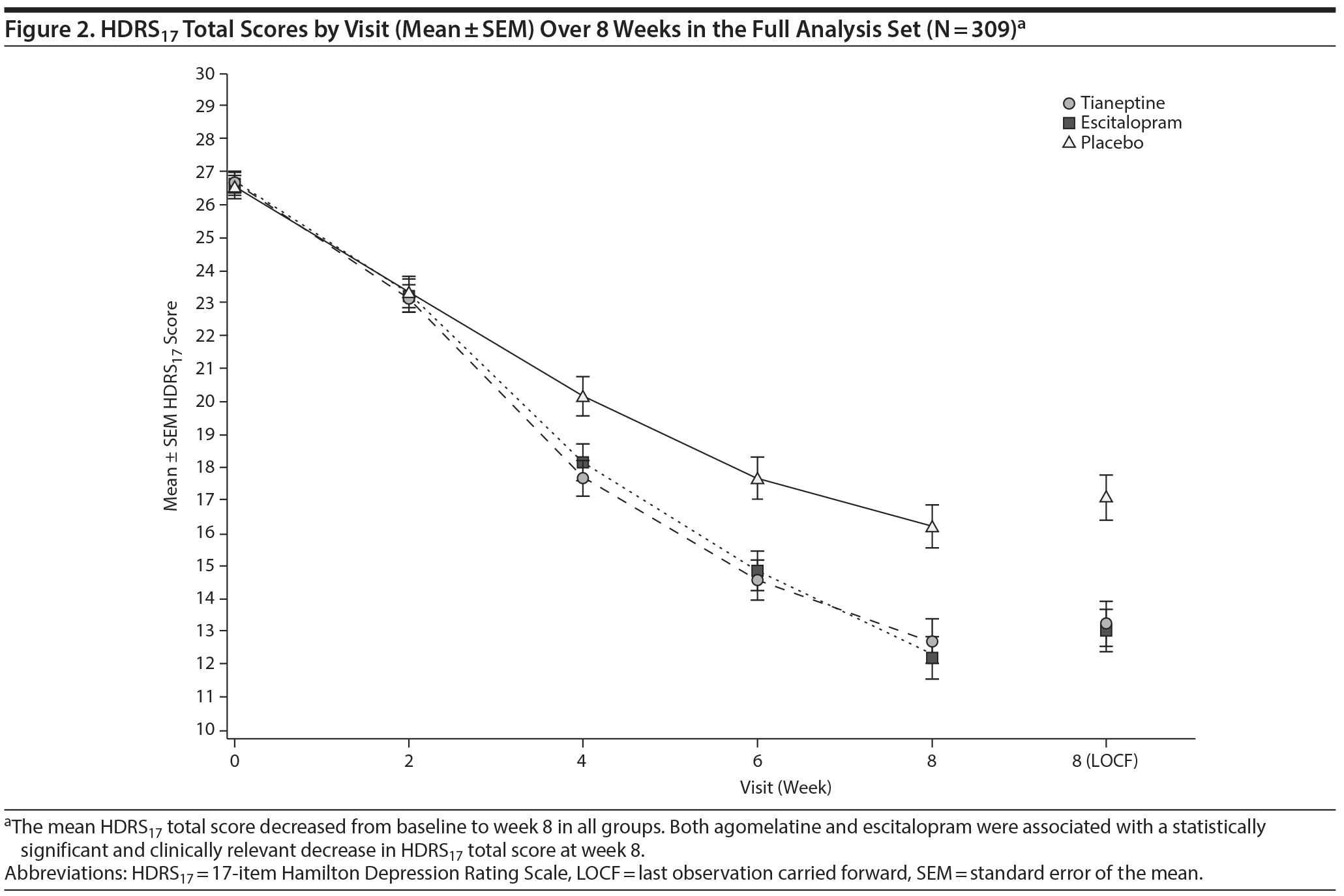
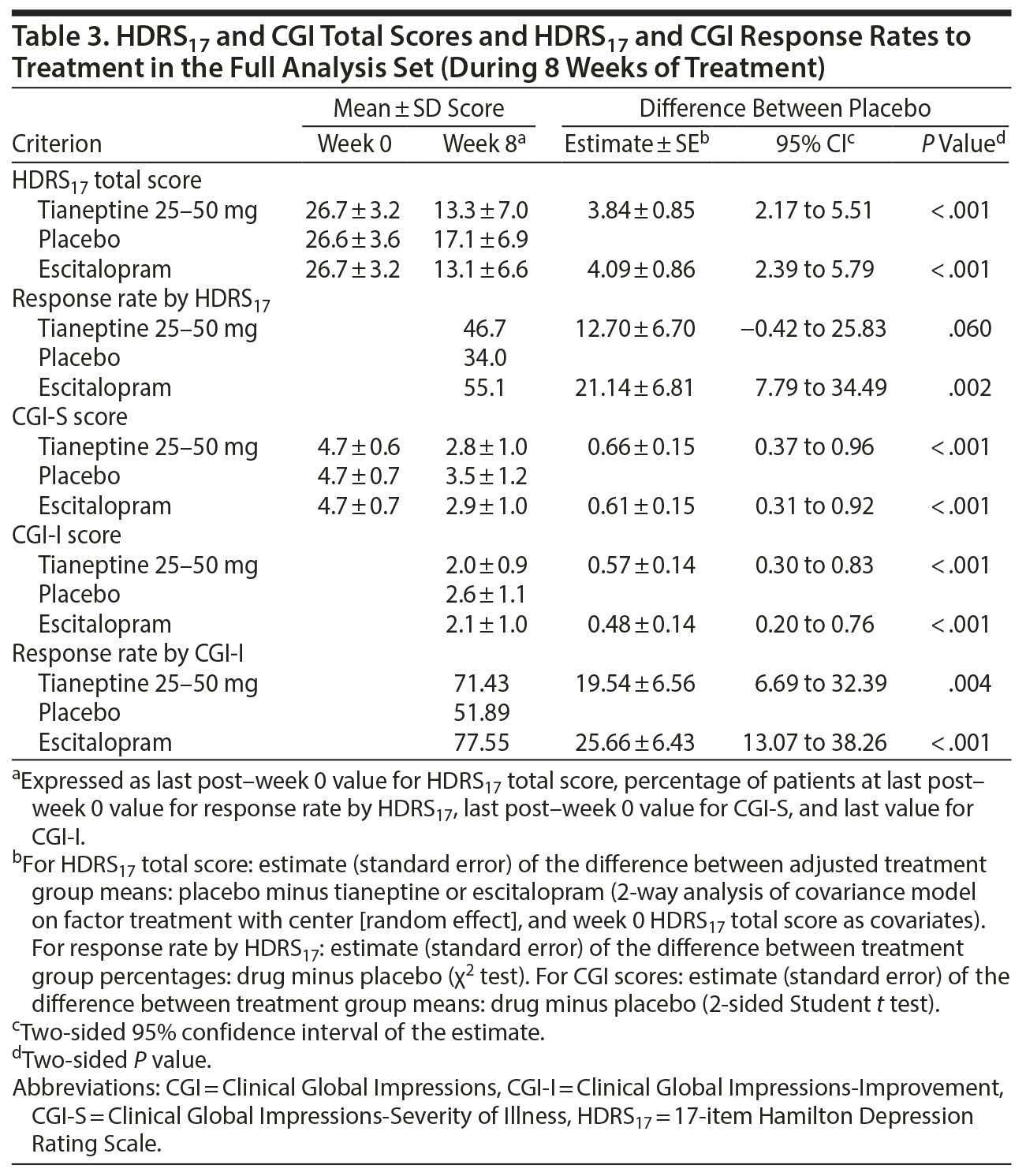
The mean HDRS17 total score decreased from week 0 to week 8 in both tianeptine and escitalopram groups (Figure 2). Compared with placebo, tianeptine was associated with a significantly greater decrease in symptoms at week 8 (main analysis: placebo minus tianeptine difference of 3.84 [0.85] points, 95% CI, 2.17 to 5.51; P < .001) (Table 3). Similarly, escitalopram was associated with a significantly greater decrease in HDRS17 total score at week 8 (difference vs placebo of 4.09 [0.86] points, 95% CI, 2.39 to 5.79; P < .001). The MMRM sensitivity analysis provided results consistent with the main analysis: a placebo minus tianeptine difference of 3.65 (0.92) (95% CI, 1.84 to 5.47; P < .001) and a placebo minus escitalopram difference of 4.53 (0.95) (95% CI, 2.67 to 6.40; P < .001).
In the FAS, at last post-week 0 value, the difference versus placebo in terms of response rate was 12.70% (6.70) for tianeptine (95% CI, −0.42 to 25.83; P = .06) and 21.14% (6.81) for escitalopram (95% CI, 7.79 to 34.49; P = .002) (Table 3).
The differences of the mean CGI-S and CGI-I scores between tianeptine and placebo were statistically significant at week 8. The placebo minus tianeptine difference was 0.66 (0.15) for CGI-S (95% CI, 0.37 to 0.96; P < .001) and 0.57 (0.14) for CGI-I (95% CI, 0.30 to 0.83; P < .001) (Table 3). The percentage of responders according to CGI-I was significantly higher in the tianeptine (71.4%) and escitalopram (77.6%) groups than in the placebo group (51.9%) (estimate [SE] = 19.54% [6.56], P = .004 and estimate [SE] = 25.66% [6.43], P < .001, respectively) (Table 3).
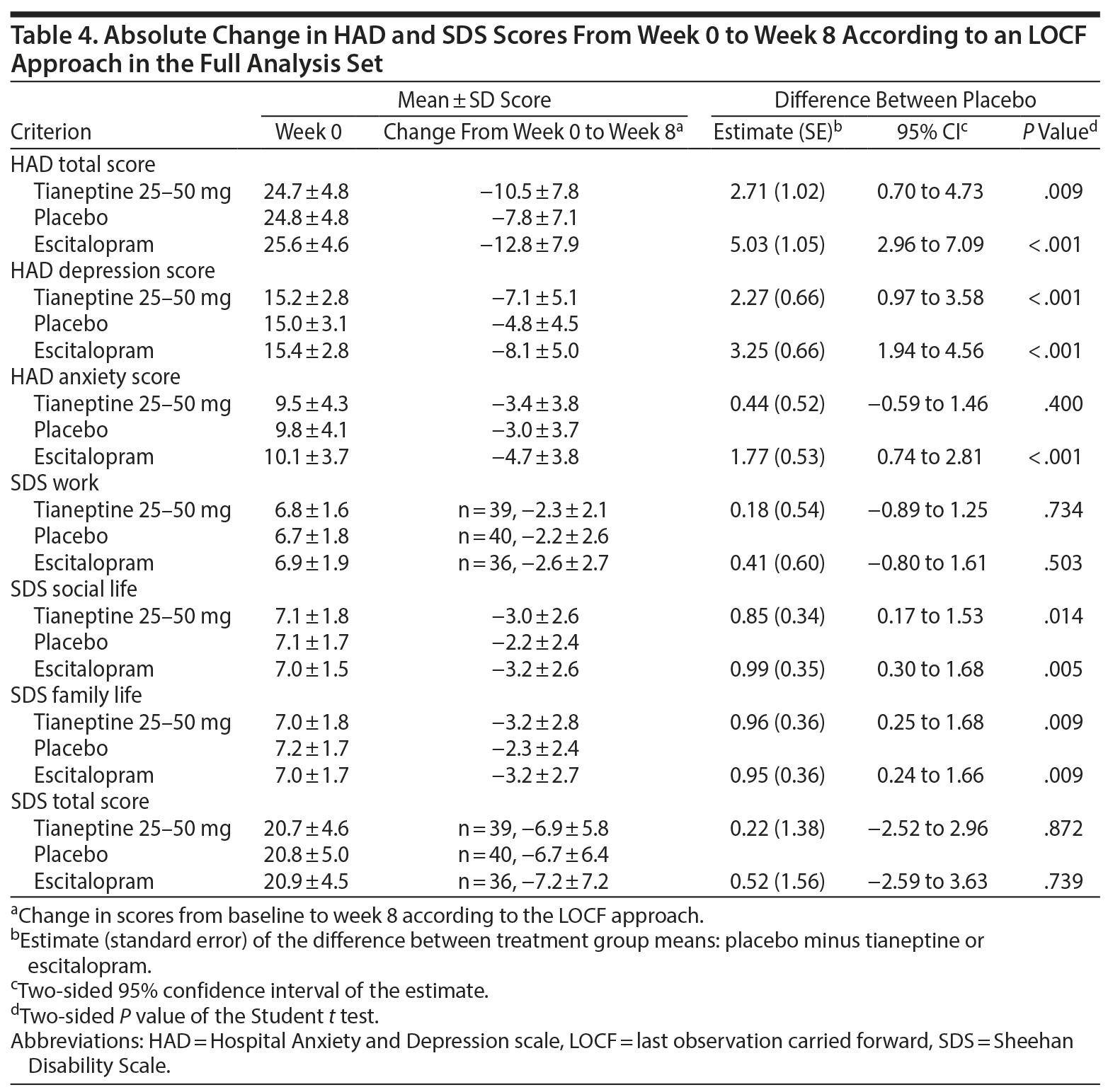
Absolute changes in HAD scores over 8 weeks were statistically greater in both treatment groups compared to placebo (except for the HAD anxiety score for tianeptine vs placebo). Patients reported statistically fewer symptom-related impairments for both tianeptine and escitalopram vs placebo for SDS social life and SDS family life scores, but not for SDS work and SDS total scores (Table 4).
Tolerability
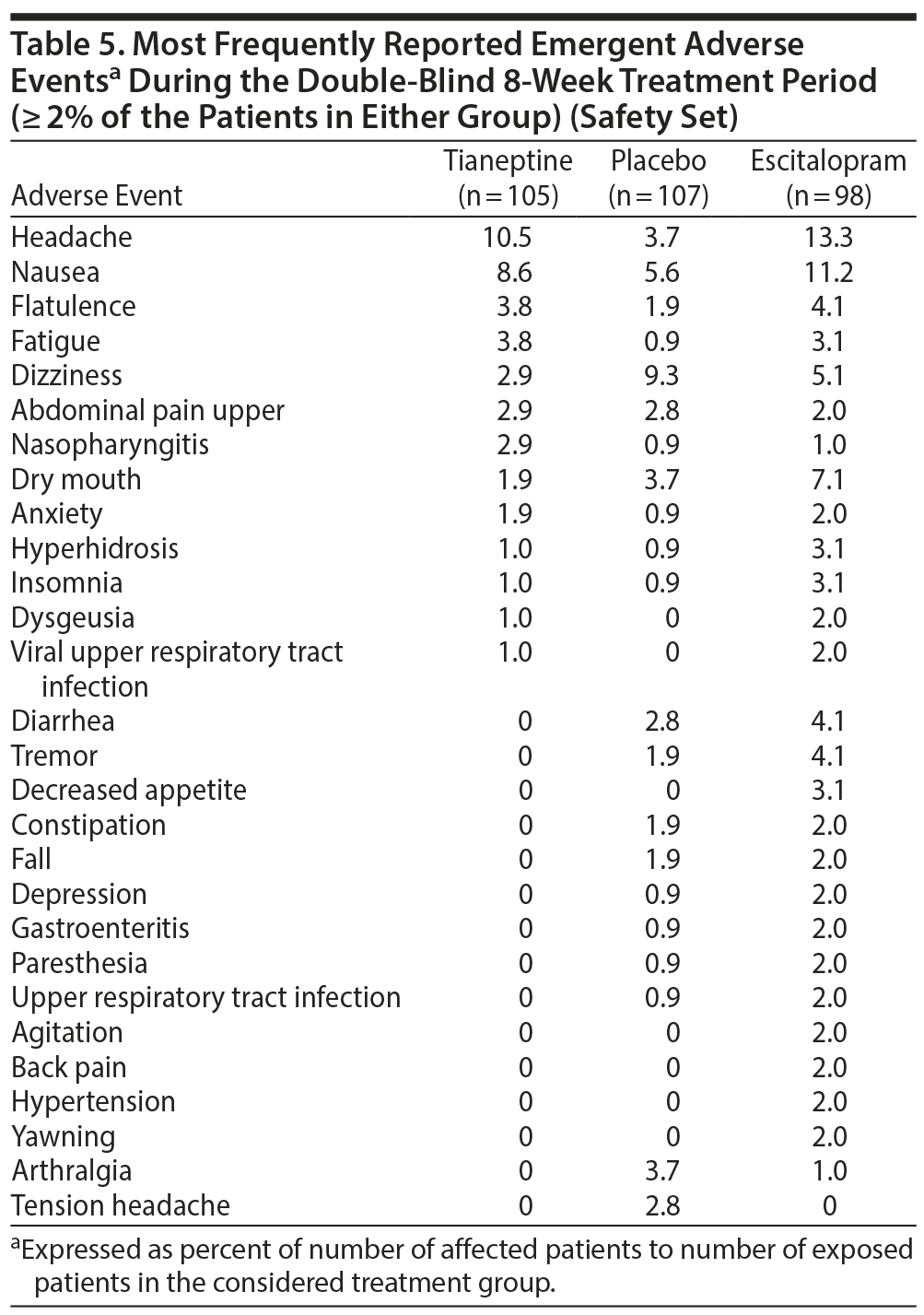
In the safety set, the percentage of patients with at least 1 EAE was 42.9% in the tianeptine group, 54.1% in the escitalopram group, and 41.1% in the placebo group. For EAEs related to the study drug, percentages of patients were 22.9% in the tianeptine group, 40.8% in the escitalopram group, and 20.6% in the placebo group. This equated to a tianeptine minus placebo difference of 2.30 (5.66) (95% CI, −8.80 to 13.39; P = .685, post hoc analysis) and an escitalopram minus placebo difference of 20.26 (6.32) (95% CI, 7.87 to 32.64; P = .002, post hoc analysis).
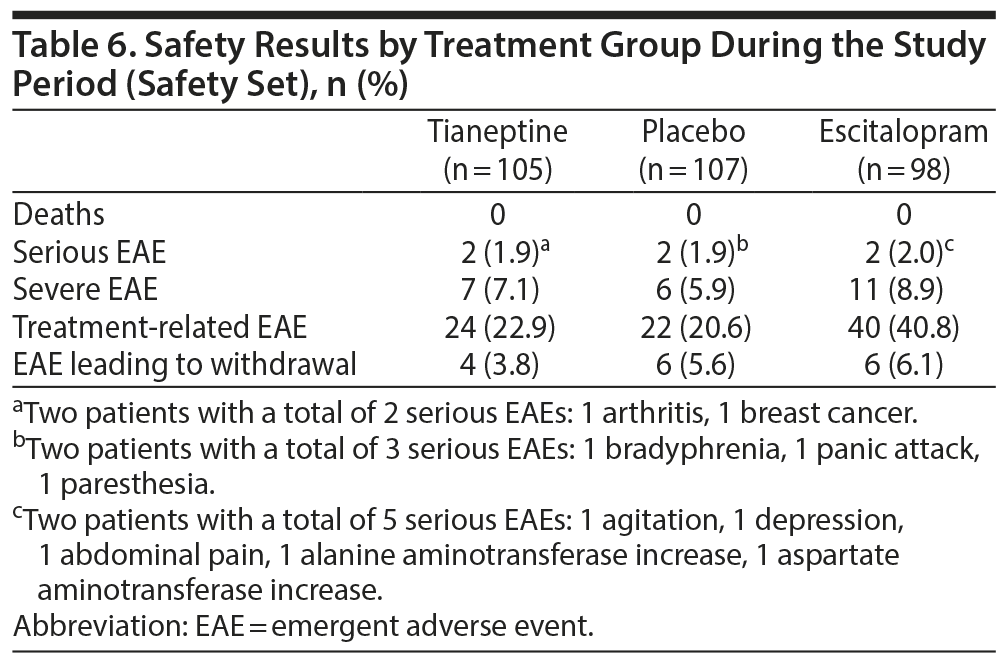
Headache, nausea, flatulence, fatigue, and dizziness were the most frequent EAEs reported by patients in treatment groups (Table 5). In the escitalopram group, several EAEs (eg, dry mouth, headache, nausea) were more frequently reported by patients than in other groups (Table 5). The majority of EAEs were rated as mild or moderate. The percentage of patients who experienced at least 1 EAE rated as severe was 7.1% in the tianeptine group, 8.9% in the escitalopram group, and 5.9% in the placebo group. Two patients in each treatment group reported serious adverse events (Table 6).
Frequencies of nonserious adverse events leading to premature discontinuation were 2.9% in the tianeptine group (3 patients), 4.1% in escitalopram group (4 patients), and 5.6% in placebo group (6 patients). Serious EAEs led to premature treatment withdrawal in 1 patient (1.0%) in the tianeptine group and 2 patients (2.0%) in the escitalopram group. No deaths were reported.
There were no clinically relevant between-group differences nor changes from week 0 to the last value on treatment for the biochemical and hematologic parameters, blood pressure, and weight.
DISCUSSION
The present study is one of few placebo-controlled studies with an active control group to demonstrate the efficacy and safety of an antidepressant in elderly depressed patients. According to the European Medicines Agency, a double-blind, randomized, placebo-controlled study with an active control and parallel groups represents an optimal design for the demonstration of an antidepressant efficacy. The 8-week treatment with tianeptine 25-50 mg was both effective and well tolerated in elderly depressed patients aged 65 years or older. The efficacy demonstrated on the HDRS17 total score is notable, with a placebo-tianeptine difference of 3.84 points in change from week 0 to week 8, and is supported by clinical response to treatment and consistent findings on CGI and HAD variables. The positive results obtained with escitalopram validate the sensitivity of the studied population and the robustness of the tianeptine data.
There are relatively few placebo-controlled studies reporting antidepressant efficacy for patients aged over 60-65 years.13-17,22,23 According to a meta-analysis of second-generation antidepressants,26 the efficacy was generally modest, heterogeneous across studies, and less pronounced in the more elderly. In addition, no treatment effect was noted for patients aged over 65 years, and the difference between response to placebo and active treatments was around 3%. In the present study, while not statistically significant, the tianeptine minus placebo response by HDRS17 was around 13%.
An excessively high response rate in the placebo arm may negate the ability of clinical studies of antidepressants to demonstrate efficacy. It is therefore critically important to minimize this risk. The placebo response rate observed in our study is in the range of what has been observed in previous trials conducted in late life depression and may be the result of several measures that were applied to improve the quality of MDD diagnosis and increase the sensitivity of the trial. In addition to the requirement of a minimum entry score on HDRS, the use of specific diagnostic criteria, ratings by the investigator, and self-evaluation by the patients helped to exclude unsuitable mildly ill patients. Furthermore, all clinicians underwent specific training for the diagnostic and outcome assessment instruments to ensure accurate evaluation and interrater reliability.
As is the case for younger adults, effective treatment of depression in older people should also result in the improvement of their functional status.49 Our study is the second to apply the SDS to a geriatric population.13 We were able to demonstrate that tianeptine improved some symptom-related functional impairments in elderly patients. Given that changes in general, core family, and social functioning place additional burden on patients and can contribute to a diminished quality of life, the positive effect of tianeptine on the functional status of patients is therefore of considerable interest.
An important consideration in the choice of an antidepressant for the elderly population is its safety and tolerability. Elderly patients have increasingly complex medication regimens that can interfere with the effectiveness of antidepressant drugs; they can be more sensitive to potential adverse effects of treatment and drug interactions.50 Elderly people may also be unwilling to take their medicines because side effects that may be considered minor in younger patients may carry more significant risk for them.51 Tianeptine 25-50 mg is well tolerated by elderly patients, with no significant difference from placebo in terms of the rate of EAEs (in contrast to escitalopram). Only 3.8% of tianeptine-treated patients discontinued the trial due to EAEs, a rate lower (although not significantly) than those noted in the 2 other groups and comparable with discontinuation rates due to EAEs among tianeptine-treated nonelderly adult patients (aged < 65 years). Tianeptine differs from most antidepressants in that it is not primarily metabolized by the hepatic cytochrome P450 system,52 so it is devoid of drug interactions. This may be particularly relevant for medically complex patients and may facilitate tolerability and adherence to treatment.
Since elderly people are more sensitive to medicines, it is generally recommended that doctors prescribe lower doses at first.53 For that reason, while the usual therapeutic dose of tianeptine is 37.5 mg/d, an initiation dose of 25 mg/d has been recommended. However, the majority of tianeptine-treated patients (77.1%) in the present study received 50 mg from the second week, and this dose was efficacious, safe, and well tolerated. This finding is similar to the results obtained from 2 positive placebo-controlled studies in young adults28,29 and consistent with the good acceptability observed over 1 year in an open study.54 However, as the present trial was of short duration, we were unable to assess any late-onset side effects that are well described with antidepressants and may affect patients’ physical health, quality of life, social functioning, and treatment adherence.55
The use of a relatively large number of exclusion criteria in this study can be considered as a limitation. In particular, the exclusion of patients with comorbid Axis I disorders (including comorbid anxiety disorders other than generalized anxiety disorder), mild cognitive impairment, depression lasting less than 12 months, and failure to respond to 2 antidepressant classes in the current episode limits generalizability of the findings as our participants were not representative of the typical older depressed patient seen in clinical practice. However, similar exclusion criteria have been used in other trials, so the results are at least comparable to the existing literature.
In conclusion, given that depression is very common in elderly people, carries an increased risk of morbidity and mortality, and is still undertreated, the results of this study are of importance. The efficacy of tianeptine on depressive symptoms together with improved patient functioning and a good safety profile make this antidepressant an attractive option for treating this medically complex population.
Submitted: June 9, 2017; accepted November 27, 2017.
Published online: July 3, 2018.
Author contributions: The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Potential conflicts of interest: In the past 3 years, Dr Emsley has participated in speakers/advisory boards and received honoraria from AstraZeneca, Janssen, Lundbeck, Servier, and Otsuka and has received research funding from Janssen and Lundbeck. Drs Blanchot, Crutel, Antoine, and Penelaud are employees of Servier. Drs Ahokas, Suarez, Marinescu, Dóci, Lehmets, Milanova, Lee, Didi, and Sulaiman and Mr Araszkiewicz declare no financial conflicts of interest.
Funding/support: This study was sponsored by Servier (Suresnes, France).
Role of the sponsor: The sponsor of the study participated in study design, data collection, data interpretation, and writing of the report.
REFERENCES
1. Bergdahl E, Gustavsson JM, Kallin K, et al. Depression among the oldest old: the Ume×¥ 85+ study. Int Psychogeriatr. 2005;17(4):557-575. PubMed CrossRef
2. Luppa M, Sikorski C, Luck T, et al. Age- and gender-specific prevalence of depression in latest-life—systematic review and meta-analysis. J Affect Disord. 2012;136(3):212-221. PubMed CrossRef
3. Lyness JM, Caine ED, King DA, et al. Depressive disorders and symptoms in older primary care patients: one-year outcomes. Am J Geriatr Psychiatry. 2002;10(3):275-282. PubMed CrossRef
4. Baldessarini RJ, Forte A, Selle V, et al. Morbidity in depressive disorders. Psychother Psychosom. 2017;86(2):65-72. PubMed CrossRef
5. Falagas ME, Vardakas KZ, Vergidis PI. Under-diagnosis of common chronic diseases: prevalence and impact on human health. Int J Clin Pract. 2007;61(9):1569-1579. PubMed CrossRef
6. Lapid MI, Rummans TA. Evaluation and management of geriatric depression in primary care. Mayo Clin Proc. 2003;78(11):1423-1429. PubMed CrossRef
7. Montano CB. Primary care issues related to the treatment of depression in elderly patients. J Clin Psychiatry. 1999;60(suppl 20):45-51. PubMed
8. Gleason OC, Pierce AM, Walker AE, et al. The two-way relationship between medical illness and late-life depression. Psychiatr Clin North Am. 2013;36(4):533-544. PubMed CrossRef
9. Taylor WD, Krishnan KR. Management of late-life depression: focus on comorbid conditions. Psychopharmacol Bull. 2002;36(4):113-130. PubMed
10. Neuropsychiatr Dis Treat. 2016;12:2105-2114. PubMed CrossRef
11. Allard P, Gram L, Timdahl K, et al. Efficacy and tolerability of venlafaxine in geriatric outpatients with major depression: a double-blind, randomised 6-month comparative trial with citalopram. Int J Geriatr Psychiatry. 2004;19(12):1123-1130. PubMed CrossRef
12. Bose A, Li D, Gandhi C. Escitalopram in the acute treatment of depressed patients aged 60 years or older. Am J Geriatr Psychiatry. 2008;16(1):14-20. PubMed CrossRef
13. Heun R, Ahokas A, Boyer P, et al; Agomelatine Study Group. The efficacy of agomelatine in elderly patients with recurrent major depressive disorder: a placebo-controlled study. J Clin Psychiatry. 2013;74(6):587-594. PubMed CrossRef
14. Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol. 2012;27(4):215-223. PubMed CrossRef
15. Rapaport MH, Schneider LS, Dunner DL, et al. Efficacy of controlled-release paroxetine in the treatment of late-life depression. J Clin Psychiatry. 2003;64(9):1065-1074. PubMed CrossRef
16. Rapaport MH, Lydiard RB, Pitts CD, et al. Low doses of controlled-release paroxetine in the treatment of late-life depression: a randomized, placebo-controlled trial. J Clin Psychiatry. 2009;70(1):46-57. PubMed CrossRef
17. Raskin J, Wiltse CG, Siegal A, et al. Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: an 8-week, double-blind, placebo-controlled trial. Am J Psychiatry. 2007;164(6):900-909. PubMed CrossRef
18. Raskin J, Wiltse CG, Dinkel JJ, et al. Safety and tolerability of duloxetine at 60 mg once daily in elderly patients with major depressive disorder. J Clin Psychopharmacol. 2008;28(1):32-38. PubMed CrossRef
19. Roose SP, Sackeim HA, Krishnan KR, et al; Old-Old Depression Study Group. Antidepressant pharmacotherapy in the treatment of depression in the very old: a randomized, placebo-controlled trial. Am J Psychiatry. 2004;161(11):2050-2059. PubMed CrossRef
20. Rossini D, Serretti A, Franchini L, et al. Sertraline versus fluvoxamine in the treatment of elderly patients with major depression: a double-blind, randomized trial. J Clin Psychopharmacol. 2005;25(5):471-475. PubMed CrossRef
21. Schatzberg A, Roose S. A double-blind, placebo-controlled study of venlafaxine and fluoxetine in geriatric outpatients with major depression. Am J Geriatr Psychiatry. 2006;14(4):361-370. PubMed CrossRef
22. Schneider LS, Nelson JC, Clary CM, et al; Sertraline Elderly Depression Study Group. An 8-week multicenter, parallel-group, double-blind, placebo-controlled study of sertraline in elderly outpatients with major depression. Am J Psychiatry. 2003;160(7):1277-1285. PubMed CrossRef
23. Sheikh JI, Cassidy EL, Doraiswamy PM, et al. Efficacy, safety, and tolerability of sertraline in patients with late-life depression and comorbid medical illness. J Am Geriatr Soc. 2004;52(1):86-92. PubMed CrossRef
24. Kennedy SH, Andersen HF, Lam RW. Efficacy of escitalopram in the treatment of major depressive disorder compared with conventional selective serotonin reuptake inhibitors and venlafaxine XR: a meta-analysis. J Psychiatry Neurosci. 2006;31(2):122-131. PubMed
25. Nelson JC, Delucchi K, Schneider LS. Efficacy of second generation antidepressants in late-life depression: a meta-analysis of the evidence. Am J Geriatr Psychiatry. 2008;16(7):558-567. PubMed CrossRef
26. Tedeschini E, Levkovitz Y, Iovieno N, et al. Efficacy of antidepressants for late-life depression: a meta-analysis and meta-regression of placebo-controlled randomized trials. J Clin Psychiatry. 2011;72(12):1660-1668. PubMed CrossRef
27. Mulsant BH, Blumberger DM, Ismail Z, et al. A systematic approach to pharmacotherapy for geriatric major depression. Clin Geriatr Med. 2014;30(3):517-534. PubMed CrossRef
28. Cassano GB, Heinze G, L×´o H, et al; Study Group. A double-blind comparison of tianeptine, imipramine and placebo in the treatment of major depressive episodes. Eur Psychiatry. 1996;11(5):254-259. PubMed CrossRef
29. Costa e Silva JA, Ruschel SI, Caetano D, et al. Placebo-controlled study of tianeptine in major depressive episodes. Neuropsychobiology. 1997;35(1):24-29. PubMed CrossRef
30. Daléry J, Dagens-Lafant V, De Bodinat C. Value of tianeptine in treating major recurrent unipolar depression: study versus placebo for 16 1/2 months of treatment [in French]. Encephale. 1997;23(1):56-64. PubMed
31. Kasper S, Olié JP. A meta-analysis of randomized controlled trials of tianeptine versus SSRI in the short-term treatment of depression. Eur Psychiatry. 2002;17(suppl 3):331-340. PubMed CrossRef
32. Lepine JP, Altamura C, Ansseau M, et al. Tianeptine and paroxetine in major depressive disorder, with a special focus on the anxious component in depression: an international, 6-week double-blind study dagger. Hum Psychopharmacol. 2001;16(3):219-227. PubMed CrossRef
33. L×´o H, Saiz-Ruiz J, Ansseau M, et al; Costa e Silva JACE. Efficacy and safety of tianeptine in the treatment of depressive disorders in comparison with fluoxetine. J Affect Disord. 1999;56(2-3):109-118. PubMed CrossRef
34. Nickel T, Sonntag A, Schill J, et al. Clinical and neurobiological effects of tianeptine and paroxetine in major depression. J Clin Psychopharmacol. 2003;23(2):155-168. PubMed CrossRef
35. ;Novotny V, Faltus F. Tianeptine and fluoxetine in major depression: a 6-week randomised double-blind study. Hum Psychopharmacol. 2002;17(6):299-303. PubMed CrossRef
36. Waintraub L, Septien L, Azoulay P. Efficacy and safety of tianeptine in major depression: evidence from a 3-month controlled clinical trial versus paroxetine. CNS Drugs. 2002;16(1):65-75. PubMed CrossRef
37. Brion S, Audrain S, de Bodinat C. Major depressive episodes in patients over 70 years of age: evaluation of the efficiency and acceptability of tianeptine and mianserin [in French]. Presse Med. 1996;25(9):461-468. PubMed
38. Chapuy P, Cuny G, Delomier Y, et al. Depression in elderly patients: value of tianeptine in 140 patients treated for 1 year [in French]. Presse Med. 1991;20(37):1844-1852. PubMed
39. Saiz-Ruiz J, Montes JM, Alvarez E, et al. Tianeptine therapy for depression in the elderly. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22(2):319-329. PubMed CrossRef
40. McEwen BS, Chattarji S, Diamond DM, et al. The neurobiological properties of tianeptine (Stablon): from monoamine hypothesis to glutamatergic modulation. Mol Psychiatry. 2010;15(3):237-249. PubMed CrossRef
41. Gassaway MM, Rives ML, Kruegel AC, et al. The atypical antidepressant and neurorestorative agent tianeptine is a μ-opioid receptor agonist. Transl Psychiatry. 2014;4(7):e411. PubMed CrossRef
42. Samuels BA, Nautiyal KM, Kruegel AC, et al. The behavioral effects of the antidepressant tianeptine require the mu-opioid receptor. Neuropsychopharmacology. 2017;42(10):2052-2063. PubMed CrossRef
43. Mutlu O, Gumuslu E, Ulak G, et al. Effects of fluoxetine, tianeptine and olanzapine on unpredictable chronic mild stress-induced depression-like behavior in mice. Life Sci. 2012;91(25-26):1252-1262. PubMed CrossRef
44. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22-33, quiz 34-57. PubMed
45. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56-62. PubMed CrossRef
46. Guy W. ECDEU Assessment Manual for Psychopharmacology. Revised Edition. Washington, DC: US Department of Health, Education, and Welfare; 1976.
47. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361-370. PubMed CrossRef
48. Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. Int Clin Psychopharmacol. 1996;11(suppl 3):89-95. PubMed CrossRef
49. Braam AW, Prince MJ, Beekman AT, et al. Physical health and depressive symptoms in older Europeans: results from EURODEP. Br J Psychiatry. 2005;187(1):35-42. PubMed CrossRef
50. Lotrich FE, Pollock BG. Aging and clinical pharmacology: implications for antidepressants. J Clin Pharmacol. 2005;45(10):1106-1122. PubMed CrossRef
51. Pollock BG. Adverse reactions of antidepressants in elderly patients. J Clin Psychiatry. 1999;60(suppl 20):4-8. PubMed
52. Wagstaff AJ, Ormrod D, Spencer CM. Tianeptine: a review of its use in depressive disorders. CNS Drugs. 2001;15(3):231-259. PubMed CrossRef
53. McDonald WM, Salzman C, Schatzberg AF. Depression in the elderly. Psychopharmacol Bull. 2002;36(suppl 2):112-122. PubMed
54. L×´o H, Ganry H, Marey C, et al. Tolerability of tianeptine in 170 patients with depression treated during one year [in French]. Encephale. 1990;16(6):445-452. PubMed
55. Cassano P, Fava M. Tolerability issues during long-term treatment with antidepressants. Ann Clin Psychiatry. 2004;16(1):15-25. PubMed CrossRef
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Geriatric Psychiatry section. Please contact Jordan F. Karp, MD, at [email protected], or Gary W. Small, MD, at [email protected].
Save
Cite
Advertisement
GAM ID: sidebar-top