Abstract
Objective: The relationship between the duration of major depressive disorder (MDD) and therapeutic response to standard antidepressant treatment (SAT) is unknown. N-methyl-D-aspartate receptor uncompetitive antagonists are emerging drugs for MDD. We investigated whether the antidepressant effect of esmethadone (REL-1017) could be related to the duration of depression.
Methods: We analyzed data from a Phase 2a study of adjunctive treatment with esmethadone in MDD patients (DSM-5) with inadequate response to ongoing SAT (May 2018–August 2019). Patients were randomized to treatment with esmethadone 25 mg, esmethadone 50 mg, or placebo for 7 days, followed by an observation period (Days 7–14). Duration of depression was derived from 2 measures: (1) time from onset (TFO), calculated as the difference in years between age at trial enrollment and age at the onset of the first major depressive episode (MDE), and (2) TFO index, calculated by computing the years of illness duration (number of years from the beginning of MDD), divided by age and multiplied by 100. First, bivariate correlations between TFO and change from baseline (CFB) were calculated by Spearman ρ. Linear mixed-model analyses were also conducted.
Results: A total of 62 patients participated in the trial. The median values of time from MDD onset for the 62 patients were 11 years (absolute value) and 22% (percentage of life-years). Duration of depression was significantly correlated with Montgomery-Asberg Depression Rating Scale (MADRS) CFB on Day 14, even when controlling for the effect of current depression severity (MADRS baseline). In the linear mixed-model analyses, we found a significant effect of duration on reduction in MADRS score from T0 to subsequent assessments (P < .05). Number of previous MDEs and effect of esmethadone 50 mg when compared to 25 mg were not significant.
Conclusion: Esmethadone 25 and 50 mg were more effective in reducing MADRS scores in patients with shorter time from first MDE onset.
Trial Registration: ClinicalTrials.gov identifier: NCT03051256.
J Clin Psychiatry 2024;85(2):22m14735
Author affiliations are listed at the end of this article.
Major depressive disorder (MDD) is a very prevalent illness associated with significant disability, morbidity, and mortality.1 MDD is highly heterogeneous, including patients with variable duration and clinical severity of depressive symptoms. Available treatments for MDD, including serotonergic antidepressants, yield modest response and remission rates.2 Only about 1 in 3 patients with MDD experienced a remission of their depression following treatment with the selective serotonin reuptake inhibitor citalopram in the large, multicenter Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial.3 Thus, an unmet medical need exists for developing novel and more effective treatments for patients with MDD.
One possible approach for improving outcomes is to identify subgroups of depressed patients more likely to respond to a second treatment.4 Few clinically relevant tools have been developed for stratifying subgroups that predict outcomes.5 However, some clinical characteristics may help predict treatment outcomes. First, there is an inverse relationship between the duration of the major depressive episode (MDE) and treatment outcome (either response or remission).6 Specifically, a shorter duration of untreated disease—in terms of both first and recurrent episodes—is a favorable prognostic factor associated with both better treatment response and better long-term prognosis.7–11 Another clinical variable associated with treatment outcome is time to antidepressant response.5 Early improvements in depressive symptoms during treatment have been shown to predict better outcomes, including remission with an increased likelihood of symptomatic and functional recovery.9
There is limited knowledge of the relationship between time from the onset of the first MDE and antidepressant treatment response. Recently, the predictive value of early onset or late onset of depression on treatment outcome was investigated,12 and it was found that treatment outcome did not differ between patients with early or late depression onset. More severe first MDE at a younger age may correlate with recurrence and play a negative role in the long-term prognosis of MDD.13 It has been also suggested that the duration of untreated MDD may be associated with hippocampus volume loss and with lower rates of treatment response.14
In addition to the classic monoaminergic hypothesis of depression, it has been hypothesized that molecular mechanisms underlying MDD include γ-aminobutyric acid/glutamate dysfunction and astrocytic alteration as well as inflammatory mechanisms.15–18 N-methyl-D-aspartate receptor (NMDAR) hyperactivity resulting in impaired neural plasticity has been hypothesized as a potential target for esmethadone and other uncompetitive NMDAR antagonists.17–19 Synaptic plasticity, regulated by NMDAR activity, plays a crucial role in mood, learning, and cognitive function. Coherently, uncompetitive NMDAR antagonists are emerging as promising rapid antidepressants. The rapid antidepressant effects of ketamine have been replicated with esketamine,20 which has been approved for treatment-resistant depression. The dextromethorphan-bupropion combination has shown efficacy for MDD in Phase 2 and Phase 3 trials21,22 and has been recently approved for the treatment of MDD.
Esmethadone (REL-1017) is a novel uncompetitive NMDAR antagonist and rapid antidepressant candidate.23–25 Esmethadone was effective in rodent models of depressive-like behavior26,27 and may increase serum brain-derived neurotrophic factor (BDNF) levels in healthy humans.28 Preclinical studies suggest a mechanism of action related to BDNF and mammalian target of rapamycin 1 (mTORC1)–dependent neural plasticity.26 Phase 1 studies showed favorable tolerability and safety profiles,29 which were confirmed in a Phase 2a study where esmethadone showed rapid, robust, and sustained therapeutic effects in patients with MDD and inadequate response to ongoing standard antidepressant treatment (SAT).30 In the current study, we analyzed the effect of time from onset (TFO) of MDD on the therapeutic response to esmethadone.
METHODS
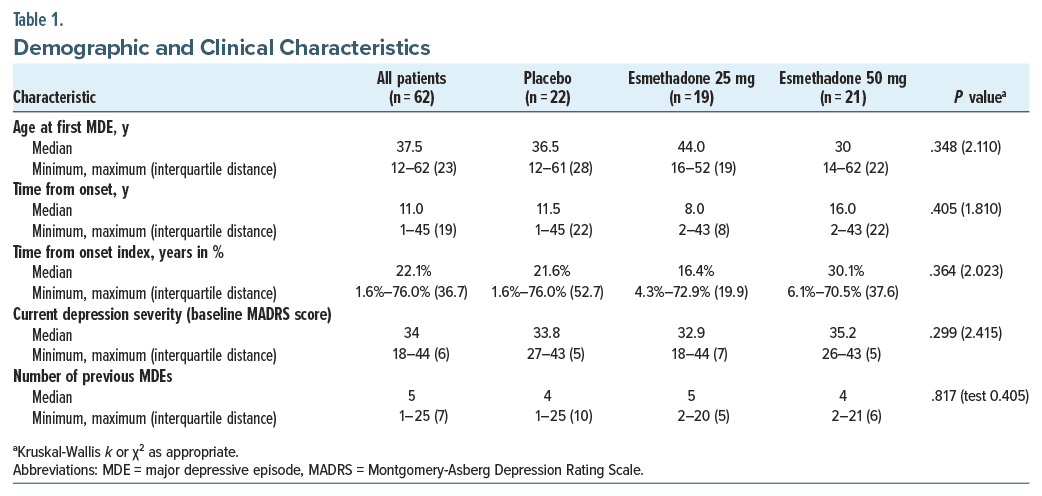
This was a post hoc analysis from a double-blind, placebo-controlled, 7-day, inpatient, 2-dose (25 and 50 mg), 3-arm, Phase 2a trial of esmethadone as adjunctive treatment in patients with MDD conducted between May 2018 and August 2019 (ClinicalTrials.gov identifier: NCT03051256).30 The study was conducted in inpatients at 10 centers in the United States from May 2018 to August 2019. Adult patients with a diagnosis of MDD experiencing a current MDE with an inadequate response to 1–3 courses of antidepressant treatment were randomized in a 1:1:1 ratio to placebo (n = 22), esmethadone 25 mg (n = 19), or esmethadone 50 mg (n = 21) in addition to ongoing SAT. Patients received treatment with REL-1017 25 mg, REL-1017 50 mg, or placebo for 7 days, followed by an observation period (Days 7–14).
Age at onset of the first MDE and number of previous MDEs were evaluated retrospectively through psychiatric history. Data were systematically collected with consistent criteria across patients. Duration of depression was derived from 2 measures: (1) TFO, defined as the number of years from the onset of MDD to the enrollment in the clinical trial and calculated as the difference between age at trial enrollment and age at the onset of the first MDE, and (2) TFO index, defined as the percentage of life-years spent from the onset of the first MDE to the enrollment in the clinical trial and calculated by computing the years of illness duration (number of years from the beginning of MDD), divided by age and multiplied by 100. Having no a priori assumption on which measures better explain the effect of the duration of depression on the therapeutic response to esmethadone, we performed the following analyses using alternatively 2 parameters (TFO and TFO index).
First, correlations between TFO or TFO index and change from baseline (CFB) in the Montgomery-Asberg Depression Rating Scale (MADRS) total score on Day 7 and Day 14 were performed for all patients (N = 62), separately for those who received esmethadone (N = 40) and for those who received placebo (N = 22). To examine correlations between TFO or TFO index and CFB in the MADRS total score while controlling for current depression severity (MADRS baseline), partial correlation analyses were conducted. In addition, 2 linear mixed models—1 parameterized with TFO and 1 parameterized with TFO index—were conducted to further investigate the impact of duration on the therapeutic response to esmethadone, while controlling for potential confounding effects (number of previous MDEs, time at assessments, and effect of esmethadone 50 mg when compared to 25 mg).
Statistical Analysis
Statistical analyses were performed using Microsoft Office 365—Excel, GraphPad Prism ver. 9.0, and IBM SPSS Statistics V26 software. The normality of continuous variables was assessed using the Shapiro-Wilk test. The normality of assumption was not met for age of onset, TFO, TFO index, and number of previous MDEs. Because of this, summary statistics were presented as median, minimum and maximum values, and interquartile distance. Differences between groups were evaluated using the Kruskal-Wallis test. Bivariate correlations were calculated by Spearman ρ. Two linear mixed models were used to evaluate the effect of the duration of depression (TFO or alternatively TFO index) on the therapeutic response to esmethadone (MADRS CFB), while controlling for potentially confounding effects (number of previous MDEs, time at assessment, and effect of esmethadone 25 mg vs 50 mg). These analyses were performed only on subjects who received esmethadone (n = 40). Subsequently, the Akaike information criterion (AIC) was utilized to determine which model better fits the data. Model 1 included TFO index, number of previous MDEs, time at assessment (Day 7, Day 14, and baseline as the reference point), and esmethadone dose (50 mg, compared to 25 mg, set as reference) as fixed effects. A random effect for the subjects in the intercept was also included. In Model 2, the analysis was replicated using TFO instead of TFO index.
RESULTS
Patient Characteristics
A total of 62 adult male and female patients (18–65 years of age) diagnosed with DSM-5–defined MDD and a current MDE unresponsive to an adequate course of standard antidepressants were enrolled and randomized. The demographic and clinical characteristics of the patients were previously reported.30 The estimated median age at the onset of the first MDE was 37.5 years and was similar between the 3 groups (P = .348; k = 2.110; Table 1). For all patients, the median TFO was 11.0 years, and the median TFO index was 22%; both medians were similar among the 3 groups (Table 1). For all patients, the median number of previous MDEs was 5 with no statistically significant differences among the groups (P = .817; k = 0.405; Table 1).
TFO and TFO Index and Response to Esmethadone vs Placebo
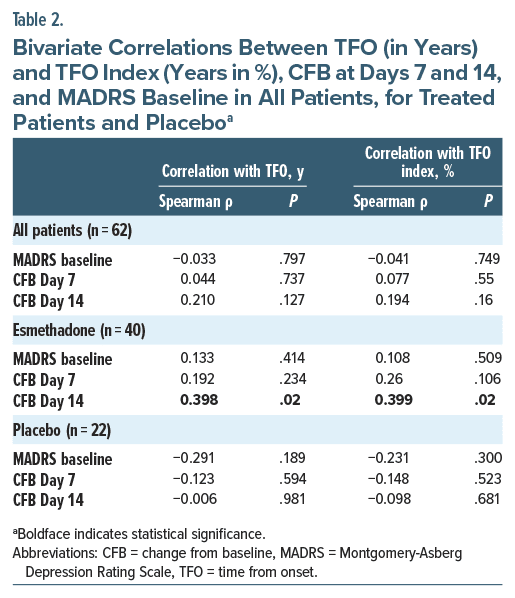
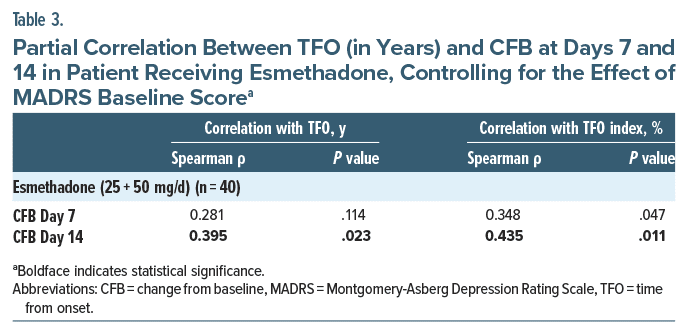
A significant correlation was found between TFO and MADRS CFB at Day 14 (Spearman ρ = 0.398; P = .02) in treated patients (Table 2). A similar finding was detected for TFO index and MADRS CFB at Day 14 (Spearman ρ = 0.399; P = .02; Table 2). The correlation between time from MDD onset (both TFO and TFO index) and CFB was not significant in the placebo group at any time point (Table 2). We further investigated whether there was an association between time from MDD onset (both TFO and TFO index) and current depression severity (MADRS baseline) and found there was no statistically significant correlation (Table 2). The correlation between TFO and MADRS CFB at Day 14 remained significant even when controlling for the effect of current depression severity (r = 0.395; P = .023) (Table 3). Similarly, TFO index and MADRS CFB at Day 14 remained significant even when controlling for the effect of current depression severity (MADRS baseline) (Spearman ρ = 0.435; P = .011; Table 3).
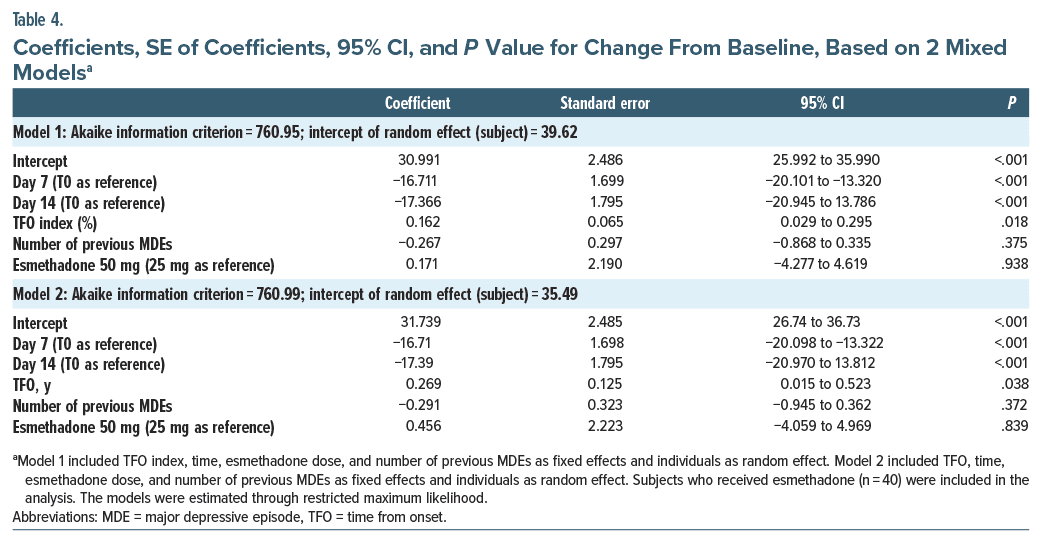
To determine which linear mixed model better fits the data, AIC was employed to determine the model that best captures data. The model incorporating TFO index demonstrated a slightly superior fit compared to the model with TFO (AIC model with TFO index 760.947 vs AIC model with TFO 760.987). Linear mixed models revealed a significant effect of both TFO index (B = 0.162; P = .018) and TFO (B = 0.269; P = .038) on MADRS score reduction from T0 to subsequent assessments, indicating better improvement on MADRS in patients with shorter time from MDD onset. In these analyses, number of previous MDEs and effect of REL 50 mg when compared to 25 mg were not significant (P = .375 and P = .938, respectively). We included random effect of subject (intercept = 39.62 for Model 1; intercept = 35.49 for Model 2). Coefficients of the model are shown in detail in Table 4.
DISCUSSION
Esmethadone is a novel, safe, and well-tolerated NMDAR antagonist with activity in rodent models of depressive-like behavior.26,27 Esmethadone may increase BDNF serum levels in healthy subjects28 and showed rapid, robust, and sustained therapeutic effects as adjunctive treatment in patients with MDD.30 This post hoc subanalysis suggests that 25 and 50 mg esmethadone orally once daily may be more effective in reducing MADRS scores as compared to placebo in patients with a shorter duration from MDD onset as compared to patients with a longer duration. Based on our results, the reported TFO index parameter is 0.162. This implies that, with all other effects in the model being constant, for every 1%-point increase in the percentage of life-years spent from the onset of depression, the predicted MADRS score increases by 0.162 units in patients who received esmethadone. In practical terms, for every 6.2% (1/0.162) reduction in the TFO index, the expected change in the MADRS score induced by esmethadone is approximately 1 point. This differential therapeutic effect related to the time of onset of MDD has not been previously reported for monoaminergic reuptake inhibitor drugs, including atypical antidepressants, nor for ketamine.31 The results of this study suggest that esmethadone may have better antidepressant effect when administered earlier compared to later in the course of MDD during a patient’s lifetime. Therefore, a shorter duration of MDD may be considered as potential predictor of better treatment response to esmethadone. Given these considerations, esmethadone might be considered a particularly promising antidepressant treatment also for children and adolescents with recent onset of MDD.
Phase 3 studies of esmethadone as adjunctive treatment for MDD are underway (ClinicalTrials.gov identifiers: NCT04688164; NCT04855747; NCT06011577). If these preliminary findings are confirmed in more extensive trials, esmethadone could become a first-line adjunctive treatment for MDD. This study has limitations. First, the study has a relatively small sample size and a short duration. Patients with ongoing antidepressant treatment received only a brief, 7-day, adjunctive treatment course with esmethadone, and we have information about the improvement of depressive episode only for 1 week after the discontinuation of the adjunctive treatment. Second, we did not analyze the data with respect to the duration of untreated illness.
Additional clinical studies in larger samples of patients treated with a longer esmethadone treatment are needed to confirm the current findings.
Article Information
Published Online: May 13, 2024. https://doi.org/10.4088/JCP.22m14735
© 2024 Physicians Postgraduate Press, Inc.
Submitted: November 19, 2022; accepted January 5, 2024.
To Cite: Guidetti C, De Martin S, Serra G, et al. Effect of time from onset of major depressive disorder on the therapeutic response to esmethadone (REL-1017).
J Clin Psychiatry. 2024;85(2):22m14735.
Author Affiliations: Child and Adolescent Neuropsychiatry Unit, Bambino Gesu’ Children’s Hospital, IRCCS, Rome, Italy (Guidetti, Serra, Apicella); Department of Pharmaceutical and Pharmacological Sciences, University of Padova, Italy (De Martin, Mattarei); Department of Psychiatry and Behavioral Sciences, University of Miami School of Medicine, Miami, Florida (Pani); Relmada Therapeutics, Coral Gables, Florida (Pani, Pappagallo, Manfredi); Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio Emilia, Modena, Italy (Pani); Department of Anesthesiology, Albert Einstein College of Medicine, Bronx, New York (Pappagallo); Department of Health Science, University of Milano, School of Medicine, Milan, Italy (Folli); Department of Psychiatry, Massachusetts General Hospital, Boston (Fava). Drs Guidetti and De Martin contributed equally to this work.
Corresponding Author: Paolo Manfredi, MD, Relmada Therapeutics, 2222 Ponce de Leon Blvd, Floor 3, Coral Gables, FL 33134 ([email protected]).
Relevant Financial Relationships: Dr Manfredi is an inventor on patents related to esmethadone. Drs Manfredi, Pappagallo, Folli, and Pani have received compensation from Relmada Therapeutics as consultants. Drs De Martin, Mattarei, Fava, and Guidetti are employed or have received compensation from companies or institutions that received funding from Relmada Therapeutics. Drs Serra and Apicella have no conflicts of interest. Dr Fava: full disclosures can be viewed at https://mghcme.org/faculty>Maurizio Fava, MD>View.
Funding/Support: This work was supported by Relmada Therapeutics, Inc, Coral Gables, Florida.
Role of the Sponsor: The sponsor contributed to the review and editing of the manuscript.
Acknowledgments: The authors would like to acknowledge the assistance of Richard Perry, PharmD, in the preparation of this manuscript, which was supported by Relmada Therapeutics, Inc.
Clinical Points
- The relationship between the duration of major depressive disorder (MDD) and therapeutic response to antidepressant treatment has been poorly investigated.
- There is a need to research potentially prognostic clinical characteristics that may predict treatment outcomes in MDD and may help select patients that are more likely to benefit from specific treatments.
References (31)

- Kessler RC, Berglund P, Demler O, et al; National Comorbidity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095–3105. PubMed CrossRef
- Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391(10128):1357–1366. PubMed CrossRef
- Trivedi MH, Rush AJ, Wisniewski SR, et al; STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. PubMed CrossRef
- Papakostas GI, Fava M. Predictors, moderators, and mediators (correlates) of treatment outcome in major depressive disorder. Dialogues Clin Neurosci. 2008;10(4):439–451. PubMed CrossRef
- Kraus C, Kadriu B, Lanzenberger R, et al. Prognosis and improved outcomes in major depression: a review. Focus (Am Psychiatr Publ). 2020;18(2):220–235. PubMed CrossRef
- Riedel M, Möller HJ, Obermeier M, et al. Clinical predictors of response and remission in inpatients with depressive syndromes. J Affect Disord. 2011;133(1–2):137–149. PubMed CrossRef
- Bukh JD, Bock C, Vinberg M, et al. The effect of prolonged duration of untreated depression on antidepressant treatment outcome. J Affect Disord. 2013;145:42–48. PubMed CrossRef
- Ghio L, Gotelli S, Marcenaro M, et al. Duration of untreated illness and outcomes in unipolar depression: a systematic review and meta-analysis. J Affect Disord. 2014;152–154:45–51. PubMed CrossRef
- Habert J, Katzman MA, Oluboka OJ, et al. Functional recovery in major depressive disorder: focus on early optimized treatment. Prim Care Companion CNS Disord. 2016;18(5).
- Hung CI, Liu CY, Yang CH. Untreated duration predicted the severity of depression at the two-year follow-up point. PLoS One. 2017;12(9):e0185119. PubMed CrossRef
- Kautzky A, Dold M, Bartova L, et al. Clinical factors predicting treatment resistant depression: affirmative results from the European multicenter study. Acta Psychiatr Scand. 2019;139(1):78–88. PubMed CrossRef
- Herzog DP, Wagner S, Engelmann J, et al. Early onset of depression and treatment outcome in patients with major depressive disorder. J Psychiatr Res. 2021;139:150–158. PubMed CrossRef
- Pereverseff RS, Beshai S, Dimova M. First episode indices associated with lifetime chronicity of depression among formerly depressed participants: an exploratory study. J Ment Health. 2020;29(6):677–683. PubMed CrossRef
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160(8):1516–1518. PubMed CrossRef
- Duman RS, Sanacora G, Krystal JH. Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron. 2019;102(1):75–90. PubMed CrossRef
- Haroon E, Miller AH, Sanacora G. Inflammation, glutamate, and glia: a trio of trouble in mood disorders. Neuropsychopharmacology. 2017;42(1):193–215. PubMed CrossRef
- Boku S, Nakagawa S, Toda H, et al. Neural basis of major depressive disorder: beyond monoamine hypothesis. Psychiatry Clin Neurosci. 2018;72(1):3–12. PubMed CrossRef
- Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–964. PubMed CrossRef
- Fava M, Stahl SM, De Martin S, et al. Esmethadone-HCl (REL-1017): a promising rapid antidepressant. Eur Arch Psychiatry Clin Neurosci. 2023. 273(7):1463–1476. PubMed CrossRef
- Popova V, Daly EJ, Trivedi M, et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatry. 2019;176(6):428–438. PubMed CrossRef
- Iosifescu DV, Jones A, O’Gorman C, et al. Efficacy and safety of AXS-05 (dextromethorphan-bupropion) in patients with major depressive disorder: a Phase 3 randomized clinical trial (GEMINI). J Clin Psychiatry. 2022;83(4):21m14345. PubMed CrossRef
- Tabuteau H, Jones A, Anderson A, et al. Effect of AXS-05 (dextromethorphan-bupropion) in major depressive disorder: a randomized double-blind controlled trial. Am J Psychiatry. 2022;179(7):490–499. PubMed CrossRef
- Bettini E, De Martin S, Mattarei A, et al. The N-methyl-D-aspartate receptor blocker REL-1017 (esmethadone) reduces calcium influx induced by glutamate, quinolinic acid, and gentamicin. Pharmaceuticals (Basel). 2022;15(7):882. PubMed CrossRef
- Bettini E, Stahl SM, De Martin S, et al. Pharmacological comparative characterization of REL-1017 (Esmethadone-HC1) and other NMDAR channel blockers in human heterodimeric N-Methyl-D-Aspartate receptors. Pharmaceuticals (Basel). 2022;15(8):997. PubMed
- Gorman AL, Elliott KJ, Inturrisi CE. The d- and l-isomers of methadone bind to the non-competitive site on the N-methyl-D-aspartate (NMDA) receptor in rat forebrain and spinal cord. Neurosci Lett. 1997;223(1):5–8. PubMed CrossRef
- Fogaça MV, Fukumoto K, Franklin T, et al. N-Methyl-D-aspartate receptor antagonist d-methadone produces rapid, mTORC1-dependent antidepressant effects. Neuropsychopharmacology. 2019;44(13):2230–2238. PubMed CrossRef
- Hanania T, Manfredi P, Inturrisi C, et al. The N-methyl-D-aspartate receptor antagonist d-methadone acutely improves depressive-like behavior in the forced swim test performance of rats. Exp Clin Psychopharmacol. 2020;28(2):196–201. PubMed CrossRef
- De Martin S, Gabbia D, Folli F, et al. REL-1017 (esmethadone) increases circulating BDNF levels in healthy subjects of a Phase 1 clinical study. Front Pharmacol. 2021;12:671859. PubMed CrossRef
- Bernstein G, Davis K, Mills C, et al. Characterization of the safety and pharmacokinetic profile of D-methadone, a novel N-methyl-D-aspartate receptor antagonist in healthy, opioid-naïve subjects: results of two phase 1 studies. J Clin Psychopharmacol. 2019;39(3):226–237. PubMed
- Fava M, Stahl S, Pani L, et al. REL-1017 (esmethadone) as adjunctive treatment in patients with major depressive disorder: a Phase 2a randomized double-blind trial. Am J Psychiatry. 2022;179(2):122–131. PubMed CrossRef
- Hirschfeld RM. Long-term nature of depression. Depress Anxiety. 1998;7(suppl 1):1–4. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite