Objective: Toxoplasma gondii (T. gondii), a protozoan parasite that persists in host tissues, including brain, has been associated with several psychiatric disorders and with suicidal behavior. We sought to test the hypothesis that latent T. gondii infection, as manifest by circulating immunoglobulin G (IgG) antibodies to T. gondii, is associated with both categorical and dimensional measures of aggression.
Method: IgG antibodies to T. gondii were collected between 1991 and 2008 from 358 adult subjects with DSM-5 intermittent explosive disorder (IED), non-IED psychiatric disorders (psychiatric controls), or no evidence of any psychiatric diagnosis (healthy controls). Assessments of aggression, anger, and impulsivity, as well as state/trait anger, depression, and anxiety were completed. T. gondii seropositive status (IgG > 12 IU) was the primary outcome measure for this study.
Results: T. gondii seropositive status (IgG > 12 IU) was associated with higher aggression (P = .022) and impulsivity (P = .05) scores. When both aggression and impulsivity scores were controlled for, however, only aggression scores were higher in seropositive subjects (P = .011). In addition, T. gondii seropositive status and marginal mean ± SE aggression scores increased from healthy controls (9.1% and −0.66 ± 0.05) to psychiatric controls (16.7% and −0.27 ± 0.05) to subjects with IED (21.8% and 1.15 ± 0.06; P ≤ .05). These findings were not accounted for by the presence of other syndromal/personality disorders or by states or traits related to depressed or anxious moods.
Conclusions: These data are consistent with previous studies suggesting a relationship between T. gondii and self-directed aggression (ie, suicidal behavior) and further add to the biological complexity of impulsive aggression both from a categorical and a dimensional perspective.
Toxoplasma gondii Infection:
Relationship With Aggression in Psychiatric Subjects
ABSTRACT
Objective: Toxoplasma gondii (T. gondii), a protozoan parasite that persists in host tissues, including brain, has been associated with several psychiatric disorders and with suicidal behavior. We sought to test the hypothesis that latent T. gondii infection, as manifest by circulating immunoglobulin G (IgG) antibodies to T. gondii, is associated with both categorical and dimensional measures of aggression.
Method: IgG antibodies to T. gondii were collected between 1991 and 2008 from 358 adult subjects with DSM-5 intermittent explosive disorder (IED), non-IED psychiatric disorders (psychiatric controls), or no evidence of any psychiatric diagnosis (healthy controls). Assessments of aggression, anger, and impulsivity, as well as state/trait anger, depression, and anxiety were completed. T. gondii seropositive status (IgG > 12 IU) was the primary outcome measure for this study.
Results: T. gondii seropositive status (IgG > 12 IU) was associated with higher aggression (P = .022) and impulsivity (P = .05) scores. When both aggression and impulsivity scores were controlled for, however, only aggression scores were higher in seropositive subjects (P = .011). In addition, T. gondii seropositive status and marginal mean ± SE aggression scores increased from healthy controls (9.1% and −0.66 ± 0.05) to psychiatric controls (16.7% and −0.27 ± 0.05) to subjects with IED (21.8% and 1.15 ± 0.06; P ≤ .05). These findings were not accounted for by the presence of other syndromal/personality disorders or by states or traits related to depressed or anxious moods.
Conclusions: These data are consistent with previous studies suggesting a relationship between T. gondii and self-directed aggression (ie, suicidal behavior) and further add to the biological complexity of impulsive aggression both from a categorical and a dimensional perspective.
J Clin Psychiatry 2016;77(3):334–341
dx.doi.org/10.4088/JCP.14m09621
© Copyright 2016 Physicians Postgraduate Press, Inc.
aClinical Neuroscience Research Unit, Department of Psychiatry and Behavioral Neuroscience, Pritzker School of Medicine, University of Chicago, Illinois
bCollege of Nursing, University of South Florida, Tampa
cDepartment of Psychiatry, University of Maryland College of Medicine, Baltimore
dDepartment of Psychology, University of Denver, Colorado Springs, Colorado
eVeterans Integrated Service Network 19, Mental Illness Research Education and Clinical Center, Denver, Colorado, and Veterans Integrated Service Network 5, Mental Illness Research Education and Clinical Center, Baltimore, Maryland
*Corresponding author: Emil F. Coccaro, MD, Department of Psychiatry and Behavioral Neuroscience, University of Chicago, 5841 South Maryland Ave, Chicago, IL 60637 ([email protected]).
Toxoplasma gondii (T. gondii) is a highly successful neurotropic protozoan parasite, infecting any warm-blooded animal including approximately one-third of all humans.1 Within the animal world, felids have been identified as the definitive host of T. gondii that localizes only in the gastrointestinal tract of any member of the cat family. Humans may be infected by T. gondii via ingestion of the parasite’s oocysts, which can spread from the feces of infected cats. Other routes of transmission include consumption of undercooked meat that has been infected with T. gondii cysts or ingestion of contaminated water2,3; congenital infection, occurring if a mother has a primary infection during pregnancy and transmits T. gondii to the fetus, is relatively rare. Postnatal chronic “latent” infection is very common, minimally symptomatic in the immune competent host, and with an encephalitic picture in the immunocompromised.4 When ingested by an intermediate host, the parasite uses leukocytes to travel from the intestine to other organs, finally localizing in muscle and brain. Once in the brain, T. gondii hides within neurons and glial cells, forming characteristic cystic intracellular structures under the pressure of the immune system.5 Although it is thought to be relatively harmless in immunocompetent adults, latent toxoplasmosis has been linked to several psychiatric disorders (eg, schizophrenia,6,7 bipolar disorder,7–9 personality disorders10) and with suicidal behavior.11–17
Given the strong relationship between suicidal behavior and impulsive aggressive behavior,18 either as a dimension or as a category, and in light of a recent study19 that reported that T. gondii seropositivity status may be associated with high self-reported trait aggression and impulsivity in mentally healthy individuals, we hypothesized that the categorical presence of immunoglobulin G (IgG) antibodies to T. gondii would (1) be associated with higher aggression and impulsivity scores in a sample of psychiatric and healthy control subjects and (2) be more frequent in individuals with intermittent explosive disorder (IED), a disorder of recurrent, problematic, and impulsive aggressive behavior, compared with healthy controls. In this study, we used psychometric assessments20–23 as the dimensional representation of impulsive aggression and the presence of IED as the categorical representation24 of impulsive aggression.
METHOD
Subjects
Three hundred fifty-eight physically healthy subjects participated in this study. All subjects were systematically evaluated in regard to aggressive, anxiety, and other behaviors as part of a larger program that is designed to study correlates of impulsive aggressive and other personality-related behaviors in human subjects. Subjects were recruited through public service, newspaper, and other media announcements seeking individuals who (1) reported psychosocial difficulty related to syndromal psychiatric and/or personality disorder conditions or (2) had little evidence of any psychopathology. All subjects gave informed consent and signed the informed consent document approved by the first author’s (E.F.C.) Institutional Review Board.
Diagnostic Assessment
Syndromal and personality disorder diagnoses were made according to DSM-5 criteria.25 Diagnoses were made using information from (1) the Structured Clinical Interview for DSM-IV Axis I disorders (SCID-I)26 for syndromal disorders and the Structured Interview for DSM-IV Personality (SIDP-IV)27 for personality disorders, (2) a clinical interview by a research psychiatrist, and (3) the review of all other available clinical data. The research diagnostic interviews were conducted by individuals with a master’s or doctor’s degree in clinical psychology, blinded to the study hypothesis. All diagnostic raters went through a rigorous training program that included lectures on DSM diagnoses and rating systems, videos of expert raters conducting SCID-I/SIDP-IV interviews, and practice interviews and ratings until the raters were deemed reliable by the trainer. This process resulted in good to excellent interrater reliabilities (mean ± SE κ = 0.84 ± 0.05; range, 0.79 to 0.93) across anxiety, mood, substance use, impulse control, and personality disorders. Final diagnoses were assigned by team best-estimate consensus procedures28,29 involving research psychiatrists and clinical psychologists as previously described.30 This methodology has been shown to enhance the accuracy of diagnosis over direct interview alone.31 Subjects with a current history of a substance use disorder or a life history of bipolar disorder, schizophrenia (or other psychotic disorder), or mental retardation were excluded from study.
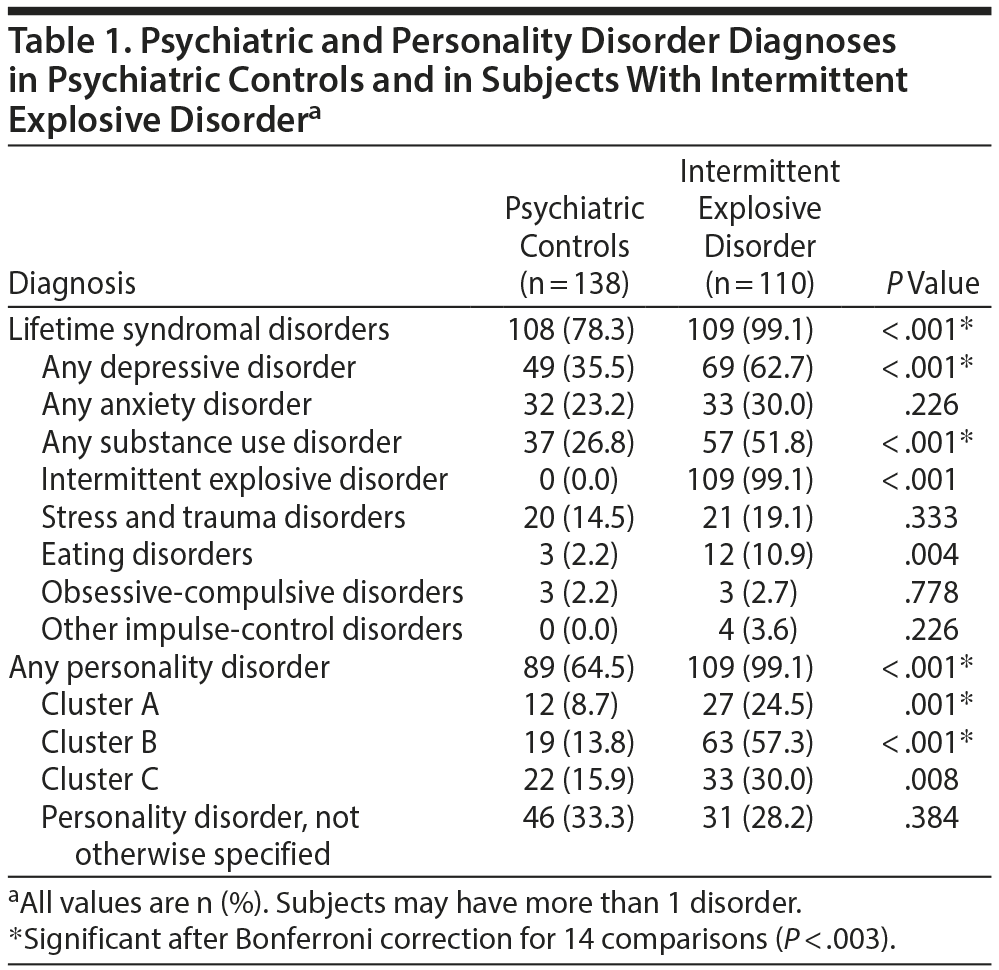
After diagnostic assignment, 110 subjects had no evidence of any psychiatric diagnosis (healthy controls); 138 subjects met criteria for a lifetime diagnosis of a syndromal psychiatric or personality disorder, but not for a lifetime diagnosis of IED (psychiatric controls); and 110 subjects met criteria for a lifetime diagnosis of IED. Of the 248 subjects with any DSM-5 diagnosis, most (72.4%) reported (1) a history of formal psychiatric evaluation and/or treatment (46.3%) or (2) a history of behavioral disturbance during which the subjects, or others, thought they should have sought mental health services but did not (26.1%). Syndromal psychiatric and personality disorder diagnoses are listed in Table 1.
Psychometric Measures of Aggression,
Impulsivity, and Related Behaviors
Aggression was assessed with the Aggression score from the Life History of Aggression (LHA)20 assessment and the Aggression (Physical and Verbal) score from the Buss-Perry Aggression Questionnaire (BPAQ).21 The LHA assesses the history of actual aggressive behavior and the BPAQ assesses aggressive tendencies as a personality trait. Impulsivity was assessed with the Barratt Impulsiveness Scale, version 11 (BIS-11)22 and the Impulsivity scale from the Eysenck Personality Inventory-2 (EPQ-2).23 Both BIS-11 and EPQ-2 Impulsivity assess a person’s disposition to act impulsively as a personality trait. Life history of suicidal and self-injurious behavior was assessed during the diagnostic interviews. An act was considered a suicide attempt if it involved behavior with the conscious (even if ambivalent) intent to die by means that the subject believed could end his or her life; an act was considered self-injurious if it involved behavior with the conscious (even if ambivalent) intent by the subject to physically harm, but not kill, himself or herself. Other assessments included the State-Trait Anger Expression Inventory-2 (STAXI-2)32 for state and trait anger, Beck Depression Inventory-II (BDI-II)33 for state depression, Depression scale from the General Behavior Inventory (GBI)34 for trait depression, Beck Anxiety Inventory (BAI)35 for state anxiety, and State-Trait Anxiety Inventory (STAI)36 for trait anxiety. The Global Assessment of Functioning (GAF)37 scale served as the variable for psychosocial functioning. Racial data, collected by diagnostic assessors, reflected self-identified racial characteristics of subjects. Socioeconomic status was assessed by the Hollingshead method (A. B. Hollingshead, Four Factor Index of Social Status, unpublished dissertation, 1975).
Asessment of T. gondii Seropositivity Status
Subjects were free of all medications for at least 4 weeks. Whole blood, anticoagulated with EDTA (ethylenediaminetetraacetic acid), was obtained between 9 am and 11 am through venipuncture of a forearm vein. Plasma was processed after centrifugation, placed in a polypropylene tube, and stored at −80°C until assay. These frozen plasma samples were collected between 1991 and 2008 and were tested for IgG antibodies to T. gondii in 2014 by solid-phase enzyme-linked immunosorbent assay (ELISA) with kits from IBL (Hamburg, Germany). All samples were run in duplicate, and quality controls were used. The coefficient of intra-assay variation was less than 10%. A subject with plasma T. gondii IgG antibodies > 12 IU was considered to be seropositive for T. gondii. Equivocal samples (8–12 IUs) were reanalyzed to accurately classify them as negative or positive. The laboratory technician was not aware of the diagnostic status of the subject. T. gondii seropositive status, rather than serointensity, was used because the latter cannot be measured in seronegative subjects. Despite the 18-year duration of sample collection, no association was observed between T. gondii seropositive status and time from the first to last study year (Spearman ρ = 0.08, P = .12). Finally, plasma levels for the proinflammatory cytokine interleukin 6 (IL-6) were available in 176 of these subjects as part of a previously published study.38
Statistical Analysis
Comparisons of between-group variables were performed by χ2, univariate (ANOVA/ANCOVA), and multivariate analysis of variance/covariance (MANOVA/MANCOVA), followed by Tukey honestly significant difference post hoc testing. Other analyses included binary logistic regression using age as a covariate. The primary biological variable in this study was T. gondii seropositive status as in previous studies.19 The primary dimensional variables included composite scores for aggression and impulsivity; these variables were created by z-transforming each of the 2 sets of aggression (LHA/BPAQ) and impulsivity (BIS-11/EPQ-2) variables and taking the mean z score of the source variables. The primary categorical variables included diagnostic status (healthy controls/psychiatric controls/subjects with IED), positive history of suicide attempt, and positive history of self-injurious behavior. A 2-tailed α value of .05 was used to denote statistical significance for all analyses. The primary analysis tested the relationship between T. gondii seropositive status and composite aggression scores, composite impulsivity scores, and history of self-directed aggression in all subjects. This was followed by analyses to determine if (1) T. gondii seropositive status was more frequent among IED subjects (subjects characterized by high levels of impulsive aggressive and suicidal behaviors), (2) T. gondii seropositive status was greater among subjects with other psychiatric and personality disorders, and (3) T. gondii seropositive individuals had higher levels of state and/or trait depression, anxiety, or anger.
RESULTS
Demographic Characteristics of the Sample
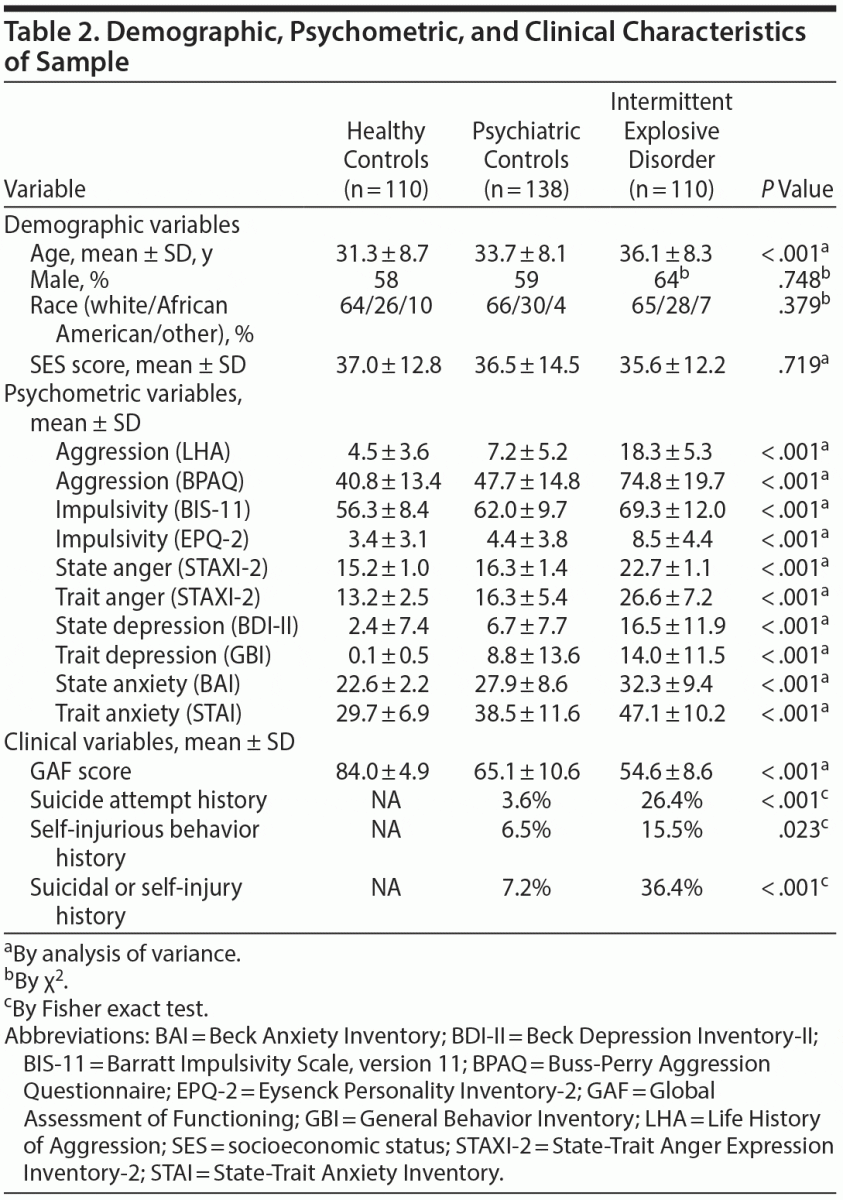
Healthy controls, psychiatric controls, and IED subjects differed only in age, with a less than 5-year age difference between IED subjects and healthy controls and a less than 3-year age difference between IED subjects and psychiatric controls. T. gondii seropositive status did not differ as a function of sex, race, or socioeconomic status (Table 2). As expected, the groups differed as a function of aggression, impulsivity, and history of suicidal and self-injurious behavior (Table 2). The rate of T. gondii seropositive subjects in this study was 15.9%, which is comparable with the most recent estimate of 14.1% for the United States.9
T. gondii Seropositive Status as a Function of Aggression and Impulsivity
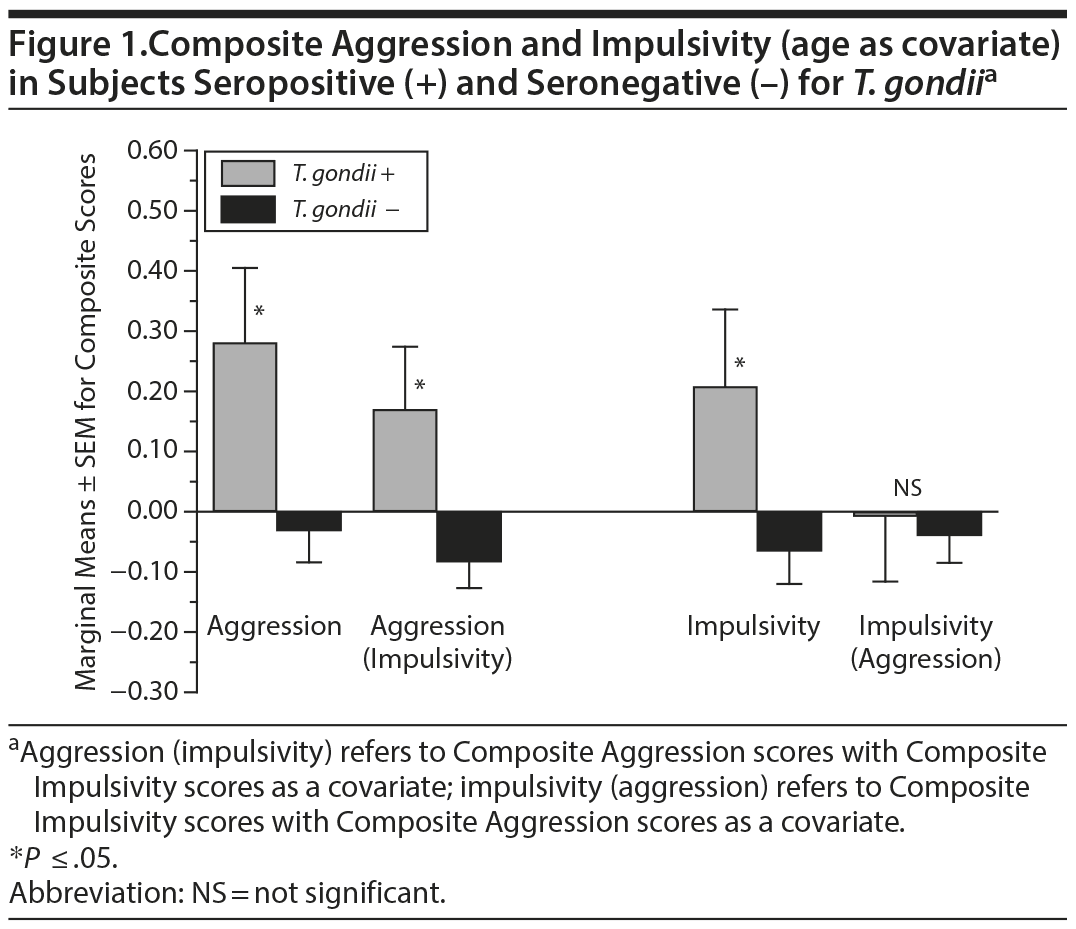
One-way ANCOVA, with age as a covariate, revealed that Composite Aggression scores were significantly higher in T. gondii seropositive subjects (F1,342 = 5.32, P = .022; Figure 1, left). Composite Impulsivity scores were also higher in T. gondii seropositive subjects (F1,325 = 3.83, P = .05; Figure 1, right). Composite Aggression scores adjusted for Composite Impulsivity scores continued to be higher among T. gondii seropositive subjects (P = .011; Figure 1, left), although the reverse was not true for Composite Impulsivity scores adjusted for Composite Aggression scores (P = .984; Figure 1, right). Similarly, when placed in the same binary logistic regression, with age as covariate, Composite Aggression (B = 0.44 ± 0.20, Wald1 = 4.77, Exp(B) = 1.55, P = .029), but not Composite Impulsivity (B = 0.02 ± 0.20, Wald1 = 0.08, Exp(B) = 1.06, P = .773), scores were associated with T. gondii seropositive status.
T. gondii Seropositive Status as a
Function of Self-Directed Aggression
Binary logistic regression, with age as covariate, revealed that T. gondii seropositive status did not predict history of suicide attempt (20.6% vs 15.4% T. gondii seropositive: B = −0.26 ± 0.46, Wald1 = 0.31, P = .577), history of self-injurious behavior (26.9% vs 15.1% T. gondii seropositive: B = −0.75 ± 0.47, Wald1 = 2.48, P = .116), or history of either type of self-directed aggressive behavior (24.0% vs 14.6% T. gondii seropositive: B = −0.58 ± 0.37, Wald1 = 2.42, P = .119).
T. gondii Seropositive Status as a Function of IED
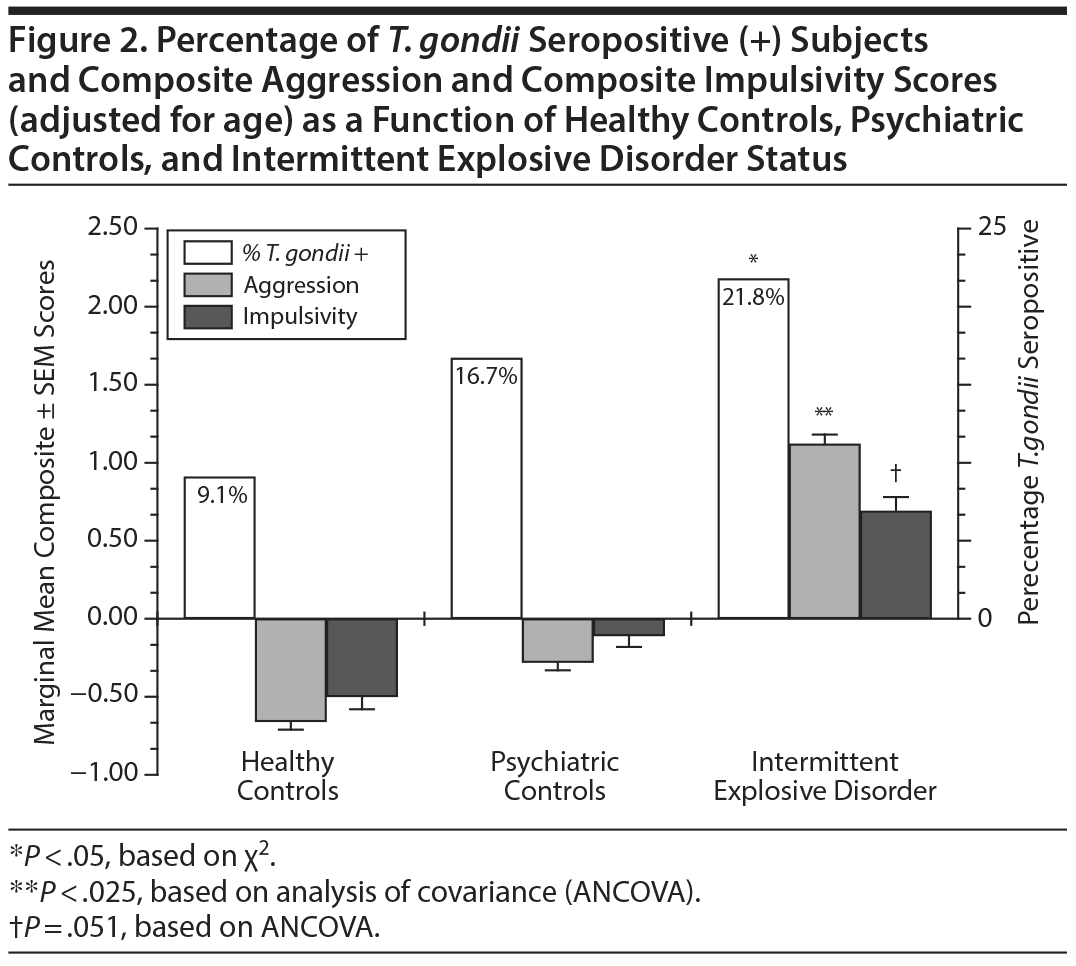
Next, we examined the relationship between T. gondii seropositive status as a function of IED, a disorder of recurrent, problematic, impulsive aggressive behavior, compared with healthy controls and psychiatric controls. A significant difference in T. gondii seropositive status was noted among the groups (linear by linear association χ21 = 6.06, P = .014; Figure 2). Binary logistic regression, with age as a covariate, yielded the same result with IED subjects (B = 0.89 ± 0.41, Wald1 = 4.68, P = .030) significantly associated with T. gondii seropositive status compared with healthy controls. Psychiatric controls were not significantly associated with T. gondii seropositive status compared with healthy controls (B = −0.63 ± 0.41, Wald1 = 2.42, P = .120). Increasing T. gondii seropositive status paralleled mean Composite Aggression (and Composite Impulsivity) scores, adjusted for age, across the groups (Figure 2). Adding Composite Aggression scores to the model eliminated the relationship for T. gondii seropositive status for IED subjects, compared with healthy controls (B = 0.26 ± 0.63, Wald1 = 0.18, P = .675).
T. gondii Seropositive Status as a
Function of Non-IED Syndromal Disorders
A significant difference in T. gondii seropositive status was also noted as a function of lifetime depressive disorder and lifetime anxiety disorder, but not lifetime substance use disorder. Binary logistic regression, with age as covariate, yielded similar results for subjects with depressive disorder (B = 0.79 ± 0.42, Wald1 = 3.51, P = .061) and subjects with anxiety disorder (B = 1.05 ± 0.47, Wald1 = 5.06, P = .025), compared with healthy controls. Adding Composite Aggression scores to the models eliminated these relationships for lifetime depressive disorder (B = 0.56 ± 0.50, Wald1 = 1.26, P = .263) and lifetime anxiety disorder (B = 0.76 ± 0.5.21, Wald1 = 2.13, P = .144).
T. gondii Seropositive Status as a
Function of Personality Disorder
A significant difference in T. gondii seropositive status was also noted as a function of the presence of borderline and/or antisocial personality disorder compared with healthy controls (healthy controls, 9.1%; psychiatric controls, 18.2%; borderline and/or antisocial personality disorder, 20.9%; linear by linear association: χ21 = 5.10, P = .024); the difference with psychiatric controls was not significant. Binary logistic regression, with age as covariate, yielded a similar result that approached, but did not reach, statistical significance (B = 0.85 ± 0.45, Wald1 = 3.47, P = .062). Adding Composite Aggression scores to the model eliminated this relationship (B = 0.32 ± 0.60, Wald1 = 0.29, P = .592). Expanding this analysis to include all personality disorders resulted in the same observation with respect to psychiatric controls having a higher rate of T. gondii seropositive status compared with healthy controls; this finding was nonsignificant after the addition of Composite Aggression scores to the model.
T. gondii Seropositive Status as a Function
of State and Trait Dysphoric Moods: Anger,
Depression, and Anxiety
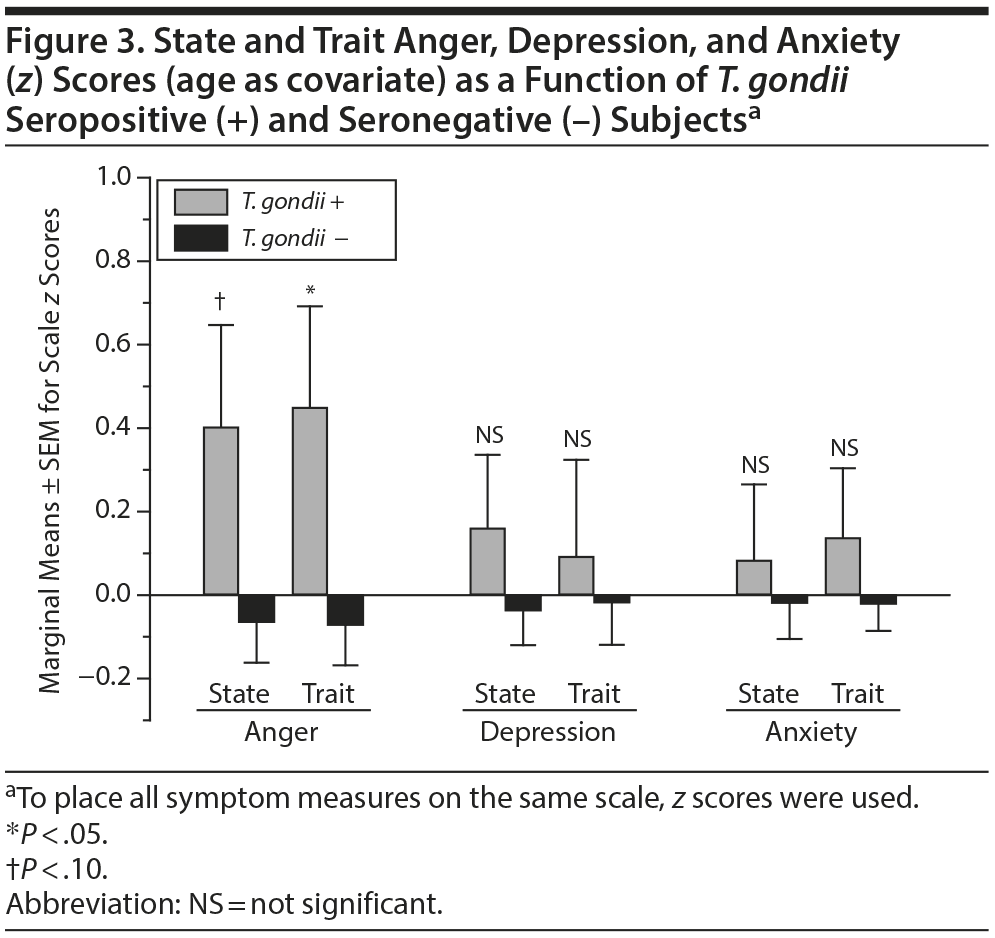
ANCOVA, with age as covariate, revealed higher state and trait anger scores as a function of T. gondii seropositive status (Figure 3, left). Similar analysis using state and trait depression and anxiety scores, however, revealed no significant differences as a function of T. gondii seropositive status (Figure 3, center and right).
Circulating IL-6 Levels as a
Function of T. gondii Seropositive Status
ANCOVA, with age as covariate, revealed no difference in circulating levels of IL-6 as a function of T. gondii seropositive status in the subgroup in which these data were available (T. gondii seropositive: 0.98 ± 1.64 pg/mL vs T. gondii seronegative: 1.41 ± 1.67 pg/mL; F1,172 = 1.27, P = .262). As previously reported, IL-6 (log IL-6: r = –0.39, P < .001) levels were inversely correlated with Composite Aggression scores in the subjects in this study.39
DISCUSSION
The results of this study suggest a relationship between latent infection with T. gondii and impulsive aggression from both a dimensional and categorical perspective. Specifically, T. gondii seropositive status was associated with higher scores on the psychometric measures for both Aggression and Impulsivity. Between aggression and impulsivity, these data suggest that T. gondii seropositive status is primarily related to aggression than to impulsivity in that the variance associated with impulsivity overlaps with the variance associated with aggression. In addition, the rate of T. gondii seropositivity in IED subjects was significantly greater than that in healthy controls, though not significantly greater than that in psychiatric controls without IED. The nonsignificant difference in seropositivity rate between IED subjects and psychiatric controls may be due to the fact that Aggression scores in psychiatric controls were intermediate between healthy controls and IED subjects, that this sample did not have the power to detect this difference to a statistically significant degree, or that other behaviors, such as depression or anxiety, are also associated with latent toxoplasmosis. These results are supported by findings from animal studies39 that showed a relationship between T. gondii infection and elevated aggression-related behaviors and a recent study19 of 1,000 psychiatrically healthy subjects that documented elevated trait aggression and impulsivity as a function of T. gondii seropositivity.
Typically, other-directed aggression is strongly associated with self-directed aggressive behavior in psychiatric subjects,18 and greater rates of T. gondii seropositive status have been reported among those with a history of suicidal behavior.11–15 Despite these previous findings, we did not find an association between T. gondii seropositive status and self-directed aggression in our sample. The proportion of subjects with lifetime histories of suicidal or self-injurious behavior was small, however, and the present study had limited statistical power to detect relationships reported from previous, and larger, samples. It is also possible that the psychiatric diagnostic composition of the sample (psychiatric controls) and tendency to direct aggression outward (IED) reduced an association with suicidal self-directed aggression. Consistently, in the largest study14 on T. gondii seropositive status and suicidal self-directed violence, performed on a cohort of Danish women, the association was significantly weaker in those women who had a concurrent diagnosis of mood disorder, psychotic disorder, or personality disorder.
Individuals with a lifetime history of depressive and anxiety disorder also had higher rates of T. gondii seropositive status compared with healthy controls. While higher depression and anxiety scores should be observed as a function of T. gondii seropositive status, no significant differences in state or trait scores for depression or anxiety were observed. The observed effect size for depression or anxiety scores was modest (d = 0.10 to 0.20), and it is possible that a larger sample would have yielded different results. However, studies in much larger samples report no significant association between T. gondii seropositive status and unipolar major depression or dysthymia,9 generalized anxiety disorder, panic disorder, or posttraumatic stress disorder,39 suggesting that mood and anxiety disorders are not accounting for the findings in our study. In contrast, composite aggression and state and trait anger scores were significantly elevated as a function of T. gondii seropositive status and, in every case, eliminated all differences as a function of T. gondii seropositive status. Thus, we posit that the higher T. gondii seropositive rates observed in individuals with depressive/anxiety disorder, compared with healthy controls, were due to their comorbidity with IED or a correlation between aggression, depression, and anxiety scores. In the current sample, IED was highly comorbid with lifetime depressive disorder (64% vs 35%, P < .001), and aggression scores correlated with both trait depression (r = 0.38, P = .001) and anxiety (r = 0.52, P < .001) scores, though not as strongly as depression correlated with anxiety (r = 0.74, P < .001).
Several factors may account for these findings. First, chronic latent infection with T. gondii may lead to a low-grade chronic immune activation within the brain, with (or without) downstream effects on neurotransmitter systems involved in aggressive behavior.40 Second, chronic T. gondii infection may alter the structure and function of corticolimbic circuits that are known to modulate impulsive aggressive behavior.41 Specifically, persistent T. gondii infection in mice is associated with neuronal tissue lesions, altered neuronal function, ventricular dilation, and neuroinflammation.42 In addition, several, though not all, studies suggest that T. gondii–containing cysts localize primarily in the prefrontal cortex and amygdala43,44 and that latent infection with T. gondii induces dendritic retraction in the basolateral amygdala.45 Third, as shown experimentally in rats,46 T. gondii infection increases testicular expression of genes involved in the production of testosterone. In addition, there is evidence that T. gondii–infected males, though not females, have higher circulating levels of testosterone compared with controls.47 However, while a number of studies report a relationship between elevated levels of testosterone and aggression,48 the magnitude of this relationship is small. Thus, it is unlikely that testosterone plays any more than a modest role in this regard.
Neurotransmitter mechanisms by which T. gondii may affect behavior include effects on serotonergic and glutaminergic transmission, both of which have been shown to play a role in aggressive behavior in human studies.49,50 Relevant to serotonin, conversion of tryptophan to kynurenine is controlled by indoleamine 2,3-dioxygenase ([IDO]; IDO-1 and IDO-2).51 Since IDO can be activated by inflammatory cytokines, levels of kynurenine can rise while levels of serotonin decline. In addition, increased levels of kynurenine lead to increased levels of its active metabolite quinolinic acid, a potent N-methyl-d-aspartate receptor agonist, which may increase the risk for aggressive behavior in humans.50 While this hypothesis is partially supported by reported elevations of kynurenine and quinolinic acid levels in mice with chronic T. gondii infection,52 we did not find differences in circulating levels of proinflammatory cytokines (ie, IL-6) as a function of T. gondii seropositivity. It is possible that the proinflammatory processes that keep T. gondii in a latent state are confined to the brain and are not reflected in the periphery. It is also possible that impulsively aggressive individuals engage in behaviors that increase their own risk of infection with T. gondii or that latent toxoplasmosis changes behavior, as in felids,1 so that the expression of aggression is increased. In addition, T. gondii is known to increase risk-taking behavior in rodents, evolutionarily benefiting the parasite (ie, transforming natural aversion in cats to attraction).53,54 This is an example of the general phenomenon of host manipulation by parasites, documented in nature,55 and proposed as a model with some explanatory potential for alterations in human behavior associated with parasitic infections.56,57
The strengths of this hypothesis-driven study include a well-characterized sample of healthy and psychiatric controls as well as validated measures of aggression, impulsivity, depression, and anxiety. Limitations to our study are present, as well. First, we used a cross-sectional design, and no causal, or directional, conclusions can be made from these analyses. Second, ascertainment of subjects may limit the generalizability of these findings in that these involved subjects who volunteered for a research study, rather than for clinical treatment. However, nearly three-quarters of the psychiatric subjects reported a past history of psychiatric treatment (or of having episodes of behavioral disturbance for which they, or others, thought they should have sought mental health services but did not), and, thus, most of these subjects are likely similar to individuals who would have been recruited from a clinical setting. Third, it is possible that the presented associations are nonspecific and, instead, due to other common latency-establishing neurotropic pathogens such as herpes viruses or cytomegalovirus. However, recent studies have documented that associations between T. gondii and self-directed58 and other-directed19 aggression in human subjects are not due to the presence of these other potential pathogens. Finally, because immunoglobulin M antibodies to T. gondii were not assessed, there is a possibility that a small number of seropositive subjects had an acute, rather than a chronic latent, infection at time of study.
In summary, we report a greater rate of T. gondii seropositive status in subjects with DSM-5 IED compared with healthy controls and a positive relationship with aggression and anger, but not with depression or anxiety. These findings are consistent with previous T. gondii seropositive status data, suggesting a relationship with self-directed aggression (ie, suicidal behavior) and a relationship involving schizophrenia or mania—disorders in which many individuals are often aggressive.59,60 Our results further add to the biological complexity of impulsive aggression, from both a categorical and a dimensional perspective.
Submitted: October 29, 2014; accepted February 26, 2015.
Potential conflicts of interest: Dr Coccaro reports being a consultant and on the Scientific Advisory Board of and having stock options from Azevan Pharmaceuticals. Dr Lee reports being the recipient of a research grant from Azevan Pharmaceuticals. Drs Groer, Can, Coussons-Read, and Postolache have no potential conflicts of interest.
Funding/support: This work was supported, in part, by grants from the National Institute of Mental Health: RO1 MH60836, RO1 MH63262, RO1 MH66984 (Dr Coccaro), a Project Pilot Grant from the University of Colorado, Denver (Dr Coussons-Read), and the Distinguished Investigator Award from the American Foundation for Suicide Prevention (Dr Postolache). Dr Postolache’s contribution was additionally supported by the Veterans Integrated Service Network 19 Mental Illness Research, Education and Clinical Center, Denver, Colorado.
Role of the sponsor: The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the manuscript.
Disclaimer: The views, opinions, and findings contained in this article are those of the authors and do not necessarily represent the official policy or position of the Department of Veterans Affairs or the US Government.
REFERENCES
1. Dubey JP, Jones JL. Toxoplasma gondii infection in humans and animals in the United States. Int J Parasitol. 2008;38(11):1257–1278. doi:10.1016/j.ijpara.2008.03.007 PubMed
2. Jones JL, Dargelas V, Roberts J, et al. Risk factors for Toxoplasma gondii infection in the United States. Clin Infect Dis. 2009;49(6):878–884. doi:10.1086/605433 PubMed
3. Jones JL, Kruszon-Moran D, Wilson M, et al. Toxoplasma gondii infection in the United States: seroprevalence and risk factors. Am J Epidemiol. 2001;154(4):357–365. doi:10.1093/aje/154.4.357 PubMed
4. Ajioka JW, Soldati D. Toxoplasma: Molecular and Cellular Biology. Norfolk, UK: Horizon Bioscience; 2007.
5. Garcia SL, Bruckner AD. Parasitic infections in the compromised host (Toxoplasma gondii). In: Garcia S, Bruckner AD, eds. Diagnostic Medical Parasitology. Washingtion, DC: American Society for Microbiology; 1997:423–424.
6. Prasad KM, Watson AM, Dickerson FB, et al. Exposure to herpes simplex virus type 1 and cognitive impairments in individuals with schizophrenia. Schizophr Bull. 2012;38(6):1137–1148. doi:10.1093/schbul/sbs046 PubMed
7. Torrey EF, Bartko JJ, Yolken RH. Toxoplasma gondii and other risk factors for schizophrenia: an update. Schizophr Bull. 2012;38(3):642–647. doi:10.1093/schbul/sbs043 PubMed
8. Tedla Y, Shibre T, Ali O, et al. Serum antibodies to Toxoplasma gondii and Herpesvidae family viruses in individuals with schizophrenia and bipolar disorder: a case-control study. Ethiop Med J. 2011;49(3):211–220. PubMed
9. Pearce BD, Kruszon-Moran D, Jones JL. The relationship between Toxoplasma gondii infection and mood disorders in the third National Health and Nutrition Survey. Biol Psychiatry. 2012;72(4):290–295. doi:10.1016/j.biopsych.2012.01.003 PubMed
10. Hinze-Selch D, Däubener W, Erdag S, et al. The diagnosis of a personality disorder increases the likelihood for seropositivity to Toxoplasma gondii in psychiatric patients. Folia Parasitol (Praha). 2010;57(2):129–135. doi:10.14411/fp.2010.016 PubMed
11. Arling TA, Yolken RH, Lapidus M, et al. Toxoplasma gondii antibody titers and history of suicide attempts in patients with recurrent mood disorders. J Nerv Ment Dis. 2009;197(12):905–908. doi:10.1097/NMD.0b013e3181c29a23 PubMed
12. Okusaga O, Langenberg P, Sleemi A, et al. Toxoplasma gondii antibody titers and history of suicide attempts in patients with schizophrenia. Schizophr Res. 2011;133(1–3):150–155. doi:10.1016/j.schres.2011.08.006 PubMed
13. Yagmur F, Yazar S, Temel HO, et al. May Toxoplasma gondii increase suicide attempt-preliminary results in Turkish subjects? Forensic Sci Int. 2010;199(1–3):15–17. doi:10.1016/j.forsciint.2010.02.020 PubMed
14. Pedersen MG, Mortensen PB, Norgaard-Pedersen B, et al. Toxoplasma gondii infection and self-directed violence in mothers. Arch Gen Psychiatry. 2012;69(11):1123–1130. doi:10.1001/archgenpsychiatry.2012.668 PubMed
15. Ling VJ, Lester D, Mortensen PB, et al. Toxoplasma gondii seropositivity and suicide rates in women. J Nerv Ment Dis. 2011;199(7):440–444. doi:10.1097/NMD.0b013e318221416e PubMed
16. Samojłowicz D, Borowska-Solonynko A, Gołab E. Prevalence of Toxoplasma gondii parasite infection among people who died due to sudden death in the capital city of Warsaw and its vicinity. Przegl Epidemiol. 2013;67(1):29–33, 115–118. PubMed
17. Alvarado-Esquivel C, Sánchez-Anguiano LF, Arnaud-Gil CA, et al. Toxoplasma gondii infection and suicide attempts: a case-control study in psychiatric outpatients. J Nerv Ment Dis. 2013;201(11):948–952. doi:10.1097/NMD.0000000000000037 PubMed
18. McCloskey MS, Ben-Zeev D, Lee R, et al. Prevalence of suicidal and self-injurious behavior among subjects with intermittent explosive disorder. Psychiatry Res. 2008;158(2):248–250. doi:10.1016/j.psychres.2007.09.011 PubMed
19. Cook TB, Brenner LA, Cloninger CR, et al. “Latent” infection with Toxoplasma gondii: association with trait aggression and impulsivity in healthy adults. J Psychiatr Res. 2015;60:87–94. doi:10.1016/j.jpsychires.2014.09.019 PubMed
20. Coccaro EF, Berman ME, Kavoussi RJ. Assessment of Life History of Aggression: development and psychometric characteristics. Psychiatry Res. 1997;73(3):147–157. doi:10.1016/S0165-1781(97)00119-4 PubMed
21. Buss AH, Perry M. The Aggression Questionnaire. J Pers Soc Psychol. 1992;63(3):452–459. doi:10.1037/0022-3514.63.3.452 PubMed
22. Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol. 1995;51(6):768–774. doi:10.1002/1097-4679(199511)51:6<768::AID-JCLP2270510607>3.0.CO;2-1 PubMed
23. Eysenck H Jr, Eysenck SBG. Manual of the Eysenck Personality Scales (EPS Adult). London, UK: Hodder & Stoughton; 1991.
24. Coccaro EF. Intermittent explosive disorder as a disorder of impulsive aggression for DSM-5. Am J Psychiatry. 2012;169(6):577–588. doi:10.1176/appi.ajp.2012.11081259 PubMed
25. American Association of Psychiatry. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. Washington, DC: American Pyschiatric Press, Inc; 2013.
26. First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I). New York, NY: Psychiatric Institute, Biometrics Research; 1997.
27. Pfohl B, Blum N, Zimmerman M, University of Iowa, Department of Psychiatry. Structured Interview for DSM-IV Personality: SIDP-IV. Washington, DC: American Psychiatric Press; 1997.
28. Kosten TA, Rounsaville BJ. Sensitivity of psychiatric diagnosis based on the best estimate procedure. Am J Psychiatry. 1992;149(9):1225–1227. doi:10.1176/ajp.149.9.1225 PubMed
29. Leckman JF, Sholomskas D, Thompson WD, et al. Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch Gen Psychiatry. 1982;39(8):879–883. doi:10.1001/archpsyc.1982.04290080001001 PubMed
30. Coccaro EF, Nayyer H, McCloskey MS. Personality disorder—not otherwise specified evidence of validity and consideration for DSM-5. Compr Psychiatry. 2012;53(7):907–914. doi:10.1016/j.comppsych.2012.03.007 PubMed
31. Klein DN, Ouimette PC, Kelly HS, et al. Test-retest reliability of team consensus best-estimate diagnoses of axis I and II disorders in a family study. Am J Psychiatry. 1994;151(7):1043–1047. doi:10.1176/ajp.151.7.1043 PubMed
32. Spielberger CD. The State-Trait Anger Expression Inventory-2 (STAXI-2): Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc.; 1999.
33. Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation. 1996.
34. Depue RA, Kleiman RM, Davis P, et al. The behavioral high-risk paradigm and bipolar affective disorder, VIII: serum free cortisol in nonpatient cyclothymic subjects selected by the General Behavior Inventory. Am J Psychiatry. 1985;142(2):175–181. doi:10.1176/ajp.142.2.175 PubMed
35. Beck AT, Epstein N, Brown G, et al. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–897. doi:10.1037/0022-006X.56.6.893 PubMed
36. Spielberger CD, Gorssuch RL, Lushene PR, et al. Manual for the State-Trait Anxiety Inventory. Mountain View, CA: Consulting Psychologists Press, Inc.; 1983.
37. American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
38. Coccaro EF, Lee R, Coussons-Read M. Elevated plasma inflammatory markers in individuals with intermittent explosive disorder and correlation with aggression in humans. JAMA Psychiatry. 2014;71(2):158–165. doi:10.1001/jamapsychiatry.2013.3297 PubMed
39. Gale SD, Brown BL, Berrett A, et al. Association between latent toxoplasmosis and major depression, generalised anxiety disorder and panic disorder in human adults. Folia Parasitol (Praha). 2014;61(4):285–292. PubMed
40. Flegr J. How and why Toxoplasma makes us crazy. Trends Parasitol. 2013;29(4):156–163. doi:10.1016/j.pt.2013.01.007 PubMed
41. Coccaro EF, Sripada CS, Yanowitch RN, et al. Corticolimbic function in impulsive aggressive behavior. Biol Psychiatry. 2011;69(12):1153–1159. doi:10.1016/j.biopsych.2011.02.032 PubMed
42. Hermes G, Ajioka JW, Kelly KA, et al. Neurological and behavioral abnormalities, ventricular dilatation, altered cellular functions, inflammation, and neuronal injury in brains of mice due to common, persistent, parasitic infection. J Neuroinflammation. 2008;5(1):48. doi:10.1186/1742-2094-5-48 PubMed
43. Berenreiterová M, Flegr J, Kuběna AA, et al. The distribution of Toxoplasma gondii cysts in the brain of a mouse with latent toxoplasmosis: implications for the behavioral manipulation hypothesis. PLoS ONE. 2011;6(12):e28925. doi:10.1371/journal.pone.0028925 PubMed
44. McConkey GA, Martin HL, Bristow GC, et al. Toxoplasma gondii infection and behaviour – location, location, location? J Exp Biol. 2013;216(pt 1):113–119. doi:10.1242/jeb.074153 PubMed
45. Mitra R, Sapolsky RM, Vyas A. Toxoplasma gondii infection induces dendritic retraction in basolateral amygdala accompanied by reduced corticosterone secretion. Dis Model Mech. 2013;6(2):516–520. doi:10.1242/dmm.009928 PubMed
46. Lim A, Kumar V, Hari Dass SA, et al. Toxoplasma gondii infection enhances testicular steroidogenesis in rats. Mol Ecol. 2013;22(1):102–110. doi:10.1111/mec.12042 PubMed
47. Flegr J, Lindová J, Kodym P. Sex-dependent toxoplasmosis-associated differences in testosterone concentration in humans. Parasitology. 2008;135(4):427–431. doi:10.1017/S0031182007004064 PubMed
48. Book A, Starzyk K, Quinsy V. The relationship between testosterone and aggression: a meta-analysis. Aggress Violent Behav. 2001;6(6):579–599. doi:10.1016/S1359-1789(00)00032-X
49. Coccaro EF, Lee R, Kavoussi RJ. Aggression, suicidality, and intermittent explosive disorder: serotonergic correlates in personality disorder and healthy control subjects. Neuropsychopharmacology. 2010;35(2):435–444. doi:10.1038/npp.2009.148 PubMed
50. Coccaro EF, Lee R, Vezina P. Cerebrospinal fluid glutamate concentration correlates with impulsive aggression in human subjects. J Psychiatr Res. 2013;47(9):1247–1253. doi:10.1016/j.jpsychires.2013.05.001 PubMed
51. Ball HJ, Sanchez-Perez A, Weiser S, et al. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396(1):203–213. doi:10.1016/j.gene.2007.04.010 PubMed
52. Notarangelo FM, Wilson EH, Horning KJ, et al. Evaluation of kynurenine pathway metabolism in Toxoplasma gondii-infected mice: implications for schizophrenia. Schizophr Res. 2014;152(1):261–267. PubMed
53. Vyas A, Kim SK, Giacomini N, et al. Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc Natl Acad Sci U S A. 2007;104(15):6442–6447. doi:10.1073/pnas.0608310104 PubMed
54. Berdoy M, Webster JP, Macdonald DW. Fatal attraction in rats infected with Toxoplasma gondii. Proc Biol Sci. 2000;267(1452):1591–1594. doi:10.1098/rspb.2000.1182 PubMed
55. Lafferty KD, Shaw JC. Comparing mechanisms of host manipulation across host and parasite taxa. J Exp Biol. 2013;216(pt 1):56–66. doi:10.1242/jeb.073668 PubMed
56. Webster JP, Kaushik M, Bristow GC, et al. Toxoplasma gondii infection, from predation to schizophrenia: can animal behaviour help us understand human behaviour? J Exp Biol. 2013;216(pt 1):99–112. doi:10.1242/jeb.074716 PubMed
57. Flegr J. Influence of latent Toxoplasma infection on human personality, physiology and morphology: pros and cons of the Toxoplasma-human model in studying the manipulation hypothesis. J Exp Biol. 2013;216(pt 1):127–133. doi:10.1242/jeb.073635 PubMed
58. Zhang Y, Träskman-Bendz L, Janelidze S, et al. Toxoplasma gondii immunoglobulin G antibodies and nonfatal suicidal self-directed violence. J Clin Psychiatry. 2012;73(8):1069–1076. doi:10.4088/JCP.11m07532 PubMed
59. Soyka M. Neurobiology of aggression and violence in schizophrenia. Schizophr Bull. 2011;37(5):913–920. doi:10.1093/schbul/sbr103 PubMed
60. Ballester J, Goldstein T, Goldstein B, et al. Is bipolar disorder specifically associated with aggression? Bipolar Disord. 2012;14(3):283–290. doi:10.1111/j.1399-5618.2012.01006.x PubMed
This PDF is free for all visitors!
Save
Cite

