Objective: Selective serotonin reuptake inhibitors (SSRIs) are recommended as the first-line pharmacologic treatment for obsessive-compulsive disorder (OCD). SSRI response is thought to be delayed in OCD, even more so than in major depression. We conducted a meta-analysis to examine the trajectory of treatment response to SSRIs and how this trajectory is modulated by dosage.
Data Sources: PubMed and the Cochrane Central Register of Controlled Trials (CENTRAL) were searched on May 22, 2013, for randomized, placebo-controlled SSRI trials in OCD with the search terms “serotonin uptake inhibitors” [MeSH] OR “serotonin uptake inhibitors” [Pharmacologic Action] AND “obsessive-compulsive disorder” [MeSH]. There were no language limitations on the search.
Study Selection: Randomized, placebo-controlled trials that examined the efficacy of SSRIs in the treatment of adults with OCD and utilized the Yale-Brown Obsessive Compulsive Scale (Y-BOCS) as an outcome were selected.
Data Extraction: We extracted weekly symptom data from randomized, placebo-controlled trials of SSRIs for the treatment of adults with OCD in order to characterize the trajectory of pharmacologic response. Our primary outcome was weighted mean difference on the Y-BOCS of SSRI treatment compared to placebo. We used the PROC MIXED procedure in SAS to examine 6 possible models of SSRI response. Interaction terms were utilized to examine the effect of dose, individual agent, and year of publication on SSRI response.
Results: The meta-analysis included 17 trials of SSRIs including 3,276 subjects. A statistically significant benefit of SSRIs compared to placebo was seen within 2 weeks after the start of treatment (weighted mean difference = −0.91 [95% CI, −0.54 to −1.28], P < .001). A logarithmic response curve, indicating decreasing symptom improvement over time, provided the best fit for the trajectory of OCD symptom improvement. A significantly greater response was associated with using higher doses of SSRIs (P < .0001).
Conclusions: These results suggest that the greatest incremental treatment gains in OCD are seen early on in SSRI treatment. This is consistent with a previous meta-analysis examining time course of SSRI action in major depressive disorder and contrasts with the widely held belief that SSRI response in OCD is delayed.
See letter by Diniz et al

Early Onset of Response With Selective Serotonin Reuptake Inhibitors in Obsessive-Compulsive Disorder:
A Meta-Analysis

ABSTRACT
Objective: Selective serotonin reuptake inhibitors (SSRIs) are recommended as the first-line pharmacologic treatment for obsessive-compulsive disorder (OCD). SSRI response is thought to be delayed in OCD, even more so than in major depression. We conducted a meta-analysis to examine the trajectory of treatment response to SSRIs and how this trajectory is modulated by dosage.
Data Sources: PubMed and the Cochrane Central Register of Controlled Trials (CENTRAL) were searched on May 22, 2013, for randomized, placebo-controlled SSRI trials in OCD with the search terms “serotonin uptake inhibitors” [MeSH] OR “serotonin uptake inhibitors” [Pharmacologic Action] AND “obsessive-compulsive disorder” [MeSH]. There were no language limitations on the search.
Study Selection: Randomized, placebo-controlled trials that examined the efficacy of SSRIs in the treatment of adults with OCD and utilized the Yale-Brown Obsessive Compulsive Scale (Y-BOCS) as an outcome were selected.
Data Extraction: We extracted weekly symptom data from randomized, placebo-controlled trials of SSRIs for the treatment of adults with OCD in order to characterize the trajectory of pharmacologic response. Our primary outcome was weighted mean difference on the Y-BOCS of SSRI treatment compared to placebo. We used the PROC MIXED procedure in SAS to examine 6 possible models of SSRI response. Interaction terms were utilized to examine the effect of dose, individual agent, and year of publication on SSRI response.
Results: The meta-analysis included 17 trials of SSRIs including 3,276 subjects. A statistically significant benefit of SSRIs compared to placebo was seen within 2 weeks after the start of treatment (weighted mean difference = −0.91 [95% CI, −0.54 to −1.28], P < .001). A logarithmic response curve, indicating decreasing symptom improvement over time, provided the best fit for the trajectory of OCD symptom improvement. A significantly greater response was associated with using higher doses of SSRIs (P < .0001).
Conclusions: These results suggest that the greatest incremental treatment gains in OCD are seen early on in SSRI treatment. This is consistent with a previous meta-analysis examining time course of SSRI action in major depressive disorder and contrasts with the widely held belief that SSRI response in OCD is delayed.
J Clin Psychiatry 2016;77(5):e605-e611
dx.doi.org/10.4088/JCP.14r09758
© Copyright 2016 Physicians Postgraduate Press, Inc.
aYale Child Study Center, Connecticut Mental Health Center, New Haven, Connecticut
bHarvard University, Cambridge, Massachusetts
cDepartment of Psychiatry, Yale University School of Medicine, New Haven, Connecticut
dDepartment of Psychology, Yale University School of Medicine, New Haven, Connecticut
*Corresponding author: Michael H. Bloch, MD, MS, Child Study Center, Yale University School of Medicine, PO Box 2070900, New Haven, CT 06520 ([email protected]).
Serotonin reuptake inhibitors (SRIs) and cognitive-behavioral therapy (CBT) are the recommended first-line treatments for obsessive-compulsive disorder (OCD).1,2 Meta-analysis of randomized clinical trials has clearly demonstrated that both selective serotonin reuptake inhibitors (SSRIs) and clomipramine are effective.3-10 SSRIs are generally used as initial pharmacologic treatment for OCD because of their better safety and tolerability compared to clomipramine.11 Meta-analysis has also demonstrated that higher doses of SSRIs are marginally more effective than lower doses.12 However, higher doses of SSRIs have an increased side effect burden. Current treatment guidelines recommend that patients should try CBT and go up to the maximum tolerable dose of 2 SSRIs (and/or clomipramine) before trying alternative pharmacologic treatments.1,2
Previous meta-analyses examining treatment response to SSRIs in OCD have focused exclusively on end point data.3-10 We performed the first meta-analysis of randomized, placebo-controlled trials of SSRIs to examine the time course of response in OCD. The goals of this meta-analysis were (1) to examine the trajectory of medication response in OCD clinical trials to determine the optimal length of medication trials, (2) to examine the effects of SSRI dosage on the response curve, and (3) to compare the response curve and overall efficacy of different SSRI agents.
METHODS
Search Strategy
Two reviewers (Y.I. and C.A.B.) searched PubMed and Cochrane Central Register of Controlled Trials (CENTRAL) on May 22, 2013, for eligible trials using the search terms “serotonin uptake inhibitors” [MeSH] OR “serotonin uptake inhibitors” [Pharmacologic Action] AND “obsessive-compulsive disorder” [MeSH]. Results were limited to randomized controlled trials or meta-analyses. There were no language limitations on the search. The reference lists of relevant SSRI meta-analyses were searched for additional citations of potential trials.3-10
Study Selection
Studies included in this meta-analysis (1) were randomized, double-blind, placebo-controlled clinical trials; (2) enrolled adults with OCD; (3) compared SSRI pharmacotherapy to placebo; and (4) measured OCD symptom severity using the Yale-Brown Obsessive Compulsive Scale (Y-BOCS).13,14 Trials were excluded from this analysis if they were discontinuation studies, secondary analyses of otherwise included trials, or studies that did not provide Y-BOCS data on the study population between baseline and end point.
Data Extraction
Included trials provided weekly or biweekly Y-BOCS scores or change in Y-BOCS for up to 12 weeks of treatment. Some trials explicitly reported the weekly values in a table, while others provided a graph of Y-BOCS scores or change in Y-BOCS over time. A computer program (Dexter; German Astrophysical Virtual Observatory, University of Heidelberg, Germany) was used to extract weekly numerical data from these graphs. Dexter is a computer program that allows for accurate data extraction from figures by defining length and scale of x- and y-axes and then assigning values to selected data points on this scale. Additional data were collected on type of medication utilized, maximum dosage of medication, duration of trial, and year of trial. All medication doses were transformed into imipramine-equivalent doses using previously described methodology.15 In brief, each SSRI was standardized to imipramine based on the average recommended daily dose of each medication for depression and other conditions. Conversion factors into imipramine equivalents for SSRIs were as follows: fluoxetine (5), fluvoxamine (1), sertraline (1.2), paroxetine (5), citalopram (3.33), and escitalopram (6.66). Results are presented in fluoxetine equivalents (imipramine equivalents divided by 5) for the graphical representation of the dose comparison to make them more relevant to clinicians.

- Selective serotonin reuptake inhibitors (SSRIs) are an effective treatment for obsessive-compulsive disorder (OCD). However, little research has examined time course of response of OCD symptoms. Clinical lore suggests that OCD patients may be slower to respond to SSRIs than patients with depression.
- Meta-analysis suggests that the greatest incremental improvement from SSRIs occurs early in treatment and decreases with time. On average, more than 75% of improvement in SSRI trials was observed by 6 weeks.
- The response to SSRIs in OCD appears similar to that in depression, but symptom improvement may take a longer time to become clinically noticeable in OCD.
Meta-Analysis Methods
The statistical analysis for this trial was adapted from a previously published meta-analysis16 examining the response curve of SSRIs for major depression. All statistical analyses were performed in SAS 9.2 (PROC MIXED; SAS Institute Inc; Cary, North Carolina) using code adopted from this previous meta-analysis. For each trial at each available weekly time point (up to week 12), we calculated weighted mean difference (WMD) for the difference in Y-BOCS improvement between the medication and placebo groups. Weighted mean difference can be interpreted as the true treatment effect of SSRIs and is calculated as the difference (SSRI − placebo) in Y-BOCS score at a given time point.17 We used generalized estimating equations to examine the effects of trial and treatment, modeling different forms of the treatment effect (see tested models below), accounting for different periods within trials as repeated measures, and defining a new covariance structure for each trial as a random effect (see Taylor et al16 for further details on methodology). Each trial’s point estimate of WMD was weighted by the number of randomized patients in that trial.
Treatment effects were modeled as (1) a sudden-onset treatment response equating to a step function at week 4, (2) a step function at week 4 and a linearly increasing treatment effect thereafter, (3) a linear treatment effect, (4) a logarithmically decreasing treatment effect defined as log (week + 1), (5) a decreasing treatment effect defined as the square root of week, or (6) an exponentially increasing treatment effect described as eweek. An autoregressive variance function was used, and the best-fitting model was selected using the Akaike information criterion. To provide a point of contrast for the longitudinal meta-analysis data, we additionally conducted a fixed-effects meta-analysis in Comprehensive Meta-Analysis 3.0 (Biostat; Englewood, New Jersey) using WMD as the primary outcome at any time points where at least half the included trials provided data. We also ran the same analyses examining the improvement from baseline in Y-BOCS in the placebo and SSRI treatment groups as outcomes. The shape of the response curve for the true treatment effect of SSRIs (SSRI improvement-placebo improvement) may differ from the actual improvement in the SSRI or placebo groups that includes other nonspecific effects, such as natural course and variation in the disease, regression toward the mean, other time effects, and unidentified parallel interventions.17 Once the best-fitting model of response trajectory was established for WMD, we examined several additional questions in secondary analyses. We examined (1) the effects of dose (imipramine-equivalent dose), (2) year of publication, and (3) individual SSRI medication on the SSRI response curve. In these models, we added both a main effect of study week and the interaction between the variable of interest and study week. The main effects of the variable of interest were not included in the models, as they are trivial. (Baseline differences correspond to the main effect term in a mixed model. The variables of interest should not cause differences between medication and placebo at baseline, before medication is given.) When examining the effects of dose, we additionally examined models, taking into account the possibility that the effect of dose would be delayed (as it typically takes 2-4 weeks to titrate to the maximum SSRI dose in OCD trials). We explored dose effects that started at baseline, week 2, week 4, or week 6 by adding a main effect of a dummy variable indicating whether the time point was greater than or equal to the week of interest and added that dummy variable to the interaction term to indicate at what point that term would contribute to the model. We chose to examine the effects of year of publication, as previous meta-analyses5,18,19 have demonstrated decreasing benefit of antidepressants with publication year in OCD, major depression, and anxiety disorders. This effect of publication year has been attributed to increasing placebo response rates in later trials and the effect of time-lag bias.18,20
RESULTS
Included Trials
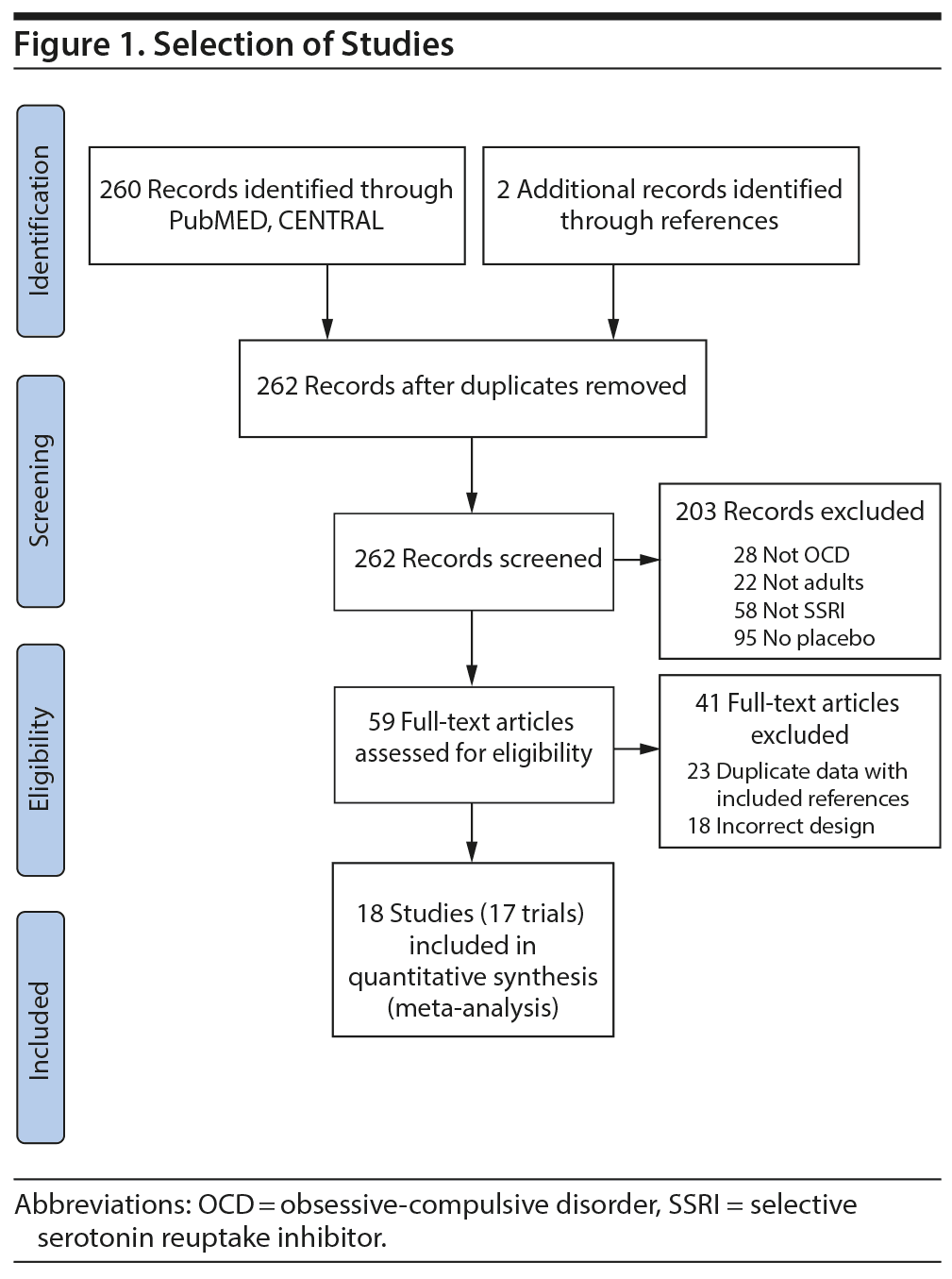
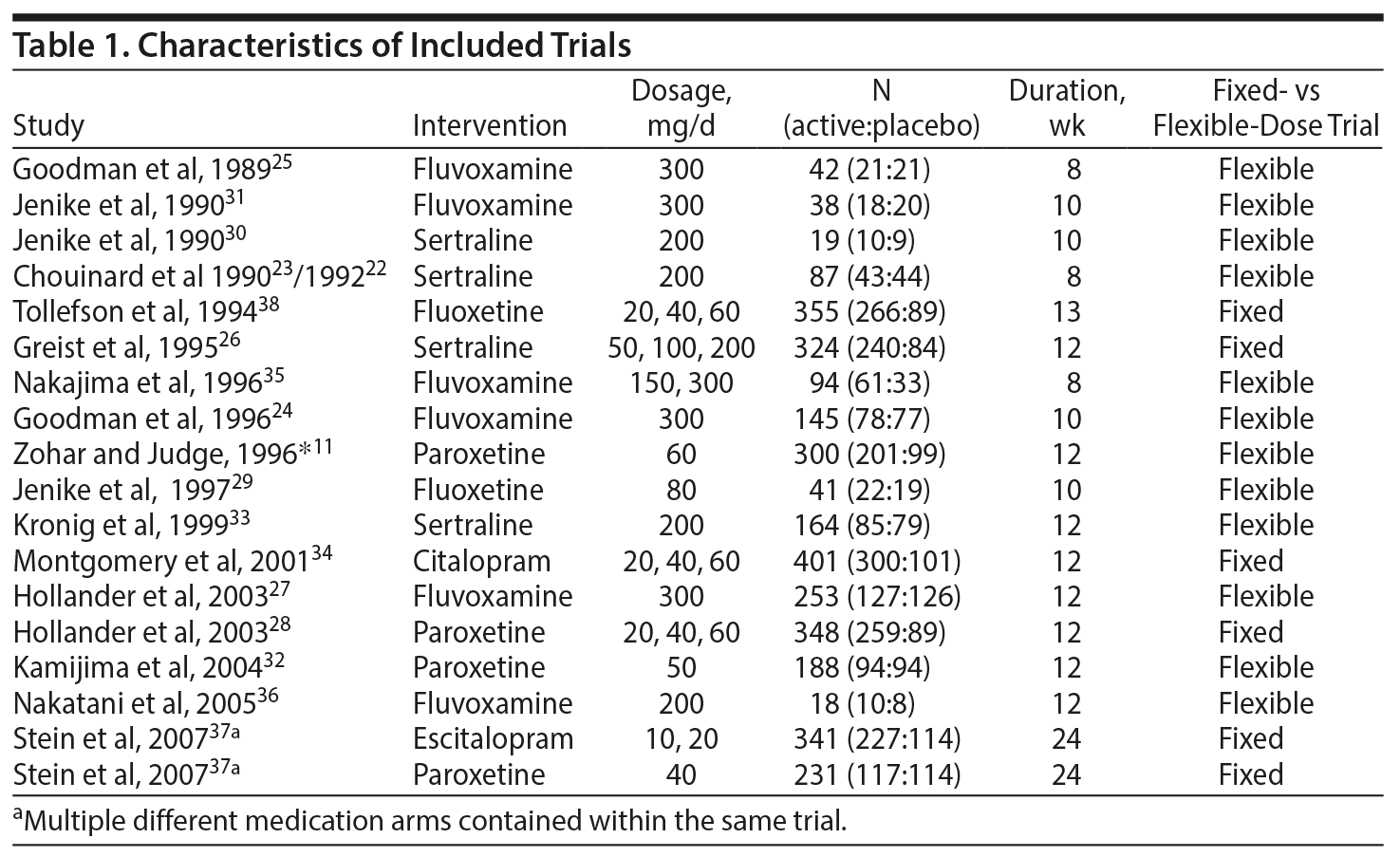
Figure 1 depicts the procedure for selection of studies. Seventeen trials involving 3,275 subjects compared SSRIs to placebo in the treatment of OCD. Table 1 depicts the characteristics of included trials. SSRIs examined included fluvoxamine (6 studies, n = 590), fluoxetine (2 studies, n = 396), paroxetine (4 studies, n = 1,067), sertraline (4 studies, n = 594), citalopram (1 study, n = 401), and escitalopram (1 study, n = 341).11,21-37
Best-Fitting Model of SSRI Response
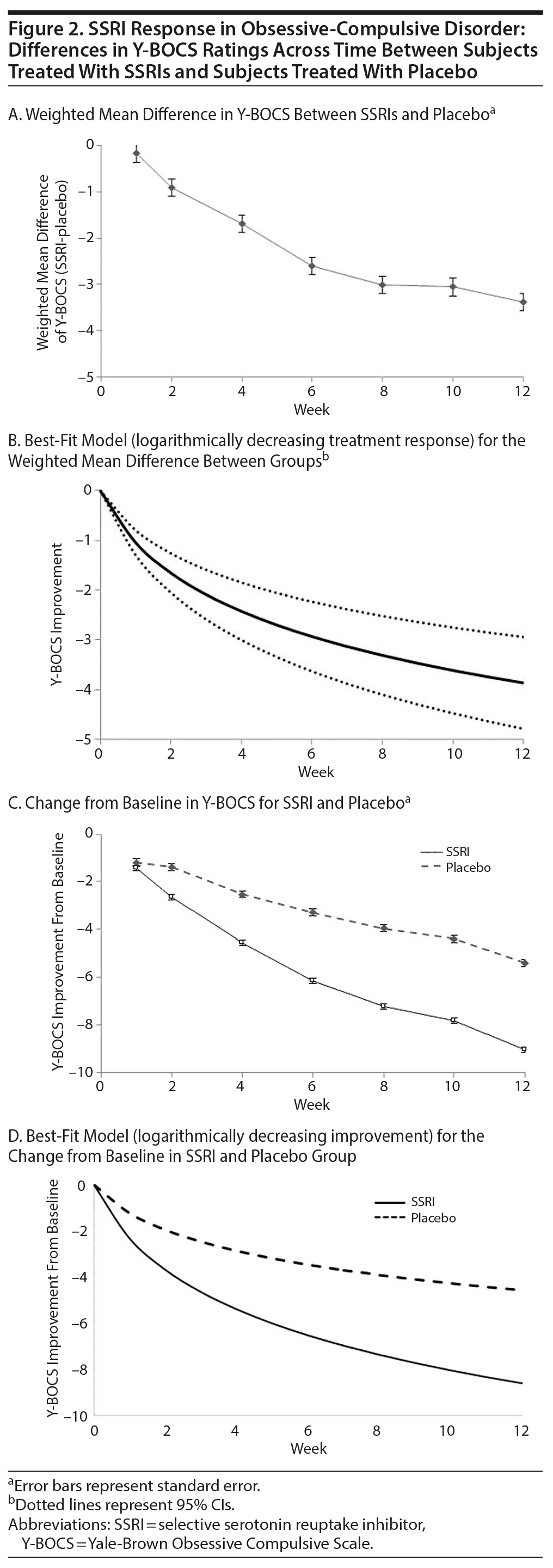
SSRIs demonstrated a significant benefit compared to placebo after 2 weeks of treatment (WMD = −0.91 [95% CI, −0.54 to −1.28], P < .001; Figure 2A). The best-fitting model for the overall SSRI treatment response was a logarithmic treatment effect. The estimate of treatment effect by log (week + 1) from the final model was 1.51 (95% CI, 1.14 to 1.87; P < .001). This response curve indicates the incremental treatment effect was greatest in the first week, with a gradual decline in the magnitude of incremental benefit week by week. Figure 2B depicts the best-fitting treatment response curve of SSRIs in OCD as well as the average improvement experienced in the first 12 weeks in included trials.
Based on Akaike information criterion, the logarithmic treatment model was nominally better than a model using the square root of week, but not to a statistically significant degree (χ2 = 0.9, P = .34). A model using square root of week similarly models a decreasing treatment effect with time. The logarithmic treatment effect model was significantly better than a model using a constant effect of time (χ2 = 5.1, P = .02), a model with a treatment response as a step-function at week 4 (χ2 = 30.9, P < .001), a model with treatment response as a step-function at week 4 and then a constant improvement thereafter (χ2 = 3.6, P = .05), and a model using an exponential effect of time (χ2 = 69.9, P < .001).
Logarithmic models also provided the best fit for describing the improvement from baseline in Y-BOCS in the placebo and SSRI treatment groups. Figure 2C provides data from meta-analysis of individual time point data in placebo and SSRI treatment groups, and Figure 2D depicts the best-fit model for improvement in Y-BOCS from baseline in each treatment group.
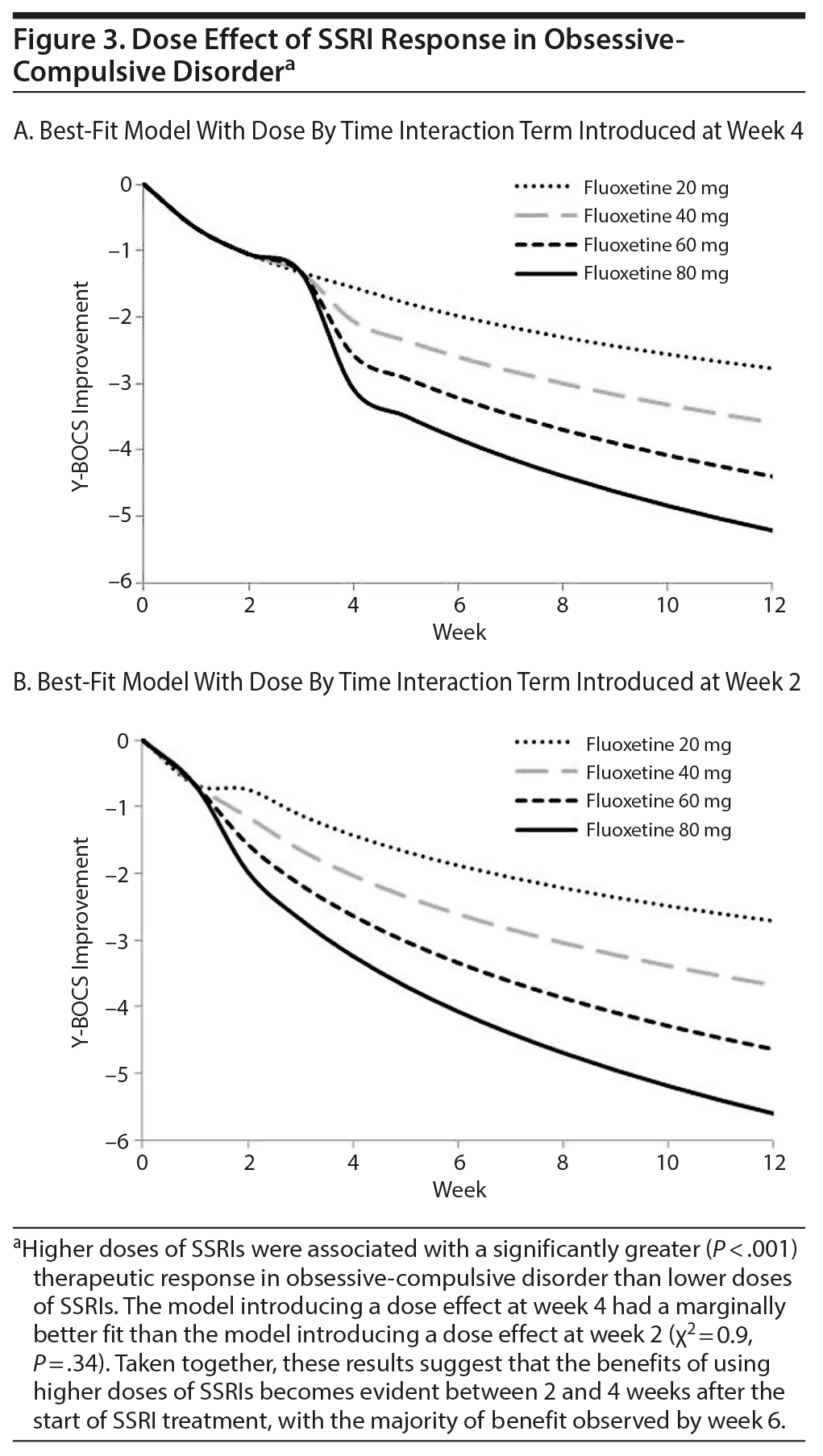
SSRI Dose
Higher SSRI doses were associated with significantly larger treatment effects. The best-fitting model indicated that the benefit of higher doses became evident at week 4. The model adding the dose effect term at week 4 was significantly better than models adding the dose effect at baseline (χ2 = 8.7, P = .003) and at week 6 (χ2 = 10.8, P = .001), but not at week 2 (χ2 = 0.9, P = .34); this may simply reflect the time taken to achieve the target dose in most studies. Figures 3A and 3B depict the best-fitting dose model for an SSRI dosing effect starting at week 4 and week 2, respectively. There was a significant effect of time (week 4: log [week + 1] = 0.96 [95% CI, 0.74 to 1.18], P < .0001; week 2: log [week + 1] = 0.97 [95% CI, 0.66 to 1.28], P < .0001), the dummy variable of week (week 4 = −0.50 [95% CI, -0.99 to −0.01], P = .046; week 2 = −0.74 [95% CI, −1.16 to −0.31], P = .007), and the interaction between dose and time (interaction = 0.0032 [95% CI, 0.0020 to 0.0043], P < .0001; week 2: interaction = 0.0038 [95% CI, 0.0024 to 0.0051], P < .0001). The significant benefit of increased SSRI dosing was robust to controlling for year of publication and to restricting analysis to individual SSRI agents.
Individual SSRI Agents
We found no significant differences between individual SSRI agents. There was no significant difference between fluoxetine, fluvoxamine, sertraline, paroxetine, citalopram, or escitalopram and other SSRIs in any of the models, whether adjustments were made or not for SSRI dosage and/or publication year.
DISCUSSION
This meta-analysis of the time course of SSRI response in OCD led to several insights into the trajectory of SSRI response in OCD. Statistically significant improvement in OCD symptoms can be observed within 2 weeks after the initiation of pharmacotherapy; this is similar to what is seen in depression16 but contrasts with the conventional wisdom that medication response in OCD is delayed. Symptom improvement to SSRIs in OCD follows a logarithmic model, with the greatest improvement happening early in treatment. Higher doses of SSRI are associated with greater improvement in OCD symptoms, as has been seen in analyses of end point data; this effect becomes most evident slightly later in OCD treatment (eg, weeks 3-6). Finally, no evidence suggests that any particular SSRI is more effective than any other in treating OCD.
The finding that the greatest drug-attributable SSRI benefit is observed early in OCD treatment has implications for neurobiological research into the mechanisms of SSRI efficacy. The primary mechanism of SSRI action—inhibition of serotonin transporters and a corresponding increase in serotonin levels—occurs rapidly both in vitro and in vivo. However, much research has focused on potential neurobiological mechanisms responsible for delayed SSRI response in OCD and depressive disorders. Neurobiological theories have focused on possible indirect mechanisms of action of SSRIs such as adaptive regulation of serotonin 1A receptors and, more recently, activation of second messengers and the resultant changes in gene expression.38,39 Specifically, increased production of brain-derived neurotrophic factor along with increased synaptic plasticity and neurogenesis have been hypothesized as indirect mechanisms of action that explain the delayed effects of antidepressants.39 Other research has focused on neuropsychological models for delayed antidepressant effects.40 Results from this meta-analysis, and a similar meta-analysis16 in depression, suggest that the greatest drug-attributable effects are seen proximal to the initiation of SSRIs. This implies that immediate mechanisms that produce early improvements in symptoms are important in SSRI action in OCD and major depressive disorder (MDD) and may be worthy of further study. The importance of (1) early effects of medication on overall response is supported by previous studies, which have observed that early side effects of clomipramine (constipation, dry mouth, nervousness, and heart palpitations) are associated with improved outcome,41 and (2) response to SSRI pharmacotherapy at 4 weeks has been previously demonstrated to be strongly associated with short-term response to treatment.42
The results of this meta-analysis are particularly relevant to OCD patients who report clinically significant benefit soon after initiating SSRI pharmacotherapy. Clinical experience suggests that a small proportion of OCD patients experience this phenomenon, but the improvement is often attributed by clinicians to the “placebo effect.” Our results suggest that an early, meaningful benefit is possible with an SSRI and that, in fact, the greatest incremental benefits from SSRI pharmacotherapy are observed soon after initiating treatment. Specifically, the response curve of SSRI pharmacologic trials demonstrates that, on average, more than half of the short-term improvement experienced by OCD patients in response to SSRIs is evident within the first 4 weeks of treatment and greater than three-quarters of the average improvement is observed by week 6.
The results of this meta-analysis may also be relevant to current OCD practice guidelines. The American Psychiatric Association (APA) Practice Guidelines for OCD currently state, “Most patients will not experience substantial improvement until 4-6 weeks after starting medication, and some who will ultimately respond will experience little improvement for as many as 10-12 weeks.”2(p12) Our results suggest that this observation is not supported by clinical trial data. Specifically, ongoing OCD symptom improvement with SSRIs decreases with time. Meta-analysis of the response curve of SSRI pharmacologic trials in OCD demonstrated that, on average, more than 80% of the short-term improvement experienced by OCD patients in response to SSRIs was evident by week 6 (and roughly 75% if patients were titrated to the highest recommend SSRI dose at the beginning of treatment). Expert consensus and APA Practice Guidelines currently recommend that SSRIs have trials lasting at least 10-12 weeks.2 Research examining the prognostic utility of early SRI response using individual patient data is needed before treatment guidelines should be changed. It remains possible that some patients experience delayed improvement on SSRIs that is largely washed out by the majority of patients who experience little change. Changing the treatment guidelines for SSRIs in OCD would be of limited practical import in the absence of novel pharmacologic alternatives that have greater efficacy and tolerability than currently available augmentation agents.43
Meta-analysis suggests that slightly more than half of adult OCD patients do not respond to SSRI pharmacotherapy.3 Standard-of-care SSRI treatment of OCD currently is at least 10-12 weeks in duration. We have few useful predictors of likelihood of response in individual OCD patients, and much time is thus wasted in long SSRI trials that ultimately prove to be without benefit. Our results raise the possibility that early SSRI response, or lack thereof, may have important prognostic value. However, examination of the prognostic utility of early SSRI response requires individual patient data, rather than the group data used in our analysis. Early improvement in both depression and anxiety symptoms has been demonstrated to be a fairly consistent and robust predictor of short-term medication response in MDD.44-50 Similarly, in OCD, early improvement (defined as a 20% reduction in Y-BOCS score at 4 weeks) significantly predicted treatment response at 12 weeks in a recent trial51 of fluoxetine. Early symptom improvement after 1 and 4 weeks of treatment with clomipramine has also been demonstrated to be the most discriminative of treatment response in clinical trials.41 Additional studies with individual patient data are needed to replicate these findings and to determine how predictive early SSRI improvement is in not only overall treatment response (which takes into account the earlier improvement from baseline) but also subsequent improvement in SSRI trials (ie, is OCD symptom improvement in the first 2 weeks of SSRI pharmacotherapy predictive of subsequent symptom improvement from weeks 2-12).
This meta-analysis supports the current treatment guidelines suggesting that raising SSRI dose is an advisable treatment strategy in the pharmacotherapy of OCD. We see a significant benefit of higher doses of SSRIs using longitudinal meta-analysis data. However, the analysis also assumed a linear relationship between SSRI dose and improvement. Previous meta-analysis12 using end point data and categorical SSRI dose categories has suggested that high-dose SSRI pharmacotherapy is marginally more effective than low-dose SSRI pharmacotherapy and that these benefits of dosage are particularly evident at the high end of the dose range. The data on high-dose SSRIs are restricted to a subset of all SSRI medications, and there is insufficient data to necessarily conclude that each of the 6 SSRI medications has an identical dose-response relationship within the US Food and Drug Administration-recommended dose range. Furthermore, there also exist warnings on the use of high-dose citalopram (United States and United Kingdom) and escitalopram (United Kingdom only) because of QTc prolongation. The current analysis extends previous SSRI dose meta-analysis12 by suggesting that appreciable treatment gains observed from high-dose SSRI dosing strategy started to occur between the second to fourth week of trials. Typically, OCD subjects are titrated to higher doses of SSRIs in fixed-dose trials and are in actuality taking lower doses during the first few weeks of treatment, which is likely to explain this result. On the basis of the data presented here, waiting 4 additional weeks after achieving the maximum tolerated dose appears to be a reasonable treatment strategy.
Given the potential clinical relevance of these findings, it is important to recognize limitations of this meta-analysis. Publication bias remains a potential limiting factor. Standard metrics of publication bias are difficult to apply to longitudinal data. However, the 17 SSRI trials cited in this meta-analysis are nearly identical to the trials included in a previous Cochrane Review3 on this subject that did not demonstrate any evidence of publication bias. This argues against any problematic effects of publication bias on our analysis. Another potential limitation is the possibility that the shape of the response curve for SSRIs across time may be influenced by the design of the underlying trials. Specifically, last-observation-carried-forward analysis of missing data could make a constant effect appear more logarithmic.16
This meta-analysis establishes that SSRI treatment gains in OCD are present within 2 weeks after the initiation of treatment and that response follows a logarithmic pattern, indicating a decreasing benefit of SSRIs with time. These findings are similar to a meta-analysis16 conducted on SSRI response in major depression that demonstrated a similar logarithmic treatment response over the first 6 weeks of treatment and statistically evident benefits after 1 week of treatment. Further comparison of the response data between MDD and OCD will be useful to examine whether the response curves of OCD and depression symptoms to SSRIs are actually different from one another.
Nonetheless, meta-analysis suggests that, on average, 75%-80% of the treatment gains of SSRIs (compared to placebo) are evident within 6 weeks, regardless of the target dose. Given that the majority of treatment gains in OCD occur early in treatment, there remains a strong possibility that early SSRI response in OCD may be of prognostic value in forecasting ultimate pharmacotherapy outcome. Pharmacologic research in OCD should focus on using individual patient data from SSRI trials to examine the prognostic utility of early SSRI response on 12-week patient outcomes. Such analyses may be useful in shortening the length of eventually futile SSRI trials for many patients with OCD.
Submitted: December 23, 2014; accepted May 21, 2015.
Drug names: citalopram (Celexa and others), clomipramine (Anafranil and others), escitalopram (Lexapro and others), fluoxetine (Prozac and others), fluvoxamine (Luvox and others), imipramine (Tofranil, Surmontil, and others), paroxetine (Paxil, Pexeva, and others), sertraline (Zoloft and others).
Potential conflicts of interest: The authors have no conflicts of interest to disclose.
Funding/support: Dr Bloch gratefully acknowledges support from the National Institute of Mental Health support of the Trichotillomania Learning Center, the Yale Child Study Center Research Training Program, the National Institutes of Health (NIH) grant 1K23MH091240, the APIRE/Eli Lilly Psychiatric Research Fellowship, the AACAP/Eli Lilly Junior Investigator Award, and NARSAD; the State of Connecticut also provided resource support via the Abraham Ribicoff Research Facilities at the Connecticut Mental Health Center; and UL1 RR024139 from the National Center for Research Resources, a component of the NIH, and NIH roadmap for Medical Research. Dr Pittenger acknowledges the support of the Allison Family Foundation and the NIH (R34MH083115).
Role of the sponsor: The sponsors had no role in the conduct or design of this study.
REFERENCES
1. Bandelow B. The medical treatment of obsessive-compulsive disorder and anxiety. CNS Spectr. 2008;13(suppl 14):37-46. PubMed
2. American Psychiatric Association. Treatment of Patients with Obsessive-Compulsive Disorder. Arlington, VA: American Psychiatric Association; 2007.
3. Soomro GM, Altman D, Rajagopal S, et al. Selective serotonin re-uptake inhibitors (SSRIs) versus placebo for obsessive compulsive disorder (OCD). Cochrane Database Syst Rev. 2008;(1):CD001765. PubMed
4. Abramowitz JS. Effectiveness of psychological and pharmacological treatments for obsessive-compulsive disorder: a quantitative review. J Consult Clin Psychol. 1997;65(1):44-52. PubMed doi:10.1037/0022-006X.65.1.44
5. Ackerman DL, Greenland S. Multivariate meta-analysis of controlled drug studies for obsessive-compulsive disorder. J Clin Psychopharmacol. 2002;22(3):309-317. PubMed doi:10.1097/00004714-200206000-00012
6. Cox BJ, Swinson RP, Morrison B, et al. Clomipramine, fluoxetine, and behavior therapy in the treatment of obsessive-compulsive disorder: a meta-analysis. J Behav Ther Exp Psychiatry. 1993;24(2):149-153. PubMed doi:10.1016/0005-7916(93)90043-V
7. Greist JH, Jefferson JW, Kobak KA, et al. Efficacy and tolerability of serotonin transport inhibitors in obsessive-compulsive disorder: a meta-analysis. Arch Gen Psychiatry. 1995;52(1):53-60. PubMed doi:10.1001/archpsyc.1995.03950130053006
8. Kobak KA, Greist JH, Jefferson JW, et al. Behavioral versus pharmacological treatments of obsessive compulsive disorder: a meta-analysis. Psychopharmacology (Berl). 1998;136(3):205-216. PubMed doi:10.1007/s002130050558
9. Piccinelli M, Pini S, Bellantuono C, et al. Efficacy of drug treatment in obsessive-compulsive disorder: a meta-analytic review. Br J Psychiatry. 1995;166(4):424-443. PubMed doi:10.1192/bjp.166.4.424
10. Stein DJ, Spadaccini E, Hollander E. Meta-analysis of pharmacotherapy trials for obsessive-compulsive disorder. Int Clin Psychopharmacol. 1995;10(1):11-18. PubMed doi:10.1097/00004850-199503000-00002
11. Zohar J, Judge R; OCD Paroxetine Study Investigators. Paroxetine versus clomipramine in the treatment of obsessive-compulsive disorder. Br J Psychiatry. 1996;169(4):468-474. PubMed doi:10.1192/bjp.169.4.468
12. Bloch MH, McGuire J, Landeros-Weisenberger A, et al. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry. 2010;15(8):850-855. PubMed doi:10.1038/mp.2009.50
13. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale, II: validity. Arch Gen Psychiatry. 1989;46(11):1012-1016. PubMed doi:10.1001/archpsyc.1989.01810110054008
14. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale, I: development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006-1011. PubMed doi:10.1001/archpsyc.1989.01810110048007
15. Bollini P, Pampallona S, Tibaldi G, et al. Effectiveness of antidepressants: meta-analysis of dose-effect relationships in randomised clinical trials. Br J Psychiatry. 1999;174(4):297-303. PubMed doi:10.1192/bjp.174.4.297
16. Taylor MJ, Freemantle N, Geddes JR, et al. Early onset of selective serotonin reuptake inhibitor antidepressant action: systematic review and meta-analysis. Arch Gen Psychiatry. 2006;63(11):1217-1223. PubMed doi:10.1001/archpsyc.63.11.1217
17. Ernst E, Resch KL. Concept of true and perceived placebo effects. BMJ. 1995;311(7004):551-553. PubMed doi:10.1136/bmj.311.7004.551
18. Bridge JA, Birmaher B, Iyengar S, et al. Placebo response in randomized controlled trials of antidepressants for pediatric major depressive disorder. Am J Psychiatry. 2009;166(1):42-49. PubMed doi:10.1176/appi.ajp.2008.08020247
19. Walsh BT, Seidman SN, Sysko R, et al. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287(14):1840-1847. PubMed doi:10.1001/jama.287.14.1840
20. Reyes MM, Panza KE, Martin A, et al. Time-lag bias in trials of pediatric antidepressants: a systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2011;50(1):63-72. PubMed doi:10.1016/j.jaac.2010.10.008
21. Chouinard G. Sertraline in the treatment of obsessive compulsive disorder: two double-blind, placebo-controlled studies. Int Clin Psychopharmacol. 1992;7(suppl 2):37-41. PubMed doi:10.1097/00004850-199210002-00007
22. Chouinard G, Goodman W, Greist J, et al. Results of a double-blind placebo controlled trial of a new serotonin uptake inhibitor, sertraline, in the treatment of obsessive-compulsive disorder. Psychopharmacol Bull. 1990;26(3):279-284. PubMed
23. Goodman WK, Kozak MJ, Liebowitz M, et al. Treatment of obsessive-compulsive disorder with fluvoxamine: a multicentre, double-blind, placebo-controlled trial. Int Clin Psychopharmacol. 1996;11(1):21-29. PubMed doi:10.1097/00004850-199603000-00003
24. Goodman WK, Price LH, Rasmussen SA, et al. Efficacy of fluvoxamine in obsessive-compulsive disorder: a double-blind comparison with placebo. Arch Gen Psychiatry. 1989;46(1):36-44. PubMed doi:10.1001/archpsyc.1989.01810010038006
25. Greist JH, Jefferson JW, Kobak KA, et al. A 1 year double-blind placebo-controlled fixed dose study of sertraline in the treatment of obsessive-compulsive disorder. Int Clin Psychopharmacol. 1995;10(2):57-65. PubMed doi:10.1097/00004850-199506000-00001
26. Hollander E, Allen A, Steiner M, et al; Paroxetine OCD Study Group. Acute and long-term treatment and prevention of relapse of obsessive-compulsive disorder with paroxetine. J Clin Psychiatry. 2003;64(9):1113-1121. PubMed doi:10.4088/JCP.v64n0919
27. Hollander E, Koran LM, Goodman WK, et al. A double-blind, placebo-controlled study of the efficacy and safety of controlled-release fluvoxamine in patients with obsessive-compulsive disorder. J Clin Psychiatry. 2003;64(6):640-647. PubMed doi:10.4088/JCP.v64n0604
28. Jenike MA, Baer L, Minichiello WE, et al. Placebo-controlled trial of fluoxetine and phenelzine for obsessive-compulsive disorder. Am J Psychiatry. 1997;154(9):1261-1264. PubMed doi:10.1176/ajp.154.9.1261
29. Jenike MA, Baer L, Summergrad P, et al. Sertraline in obsessive-compulsive disorder: a double-blind comparison with placebo. Am J Psychiatry. 1990;147(7):923-928. PubMed
30. Jenike MA, Hyman S, Baer L, et al. A controlled trial of fluvoxamine in obsessive-compulsive disorder: implications for a serotonergic theory. Am J Psychiatry. 1990;147(9):1209-1215. PubMed doi:10.1176/ajp.147.9.1209
31. Kamijima K, Murasaki M, Asai M, et al. Paroxetine in the treatment of obsessive-compulsive disorder: randomized, double-blind, placebo-controlled study in Japanese patients. Psychiatry Clin Neurosci. 2004;58(4):427-433. PubMed doi:10.1111/j.1440-1819.2004.01278.x
32. Kronig MH, Apter J, Asnis G, et al. Placebo-controlled, multicenter study of sertraline treatment for obsessive-compulsive disorder. J Clin Psychopharmacol. 1999;19(2):172-176. PubMed doi:10.1097/00004714-199904000-00013
33. Montgomery SA, Kasper S, Stein DJ, et al. Citalopram 20 mg, 40 mg and 60 mg are all effective and well tolerated compared with placebo in obsessive-compulsive disorder. Int Clin Psychopharmacol. 2001;16(2):75-86. PubMed doi:10.1097/00004850-200103000-00002
34. Nakajima T, Kudo Y, Yamashita I. Clinical usefulness of Fluvoxamine Maleate (SME3110), a selective serotonin reuptake inhibitor, in the treatment of obsessive compulsive disorder: a double blind, placebo-controlled study investigating the therapeutic dose range and the efficacy of SME3110. J Clin Therapeutics & Medicine. 1996;12(3):409-437.
35. Nakatani E, Nakagawa A, Nakao T, et al. A randomized controlled trial of Japanese patients with obsessive-compulsive disorder—effectiveness of behavior therapy and fluvoxamine. Psychother Psychosom. 2005;74(5):269-276. PubMed doi:10.1159/000086317
36. Stein DJ, Andersen EW, Tonnoir B, et al. Escitalopram in obsessive-compulsive disorder: a randomized, placebo-controlled, paroxetine-referenced, fixed-dose, 24-week study. Curr Med Res Opin. 2007;23(4):701-711. PubMed doi:10.1185/030079907X178838
37. Tollefson GD, Rampey AH Jr, Potvin JH, et al. A multicenter investigation of fixed-dose fluoxetine in the treatment of obsessive-compulsive disorder. Arch Gen Psychiatry. 1994;51(7):559-567. PubMed doi:10.1001/archpsyc.1994.03950070051010
38. Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54(7):597-606. PubMed doi:10.1001/archpsyc.1997.01830190015002
39. Manji HK, Quiroz JA, Sporn J, et al. Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression. Biol Psychiatry. 2003;53(8):707-742. PubMed doi:10.1016/S0006-3223(03)00117-3
40. Harmer CJ, Goodwin GM, Cowen PJ. Why do antidepressants take so long to work? a cognitive neuropsychological model of antidepressant drug action. Br J Psychiatry. 2009;195(2):102-108. PubMed doi:10.1192/bjp.bp.108.051193
41. Ackerman DL, Greenland S, Bystritsky A. Use of receiver-operator characteristic (ROC) curve analysis to evaluate predictors of response to clomipramine therapy. Psychopharmacol Bull. 1996;32(1):157-165. PubMed
42. da Conceiç×£o Costa DL, Shavitt RG, Castro Cesar RC, et al. Can early improvement be an indicator of treatment response in obsessive-compulsive disorder? Implications for early-treatment decision-making. J Psychiatr Res. 2013;47(11):1700-1707. PubMed doi:10.1016/j.jpsychires.2013.07.006
43. Pittenger C, Bloch MH. Pharmacological treatment of obsessive-compulsive disorder. Psychiatr Clin North Am. 2014;37(3):375-391. PubMed doi:10.1016/j.psc.2014.05.006
44. Jakubovski E, Bloch MH. Prognostic subgroups for citalopram response in the STAR*D trial. J Clin Psychiatry. 2014;75(7):738-747. PubMed doi:10.4088/JCP.13m08727
45. Gollan JK, Fava M, Kurian B, et al. What are the clinical implications of new onset or worsening anxiety during the first two weeks of SSRI treatment for depression? Depress Anxiety. 2012;29(2):94-101. PubMed doi:10.1002/da.20917
46. Henkel V, Seemüller F, Obermeier M, et al. Does early improvement triggered by antidepressants predict response/remission? analysis of data from a naturalistic study on a large sample of inpatients with major depression. J Affect Disord. 2009;115(3):439-449. PubMed doi:10.1016/j.jad.2008.10.011
47. Johansson H, Jansson JA. Therapeutic alliance and outcome in routine psychiatric out-patient treatment: patient factors and outcome. Psychol Psychother. 2010;83(pt 2):193-206. PubMed doi:10.1348/147608309X472081
48. Kemp DE, Ganocy SJ, Brecher M, et al. Clinical value of early partial symptomatic improvement in the prediction of response and remission during short-term treatment trials in 3369 subjects with bipolar I or II depression. J Affect Disord. 2011;130(1-2):171-179. PubMed doi:10.1016/j.jad.2010.10.026
49. Szegedi A, Jansen WT, van Willigenburg AP, et al. Early improvement in the first 2 weeks as a predictor of treatment outcome in patients with major depressive disorder: a meta-analysis including 6562 patients. J Clin Psychiatry. 2009;70(3):344-353. PubMed doi:10.4088/JCP.07m03780
50. Tadić A, Gorbulev S, Dahmen N, et al; EMC Study Group. Rationale and design of the randomised clinical trial comparing early medication change (EMC) strategy with treatment as usual (TAU) in patients with major depressive disorder—the EMC trial. Trials. 2010;11(1):21. PubMed doi:10.1186/1745-6215-11-21
51. da Conceiç×£o Costa DL, Shavitt RG, Castro Cesar RC, et al. Can early improvement be an indicator of treatment response in obsessive-compulsive disorder? implications for early-treatment decision-making. J Psychiatr Res. 2013;47(11):1700-1707. PubMed doi:10.1016/j.jpsychires.2013.07.006
Enjoy free PDF downloads as part of your membership!
Save
Cite
Advertisement
GAM ID: sidebar-top